Response of Benthic Diatom Assemblages to Contamination by Metals in a Marine Environment
Abstract
1. Introduction
2. Study Area
3. Materials and Methods
3.1. Diatom Mounts
3.2. Measurements of Metal Concentration
4. Results
4.1. Metal Concentrations
4.2. Normalized Enrichment Factor (NEF)
4.3. Diatom Assemblages
4.4. Diatom Species Diversity
4.5. Control Site Diatoms
5. Discussion
5.1. Metals in Sediments
5.2. Benthic Diatoms
5.3. Element Contamination Comparison with Benthic Diatoms
5.4. Deformed Frustules
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryan, G.; Hummerston, E.L. Adaptation of the polychaete Nereis diversicolor to estuarine sediments containing high concentrations of heavy metals. General observations and adaptation to copper. J. Mar. Biol. Assoc. U. K. 1971, 51, 845–863. [Google Scholar] [CrossRef]
- Méndez-Ubach, N.; Green-Ruíz, C. Advantages and disadvantages of performing ecotoxicological bioassays with larvae of polychaetes belonging to the Capitella capitata species-complex. CICIMAR-Oceánides 2006, 21, 145–151. [Google Scholar] [CrossRef]
- Siqueiros-Beltrones, D.A. Diatomeas Bentónicas de la Península de Baja California; Diversidad y Potencial Ecológico; Centro Interdisciplinario de Ciencias Marinas-Instituto Politécnico Nacional/Universidad Autónoma de Baja California Sur: La Paz, México, 2002; 102p, ISBN 970-18-7595-8. [Google Scholar]
- Siqueiros-Beltrones, D.A. Una paradoja sobre uniformidad vs. orden y estabilidad en la medida de la diversidad de especies según la teoría de la información. Ludus Vitalis 2005, 13, 1–10. [Google Scholar]
- Ivorra, N.; Hettelaar, J.; Tubbin, G.M.J.; Kraak, M.H.S.; Sabater, S.; Admiral, W. Translocation of microbenthic algal assemblages used for in situ analysis of metal pollution in rivers. Arch. Environ. Contam. Toxicol. 1999, 37, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Couillard, Y.; Wunsam, S.; Courcelles, M. Diatom taxonomic and morphological changes as indicators of metal pollution and recovery in Lac Dufault (Québec, Canada). J. Paleolimnol. 2004, 32, 163–175. [Google Scholar] [CrossRef]
- Dickman, M.D. Benthic marine diatom deformities associated with contaminated sediments in Hong Kong. Environ. Int. 1998, 24, 749–759. [Google Scholar] [CrossRef]
- Pandey, L.K.; Sharma, Y.S.; Park, J.; Choi, S.; Lee, H.; Lyu, J.; Han, T. Evaluating features of periphytic diatom communities as biomonitoring tools in fresh, brackish and marine Waters. Aquat. Toxicol. 2018, 194, 67–77. [Google Scholar] [CrossRef]
- Stauber, J.L.; Florence, T.M. Mechanism of toxicity of zinc to the marine diatom Nitzschia closterium. Mar. Biol. 1990, 105, 519–524. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Hampel, M.; Lubián, L.M.; Blasco, J. Sediment toxicity tests using benthic marine microalgae Cylindrotheca closterium (Ehremberg) Lewin and Reimann (Bacillariophyceae). Ecotoxicol. Environ. Saf. 2003, 54, 290–295. [Google Scholar] [CrossRef]
