The Case of Lionfish (Pterois miles) in the Mediterranean Sea Demonstrates Limitations in EU Legislation to Address Marine Biological Invasions
Abstract
1. Introduction
2. The Lionfish (Pterois miles) Invasion History
3. Proposal of Lionfish for Inclusion to the Union List
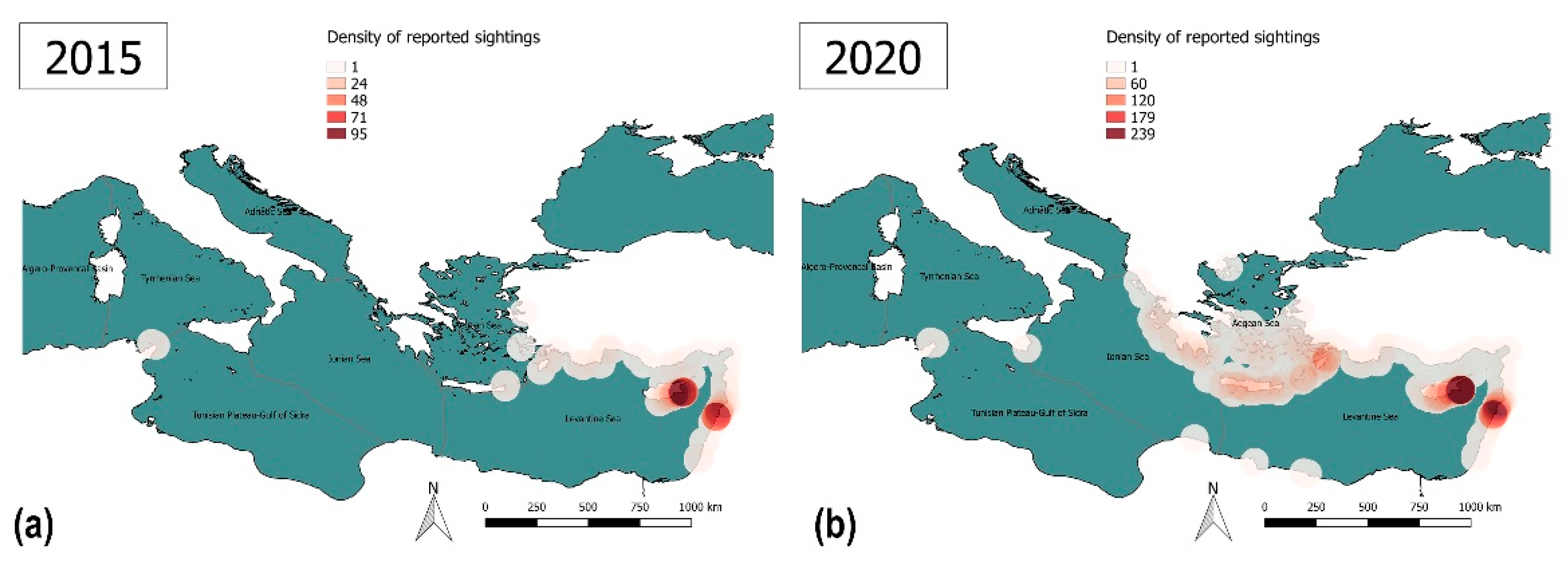
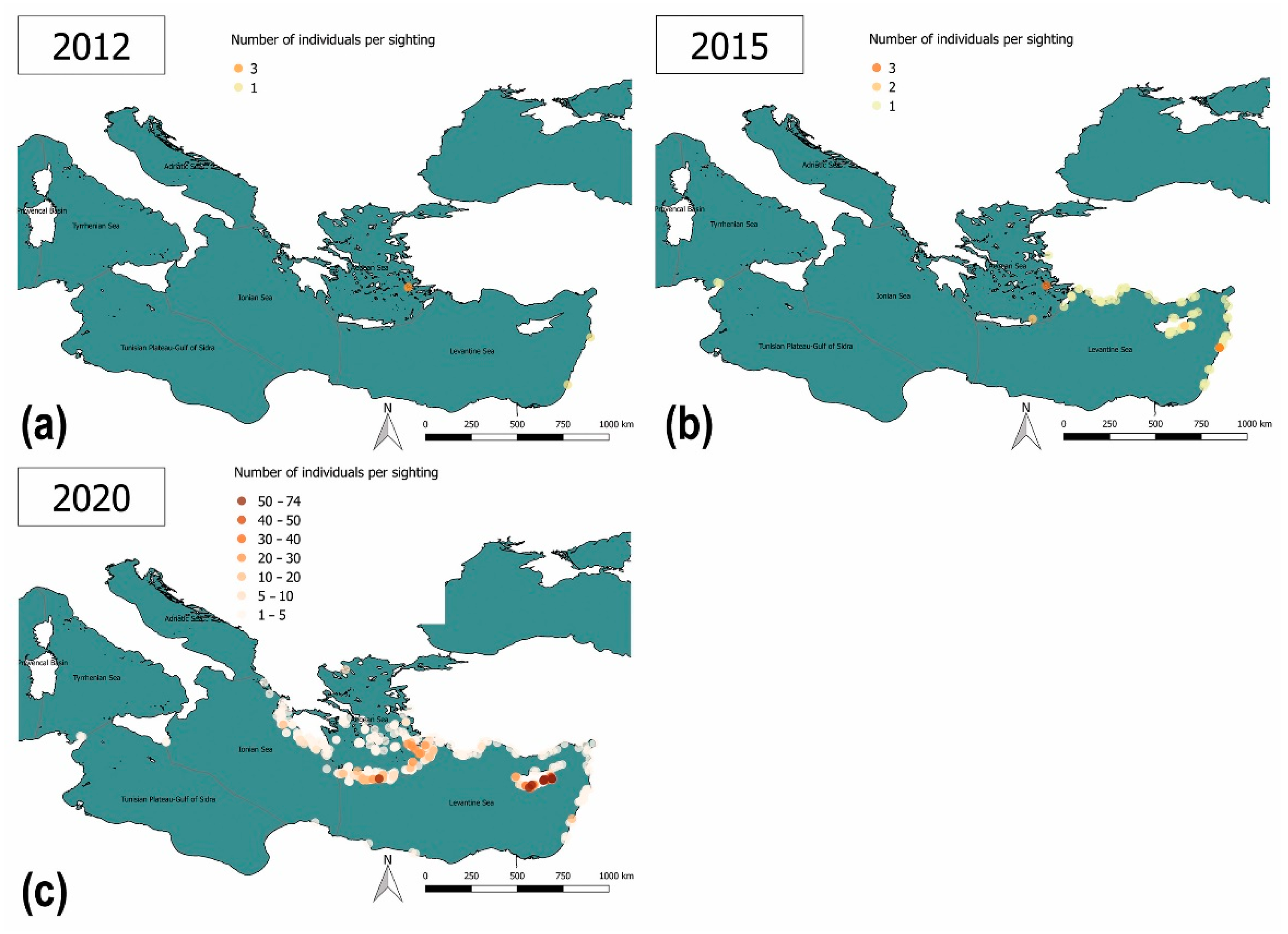
3.1. Data Collection and Species Proposal