- Adams, M.S.; Stauber, J.L. Development of a whole-sediment toxicity test using a benthic marine microalga. Environ. Toxicol. Chem. 2004, 23, 1957–1968. [Google Scholar] [CrossRef]
- Franklin, N.M.; Stauber, J.L.; Lim, R.P. Development of flow cytometry-based algal bioassays for assessing toxicity of copper in natural waters. Environ. Toxicol. Chem. 2001, 20, 160–170. [Google Scholar] [CrossRef]
- Anantharaj, K.; Govindasamy, C.; Natanamurugaraj, G.; Jeyachandran, S. Effect of Heavy Metals on Marine Diatom Amphora coffeaeformis (Agardh. Kutz.). Glob. J. Environ. Res. 2011, 5, 112–117. [Google Scholar]
- Manimaran, K.; Karthikeyan, P.; Ashokkumar, S.; Ashokprabu, V.; Sampathkumar, P. Effect of copper on growth and enzyme activities of marine diatom, Odontella mobiliensis. Bull. Environ. Contam. Toxicol. 2012, 88, 30–37. [Google Scholar] [CrossRef]
- French, M.S.; Evans, L.V. The effects of copper and zinc on growth of the fouling diatoms Amphora and Amphiprora. Biofouling 1988, 1, 3–18. [Google Scholar] [CrossRef]
- Wang, M.J.; Wang, W.X. Cadmium in three marine phytoplankton: Accumulation, subcellular fate and thiol induction. Aquat. Toxicol. 2009, 95, 99–107. [Google Scholar] [CrossRef]
- Miao, A.J.; Wang, W.X. Cadmium toxicity to two marine phytoplankton under different nutrient conditions. Aquat. Toxicol. 2006, 78, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Siqueiros-Beltrones, D.A. Diatomeas bentónicas de la Laguna Figueroa, Baja California. Cienc. Mar. 1988, 14, 85–112. [Google Scholar] [CrossRef]
- Siqueiros-Beltrones, D.A.; Argumedo-Hernández, U.; Hernández-Almeida, O.U. High species diversity (H′) of benthic diatoms in a coastal lagoon located within a natural protected area. Hidrobiológica 2017, 27, 293–300. [Google Scholar] [CrossRef]
- Cunningham, L.; Stark, J.S.; Snape, I.; McMinn, A.; Riddle, M.J. Effects of metal and petroleum hydrocarbons on benthic diatom communities near Casey Station, Antarctica: An experimental approach. J. Phycol. 2003, 39, 490–503. [Google Scholar] [CrossRef]
- Cunningham, L.; Raymond, B.; Snape, I.; Riddle, M.J. Benthic diatoms communities as indicators of anthropogenic metal contamination at Casey Station, Antarctica. J. Paleolimnol. 2005, 33, 499–513. [Google Scholar] [CrossRef]
- Petrov, A.; Nevrova, E.; Terletskaya, A.; Milyukin, M.; Demchenko, V. Structure and taxonomic diversity of benthic diatom assemblage in a polluted marine environment (Balaklava Bay, Black Sea). Pol. Bot. J. 2010, 55, 183–197. [Google Scholar]
- Rubino, F.; Cibic, T.; Belmonte, M.; Rogelja, M. Microbenthic community structure and trophic status of sediments in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea). Environ. Sci. Pollut. Res. 2016, 23, 12624–12644. [Google Scholar] [CrossRef] [PubMed]
- Potapova, M.; Desianti, N.; Enache, M. Potential effects of sediment contaminants on diatom assemblages in coastal lagoons of New Jersey and New York States. Mar. Pollut. Bull. 2016, 107, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Shumilin, E.; Rodríguez-Figueroa, G.; Bermea, O.M.; Baturina, E.L.; Hernández, E.; Meza, G.D.R. Anomalous trace element composition of coastal sediments near the copper mining district of Santa Rosalía, Peninsula of Baja California, Mexico. Bull. Environ. Contam. Toxicol. 2000, 65, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Shumilin, E.; Gordeev, V.; Rodríguez-Figueroa, G.; Demina, L.; Choumiline, K. Assessement of geochemical mobility of metals in surface sediments of the Santa Rosalía mining region, Western Gulf of California. Arch. Environ. Contam. Toxicol. 2011, 60, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Shumilin, E.; Rodríguez-Figueroa, G.; Sapozhnikov, D.; Sapozhnikov, Y.; Choumiline, K. Anthropogenic and Authugenic Uranium in marine sediments od the Central Gulf of California adjacent to the Santa Rosalía mining region. Arch. Environ. Contam. Toxicol. 2012, 63, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Shumilin, E.; Jiménez-Illescas, A.R.; López-López, S. Anthropogenic contamination of metals in sediments of the Santa Rosalía harbor, Baja California Península. Bull. Environ. Contam. Toxicol. 2012, 90, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, M.P.; Shumilin, E.; Rodríguez-Figueroa, M.G.; Rodríguez-Espinoza, P.F.; Sujitha, S.B. Potential toxicity of chemical elements in beach sediments near Santa Rosalia copper mine, Baja California Peninsula, Mexico. Estuar. Coast. Shelf Sci. 2016, 180, 91–96. [Google Scholar] [CrossRef]
- Volke-Sepúlveda, T.; Solórzano-Ochoa, G.; Rosas-Domínguez, A.; Izumikawa, C.; Velasco-Trejo, J.A. Remediación de sitios contaminados por metales provenientes de Jales mineros en los Distritos de El triunfo- San Antonio y Santa Rosalía, Baja California Sur. Cent. Nac. Investig. Capacit. Ambient. Inf. Final 2003, 1–36. [Google Scholar] [CrossRef]
- Rodríguez-Figueroa, G.; Shumilin, E.; Sánchez-Rodríguez, L. Heavy metal pollution monitoring using the brown seaweed Padina durvillaei in the coastal zone of the Santa Rosalía mining region, Baja California Peninsula, Mexico. J. Appl. Phycol. 2009, 21, 19–26. [Google Scholar] [CrossRef]
- Lavín, M.F.; Castro, R.; Beier, E.; Godínez, V.M.; Amador, A.; Guest, P. SST, thermohaline structure, and circulation in the southern Gulf of California in June 2004 during the North American Monsoon Experiment. J. Geophys. Res. 2009, 114, 1–22. [Google Scholar] [CrossRef]
- Pantoja, D.A.; Marinone, S.G.; Parés-Sierra, A.; Gómez-Valdivia, F. Numerical modeling of seasonal and mesoscale hydrography and circulation in the Mexican Central Pacific. Cienc. Mar. 2012, 38, 363–379. [Google Scholar] [CrossRef]
- Martínez, Y.J.; Siqueiros-Beltrones, D.A. New floristic records of benthic diatoms (Bacillariophyceae) from the Gulf of California. Hidrobiológica 2018, 28, 141–145. [Google Scholar] [CrossRef]
- Moreno, J.; Licea, S.; Santoyo, H. Diatomeas del golfo de California; Universidad Autónoma de Baja California Sur-SEP-FOMES-PROMARCO: La Paz, México, 1996; 273p. [Google Scholar]
- López-Fuerte, F.O.; Siqueiros-Beltrones, D.A.; Jakes-Cota, U.; Tripp-Valdéz, A. New records of epizoic diatoms from the skin of stone scorpionfish Scorpaena mystes in Mexican waters. Open J. Mar. Sci. 2019, 9, 98–112. [Google Scholar] [CrossRef]
- Peragallo, H.; Peragallo, M. Diatomees Marines de France et des Districts Marines Voisins; Grez-sur-Loing: Diatoms, France, 1908; 491p. [Google Scholar]
- Schmidt, A.; Schmidt, M.; Fricke, F.; Heiden, H.; Muller, O.; Hustedt, F. Atlas der Diatomaceenkunde; Heft 1-120, Tafeln 1-460; Reisland: Leipzig, Germany, 1984; 208p. [Google Scholar]
- Hustedt, F. Die kieselalgen Deutschlands, Österreichs and der Schweis. In Kryptogammen-Flora; VII Band, II Teil; Rabenhorst, L., Ed.; Koeltz Scientific Book: Leipzig, Germany, 1959; 845p. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom flora of marine coasts I. In Iconographia Diatomologica; Lange-Bertalot, H., Ed.; A.R.G. Gantner: Ruggell, Germany, 2000; Volme 7, 925p. [Google Scholar]
- Siqueiros-Beltrones, D.A.; Hernández-Almeida, O.U. Florística de diatomeas epifitas en macroalgas de un manchón subtropical. CICIMAR-Oceánides 2006, 21, 11–61. [Google Scholar] [CrossRef]
- Hernández-Almeida, O.U.; Siqueiros-Beltrones, D.A. Variaciones en asociaciones de diatomeas epifitas de macroalgas en una zona subtropical. Hidrobiológica 2008, 18, 51–61. [Google Scholar]
- Hernández-Almeida, O.U.; Siqueiros-Beltrones, D.A. Substrate dependent differences in the structure of epiphytic vs. epilithic diatom assemblages from the southwestern coast of the Gulf of California. Botánica Mar. 2012, 55, 149–159. [Google Scholar] [CrossRef]
- López-Fuerte, F.O.; Siqueiros-Beltrones, D.A.; Navarro, J.N. Benthic Diatoms Associated with Mangrove Environments in the Northwest Region of Mexico; CONABIO-UABCS-IPN: La Paz, Mexico, 2010; 206p. [Google Scholar]
- Siqueiros-Beltrones, D.A.; Argumedo-Hernández, U.; Murillo-Jiménez, J.M.; Marmolejo-Rodríguez, A.J. Diversidad de diatomeas bentónicas marinas en un ambiente ligeramente enriquecido con elementos potencialmente tóxicos. Rev. Mex. Biodivers. 2014, 85, 1065–1085. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crus. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Long, E.R.; MacDonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Marmolejo Rodríguez, A.J.; Sánchez-Martínez, M.A.; Romero Guadarrama, A.; Sánchez-González, A.; Magal-lanes-Ordóñez, V.R. Migration of As, Hg, Pb, and Zn in arroyo sediments from a semiarid coastal system influenced by the abandoned gold mining district at El Triunfo, Baja California Sur, Mexico. J. Environ. Monit. 2011, 13, 2182–2189. [Google Scholar] [CrossRef]
- Wilson, I.F.; Rocha, V.S. Geology and mineral deposits of the Boleo copper district, Baja California, Mexico. Geol. Surv. Prof. 1955, 273. [Google Scholar] [CrossRef]
- Buchman, M.F. NOAA Screening Quick Reference Tables; Report 08-1; NOAA OR&R, Office of Response and Restoriation Division, National Oceanic and Atmospheric Administration: Seattle, WA, USA, 2008; 34p.
- Belando, M.D.; Marín, A.; Aboal, M.; García-Fernández, A.J.; Marín-Guirao, L. Combined in situ effects of metals and nutrients on marine biofilms: Shifts in the diatom assemblage structure and biological traits. Sci. Total Environ. 2017, 574, 381–389. [Google Scholar] [CrossRef] [PubMed]
- López-Fuerte, F.O.; Siqueiros-Beltrones, D.A. A checklist of marine benthic diatoms (Bacillariophyta) from Mexico. Phytotaxa 2016, 283, 201–258. [Google Scholar] [CrossRef]
- López-Fuerte, F.O.; Siqueiros-Beltrones, D.A.; del Carmen Altamirano-Cerecedo, M. Species Composition and New Records of Diatom Taxa on Phyllodictyon pulcherrimum Chlorophyceae) from the Gulf of California. Diversity 2020, 12, 339. [Google Scholar] [CrossRef]
- Siqueiros-Beltrones, D.A. Association structure of benthic diatoms in a hypersaline environment. Cienc. Mar. 1990, 16, 101–127. [Google Scholar] [CrossRef]
- Morin, S.; Duong, T.T.; Dabrin, A.; Coynel, A.; Herlory, O.; Baudrimont, M.; Delmas, F.; Durrieu, G.; Schäfer, J.; Winterton, P.; et al. Long-term survey of heavy-metal pollution, biofilm contamination and diatom community structure in the Riou Mort watershed, South-West France. Environ. Pollut. 2008, 115, 532–542. [Google Scholar] [CrossRef]
- Morin, S.; Cordonier, A.; Lavoie, I.; Arini, A.; Blanco, S.; Duong, T.T.; Tornés, E.; Bonet, B.; Corcoll, N.; Faggiano, L.; et al. Consistency in Diatom Response to Metal-Contaminated environments. 117–147. In The Handbook of Environmental Chemistry. Emerging and Priority Pollutants in Rivers. Bringing Science into River Management Plans; Guasch, H., Ginebreda, A., Geiszinger, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 19. [Google Scholar] [CrossRef]
- Pandey, L.K.; Vinayaj, V.; Arya, A. Morphological and physiological alterations in the diatom Gomponema pseudoaugur due to heavy metal stress. Ecol. Indic. 2017, 72, 67–76. [Google Scholar] [CrossRef]
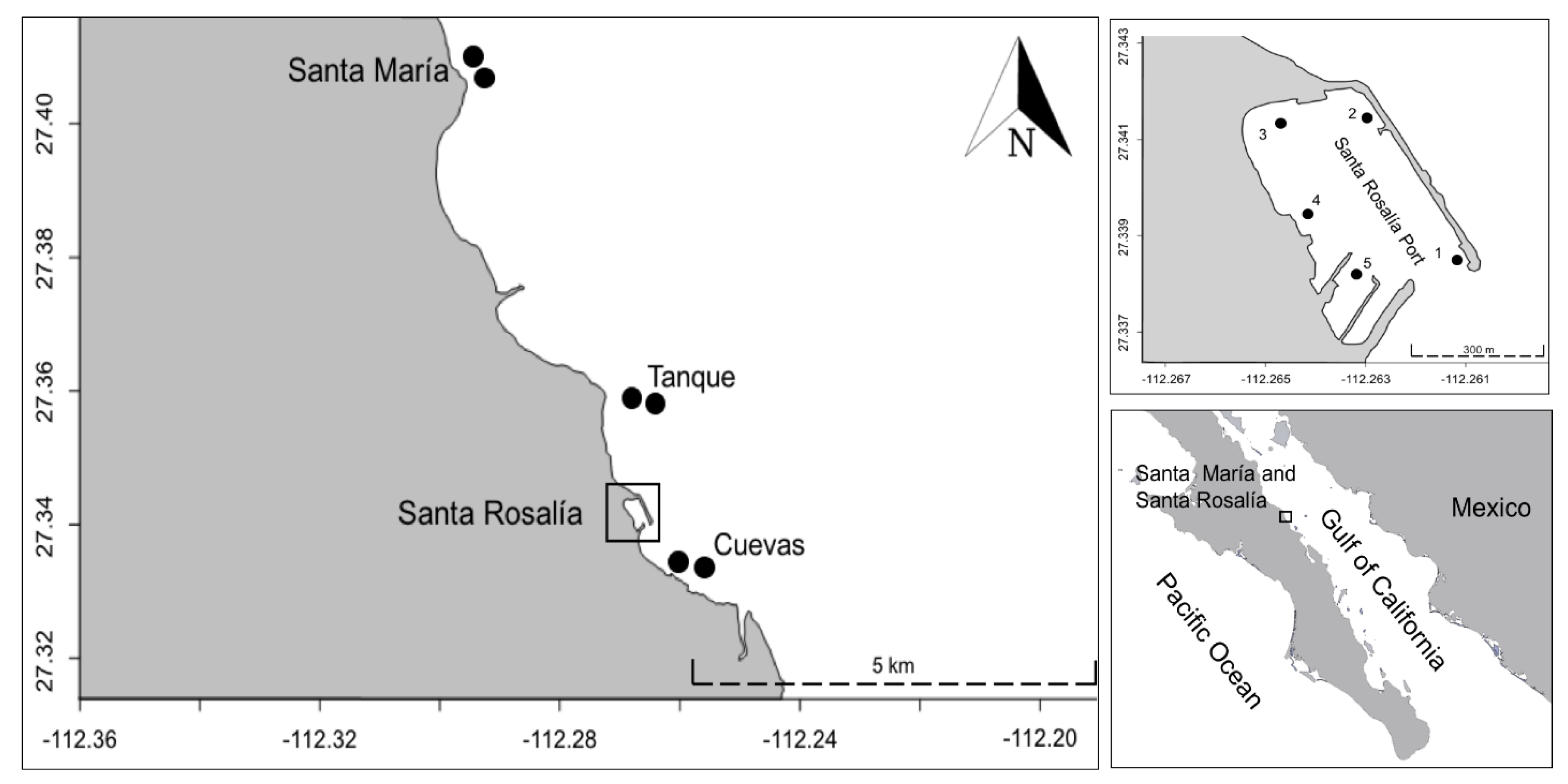
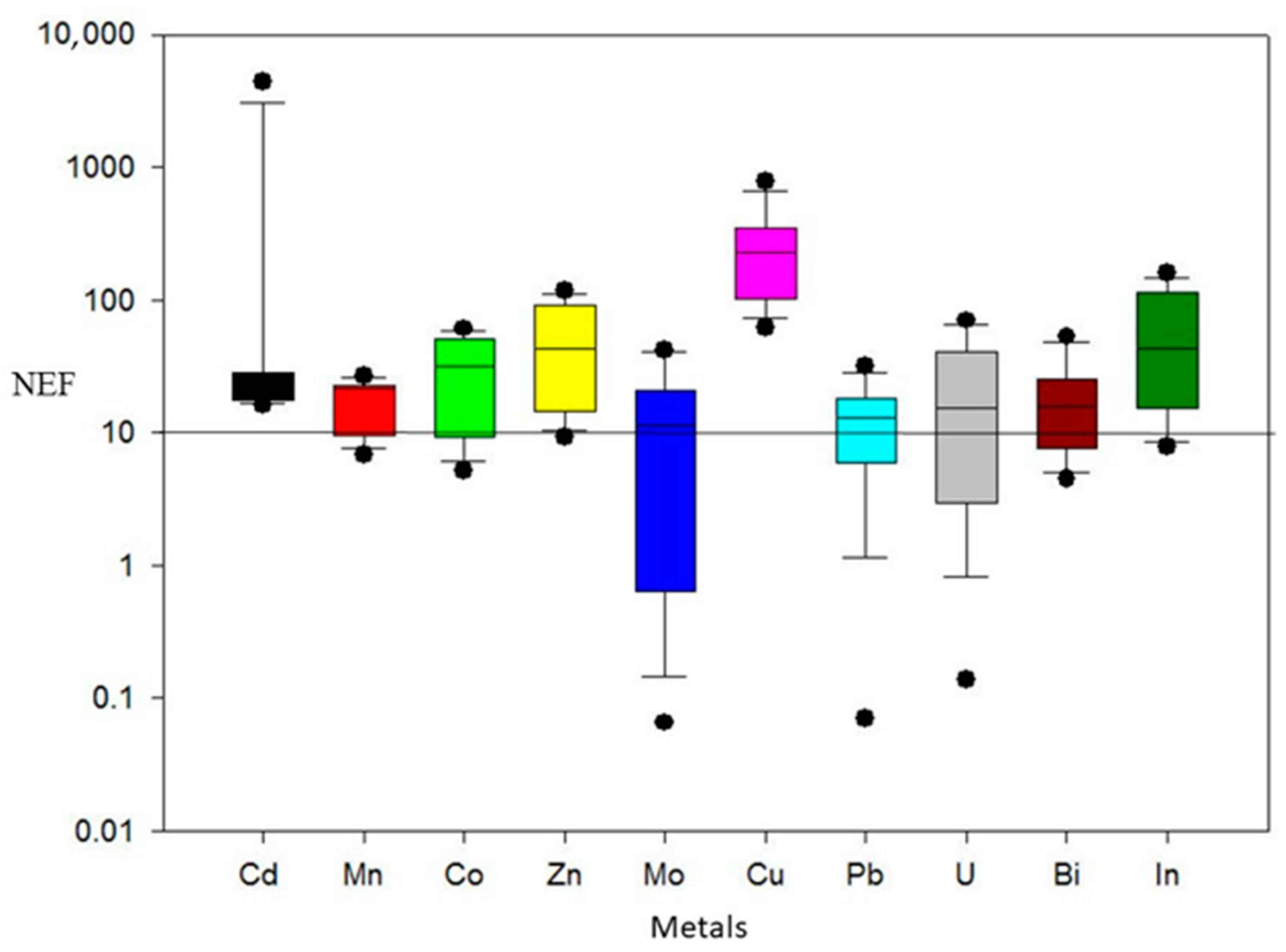
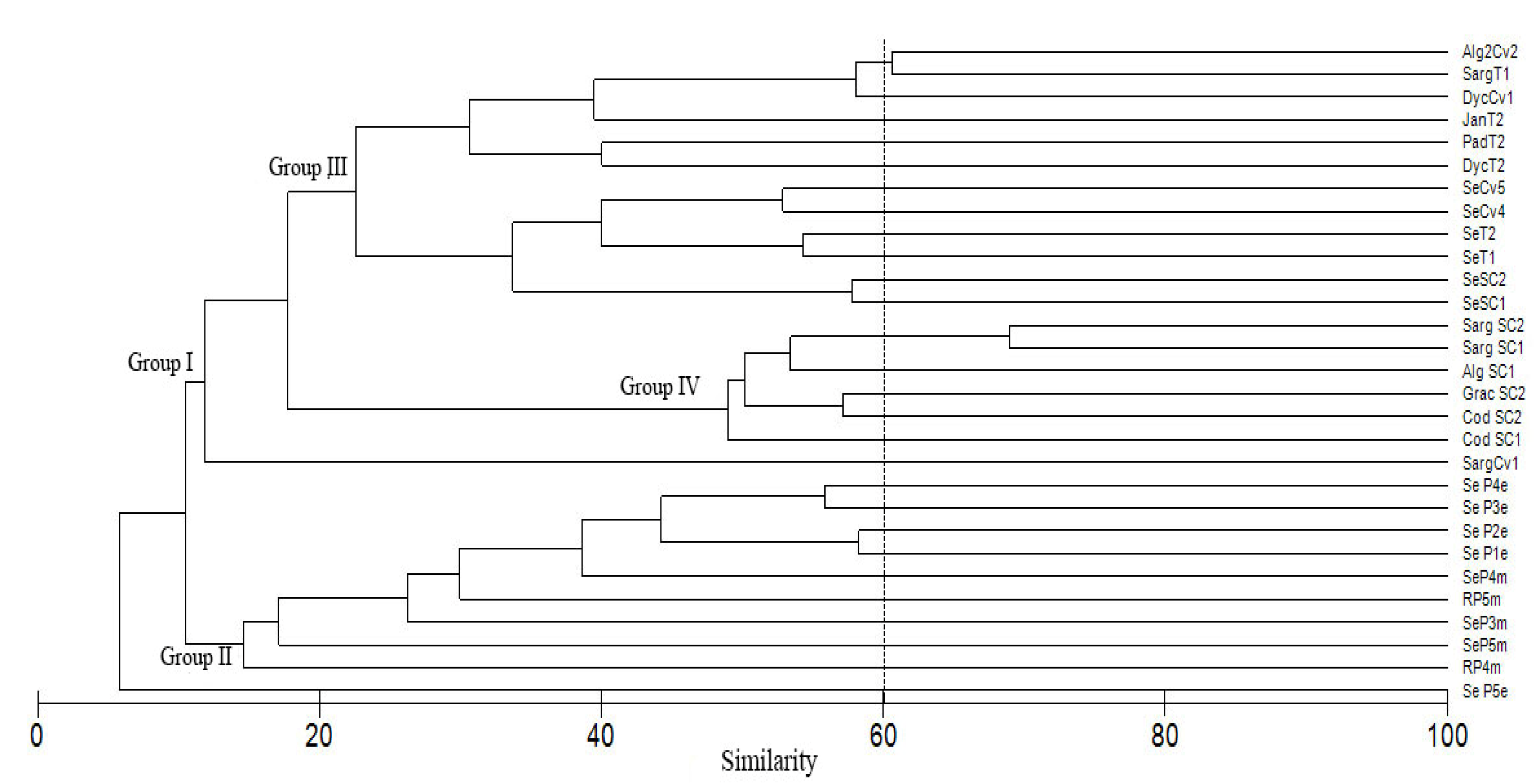

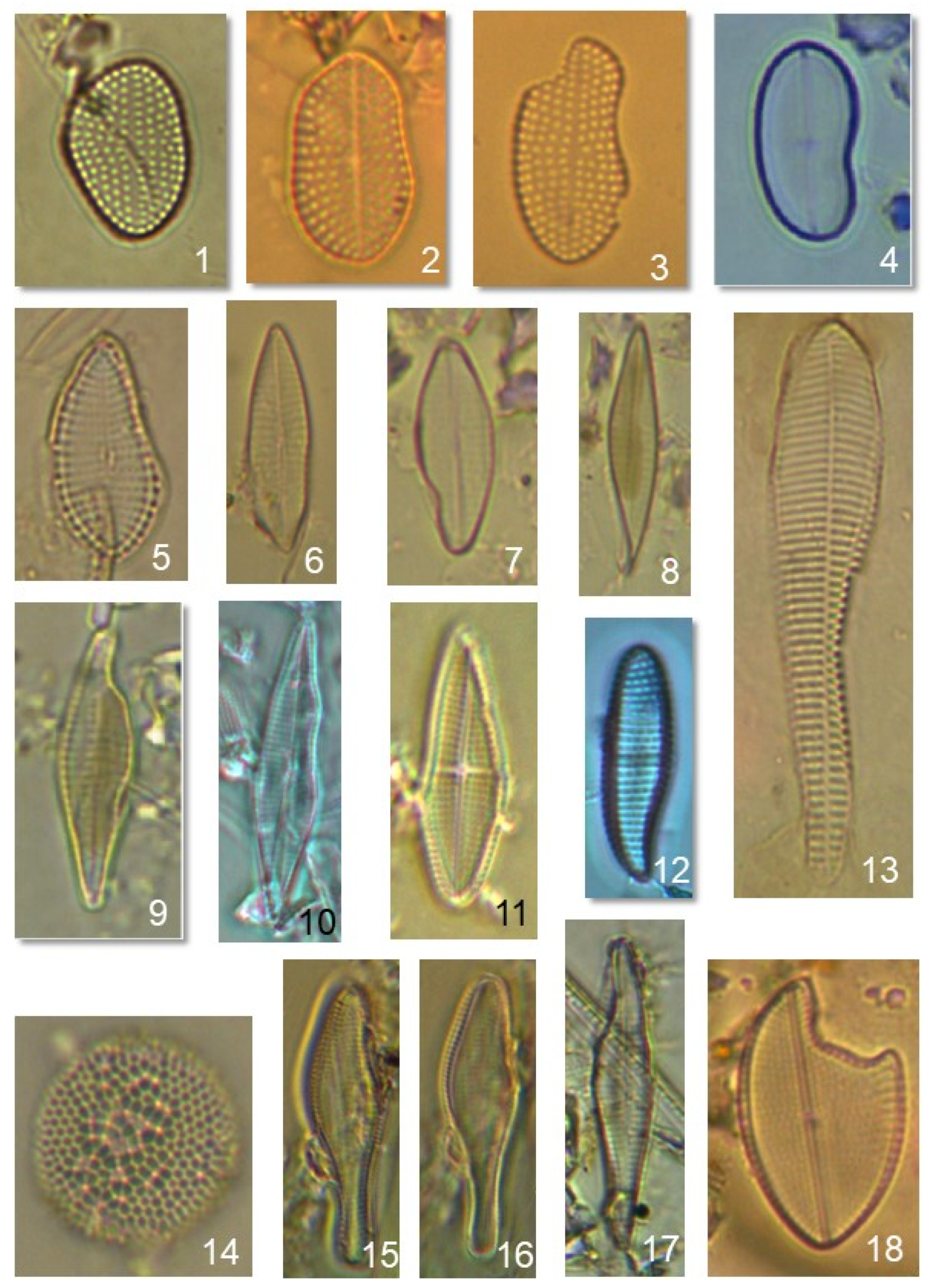
| Study Area (Santa Rosalia) | Control Site (Santa Maria) | ||||
|---|---|---|---|---|---|
| Station | Port | Costa Tanques | Costa Cuevas | 1 | 2 |
| Sampling points | 5 | 2 | 2 | ||
| Substrate marine | Sediment Rock | Sediment Algae: Sargassum sp. Dyctiota sp. Jania sp. Padina sp. | Sediment Algae: Sargassum sp. Dyctiota sp. Alga 1. | Sediment Algae: Codium sp. Sargassum sp. Alga 1 | Sediment Algae: Codium sp. Gracilaria sp. Sargassum sp. |
| Reference Standard MESS-3 | Reference Standard PACS-2 | |||||
|---|---|---|---|---|---|---|
| Metal | Certified Value | Determined Value | % Rec. | Certified Value | Determined Value | % Rec. |
| Al (%) | 8.59 | 7.48 | 87.08 | 6.62 | 5.96 | 90.03 |
| Ag | 0.18 | 0.27 | 150 | 1.22 | 1.24 | 101.64 |
| As | 21.2 | 21.9 | 103.3 | 26.2 | 26.5 | 101.15 |
| Ba | - | 1060 | - | - | 73 | - |
| Bi | - | 0.38 | - | - | 0.38 | - |
| Cd | 0.24 | 0.2 | 83.33 | 2.11 | 2.5 | 118.48 |
| Co | 14.4 | 12.7 | 88.19 | 11.5 | 10.8 | 93.91 |
| Cr | 105 | 84.9 | 80.86 | 90.7 | 66.7 | 73.54 |
| Cu | 33.9 | 35.4 | 104.42 | 310 | 328 | 105.81 |
| Fe (%) | 4.34 | 4.11 | 94.70 | 4.09 | 4 | 97.80 |
| Hg | 0.091 | 0.12 | 131 | 3.4 | 2.55 | 75 |
| In | - | 0.1 | - | - | 0.3 | - |
| Mg (%) | 1.6 | 1.52 | 95.00 | 1.47 | 1.27 | 86.39 |
| Mn | 324 | 329 | 101.54 | 440 | 427 | 97.05 |
| Mo | 2.78 | 2.76 | 99.28 | 5.43 | 5.9 | 108.66 |
| Ni | 46.9 | 38.2 | 81.45 | 39.5 | 23 | 58.23 |
| Pb | 21.1 | 22 | 104.27 | 183 | 157 | 85.79 |
| Sb | 1.02 | 0.4 | 39.22 | 11.3 | 6 | 53.1 |
| Se | 0.72 | 0.3 | 41.67 | 0.92 | 0.7 | 76.09 |
| Sn | 2.5 | 2 | 80 | 19.8 | 21 | 106.06 |
| Sr | 129 | 133 | 103.1 | 276 | 271 | 98.19 |
| U | 4 | 4 | 100 | 3 | 2.6 | 86.67 |
| V | 243 | 211 | 86.83 | 133 | 119 | 89.47 |
| Zn | 159 | 151 | 94.97 | 364 | 400 | 109.89 |
| P1 | P2 | P3 | P4 | P5 | C | T | SC | UCC | LRE | MRE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | 0.5 | 0.9 | 0.6 | 0.5 | 0.4 | 0.53 | 0.5 | 0.12 | 0.1 | 1 | 3.7 |
| Al (%) | 3.3 | 5.4 | 6.6 | 7.4 | 7.9 | 6.73 | 8.55 | 5.8 | 7.74 | - | - |
| Ba | 2290 | 660 | 4725 | 1915 | 1168 | >5000 | 1520 | 493.3 | 668 | - | - |
| Cd | 1.8 | 3.2 | 2.2 | 1.8 | 1.7 | 2.2 | 243 | 0.3 | 0.1 | 1.2 | 9.6 |
| Co | 301 | >500 | >500 | 231 | 103 | >500 | 109 | 8.96 | 11.6 | - | - |
| Cr | 45 | 59.6 | 98.4 | 65 | 52.4 | 56.9 | 62 | 39.2 | 35 | 81 | 370 |
| Cu | 3130 | 7980 | 4250 | 2220 | 1223 | 4350 | 1560 | 31.96 | 14.3 | 34 | 270 |
| Fe (%) | 4.6 | 6.3 | 9.2 | 5.7 | 4.6 | 7.425 | 5.11 | 2.9 | 3.08 | - | - |
| Hg | 60 | 60 | 50 | 105 | 70 | 25 | 40 | 48.33 | 56 | 150 | 710 |
| Li | 58 | 87.8 | 140.5 | 44.9 | 24.2 | 162.5 | 19.65 | 13.26 | 22 | - | - |
| Mn | >10,000 | >10,000 | >10,000 | 7720 | 5245 | >10,000 | 5465 | 744 | 527 | - | - |
| Mo | 12.1 | 21.5 | 47.2 | 7.8 | 8.9 | 18.5 | 0.305 | 1.15 | 1.4 | - | - |
| Ni | 401 | <0.5 | <0.5 | <0.5 | <0.5 | 16.1 | 52.9 | 0.5 | 18.6 | 20.9 | 51.6 |
| Pb | 135 | 383 | 234 | 219 | 111 | 276.5 | 48.9 | 11.16 | 17 | 46.7 | 218 |
| Sn | 4 | 15 | 6 | 5 | 8 | 3 | 2 | 1 | 2.5 | - | - |
| Sr | 1490 | 1820 | 2555 | 1259 | 984 | 7550 | 458.4 | 935.6 | 316 | - | - |
| U | 27.3 | 56.7 | 63.6 | 16.8 | 8 | 110.5 | 2.95 | 2 | 2 | - | - |
| V | 107 | 162 | 194 | 106.5 | 94.5 | 177.5 | 79.5 | 66 | 53 | - | - |
| Zn | 1990 | 3320 | 4030 | 1430 | 703 | 4755 | 740.5 | 119.9 | 52 | 150 | 410 |
| Species | CS | COAST | PORT |
|---|---|---|---|
| Amphora ocellata | 0 | 8 | 333 |
| Achnanthes javanica | 0 | 0 | 197 |
| Catenula adherens | 0 | 478 | 0 |
| Cocconeis scutellum | 256 | 1439 | 0 |
| Gomphoseptatum aestuarii | 0 | 1192 | 0 |
| Licmophora flabellata | 1431 | 0 | 0 |
| Navicula diversistriata | 70 | 503 | 6 |
| Navicula subinflatoides | 0 | 0 | 320 |
| Staurophora salina | 0 | 0 | 483 |
| Psammodyction constrictum | 0 | 22 | 270 |
| Total | 4026 | 5289 | 4183 |
| Station | Substrate | S | N | J´ | H´ | λ | 1-λ |
|---|---|---|---|---|---|---|---|
| Port May 2015 | Rock P4 | 8 | 14 | 0.89 | 2.6 | 0.13 | 0.86 |
| Rock P5 | 25 | 500 | 0.51 | 2.4 | 0.26 | 0.73 | |
| Se P3 | 30 | 169 | 0.75 | 3.6 | 0.14 | 0.85 | |
| Se P4 | 34 | 500 | 0.80 | 4.1 | 0.08 | 0.91 | |
| Se P5 | 13 | 500 | 0.53 | 1.9 | 0.32 | 0.67 | |
| Port January 2016 | Se P1 | 47 | 500 | 0.77 | 4.3 | 0.07 | 0.92 |
| Se P2 | 32 | 500 | 0.79 | 3.9 | 0.08 | 0.91 | |
| Se P3 | 41 | 500 | 0.79 | 4.2 | 0.08 | 0.91 | |
| Se P4 | 45 | 500 | 0.69 | 3.8 | 0.15 | 0.84 | |
| Se P5 | 6 | 500 | 0.45 | 1.1 | 0.52 | 0.47 | |
| Costa March 2016 | Se T1 | 26 | 500 | 0.58 | 2.7 | 0.24 | 0.75 |
| Se T2 | 33 | 277 | 0.82 | 4.1 | 0.06 | 0.93 | |
| Se C1 | 27 | 504 | 0.54 | 2.5 | 0.27 | 0.72 | |
| Se C2 | 26 | 500 | 0.54 | 2.5 | 0.33 | 0.66 | |
| Sargassum T1 | 16 | 501 | 0.36 | 1.47 | 0.60 | 0.39 | |
| Dyctiota T2 | 14 | 502 | 0.15 | 0.60 | 0.85 | 0.14 | |
| Jania T2 | 10 | 500 | 0.26 | 0.88 | 0.71 | 0.28 | |
| Padina T2 | 31 | 500 | 0.76 | 3.78 | 0.10 | 0.89 | |
| Sargassum C1 | 3 | 501 | 0.28 | 0.44 | 0.84 | 0.15 | |
| Dyctiota C1 | 18 | 504 | 0.62 | 2.6 | 0.27 | 0.72 | |
| Alga 2 C2 | 17 | 500 | 0.47 | 1.92 | 0.47 | 0.52 | |
| Control site March 2016 | Sed CS1 | 44 | 500 | 0.87 | 4.7 | 0.04 | 0.95 |
| Sed CS2 | 53 | 501 | 0.77 | 4.4 | 0.08 | 0.91 | |
| Alga CS1 | 57 | 500 | 0.82 | 4.8 | 0.05 | 0.94 | |
| Codium CS1 | 20 | 504 | 0.33 | 1.4 | 0.62 | 0.37 | |
| Sargassum CS1 | 33 | 500 | 0.69 | 3.4 | 0.13 | 0.86 | |
| Codium CS2 | 23 | 519 | 0.24 | 1.1 | 0.71 | 0.28 | |
| Gracilaria CS2 | 19 | 502 | 0.27 | 1.1 | 0.70 | 0.29 | |
| Sargassum CS2 | 25 | 500 | 0.62 | 2.8 | 0.23 | 0.76 |
| SiteStation | Substrate | DV | RA | % |
|---|---|---|---|---|
| Port SR (polluted) | MSe3 | 2 | 169 | 1.1% |
| Mse4 | 16 | 500 | 3.2% | |
| Mse5 | 6 | 500 | 1.2% | |
| MR5 | 38 | 500 | 7.6% | |
| Jse1 | 4 | 500 | 0.8% | |
| Jse2 | 8 | 500 | 1.6% | |
| Jse3 | 5 | 500 | 1.0% | |
| Jse4 | 6 | 500 | 1.2% | |
| Jse5 | 1 | 500 | 0.2% | |
| Costa SR (polluted) | Sarg T1 | 3 | 501 | 0.6 |
| Sed Tan 1 | 2 | 500 | 0.4 | |
| Sarg C1 | 2 | 501 | 0.4 | |
| Dyct C1 | 2 | 504 | 0.4 | |
| CS Non-polluted | Sed 1 | 1 | 500 | 0.2 |
| Mse3 | Mse4 | Mse5 | MR4 | MR5 | Jse1 | Jse2 | Jse3 | Jse4 | Jse5 | Sarg T1 | Sarg C1 | Dyct C1 | Sed T 1 | Sed CS1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Achnanthes javanica | 7 | ||||||||||||||
| A. longipes | 23 | ||||||||||||||
| A. parvula | 8 | ||||||||||||||
| Achnanthes sp. | 2 | 11 | 2 | 8 | 4 | 1 | |||||||||
| A. yaquinensis | 2 | ||||||||||||||
| Cocconeis sp. | 1 | ||||||||||||||
| Diploneis litoralis | 1 | 2 | |||||||||||||
| Navicula subinflatoides | 5 | ||||||||||||||
| Staurophora salina | 1 | 1 | |||||||||||||
| Thalassiosira eccentrica | 1 | ||||||||||||||
| Shionodiscus oestrupii | 3 | 2 | 1 | ||||||||||||
| Cocconeis scutellum | 1 | 2 | 1 | ||||||||||||
| Gomphoseptatum aestuarii | 1 | ||||||||||||||
| Licmophora gracilis | 1 | ||||||||||||||
| Navicula directa | 2 | ||||||||||||||
| Navicula diversistriata | 1 | ||||||||||||||
| Coconeis sp. | 1 | ||||||||||||||
| Total | 2 | 16 | 6 | 0 | 38 | 4 | 8 | 5 | 6 | 1 | 2 | 2 | 2 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, Y.J.; Siqueiros-Beltrones, D.A.; Marmolejo-Rodríguez, A.J. Response of Benthic Diatom Assemblages to Contamination by Metals in a Marine Environment. J. Mar. Sci. Eng. 2021, 9, 443. https://doi.org/10.3390/jmse9040443
Martínez YJ, Siqueiros-Beltrones DA, Marmolejo-Rodríguez AJ. Response of Benthic Diatom Assemblages to Contamination by Metals in a Marine Environment. Journal of Marine Science and Engineering. 2021; 9(4):443. https://doi.org/10.3390/jmse9040443
Chicago/Turabian StyleMartínez, Yuriko Jocselin, David Alfaro Siqueiros-Beltrones, and Ana Judith Marmolejo-Rodríguez. 2021. "Response of Benthic Diatom Assemblages to Contamination by Metals in a Marine Environment" Journal of Marine Science and Engineering 9, no. 4: 443. https://doi.org/10.3390/jmse9040443
APA StyleMartínez, Y. J., Siqueiros-Beltrones, D. A., & Marmolejo-Rodríguez, A. J. (2021). Response of Benthic Diatom Assemblages to Contamination by Metals in a Marine Environment. Journal of Marine Science and Engineering, 9(4), 443. https://doi.org/10.3390/jmse9040443






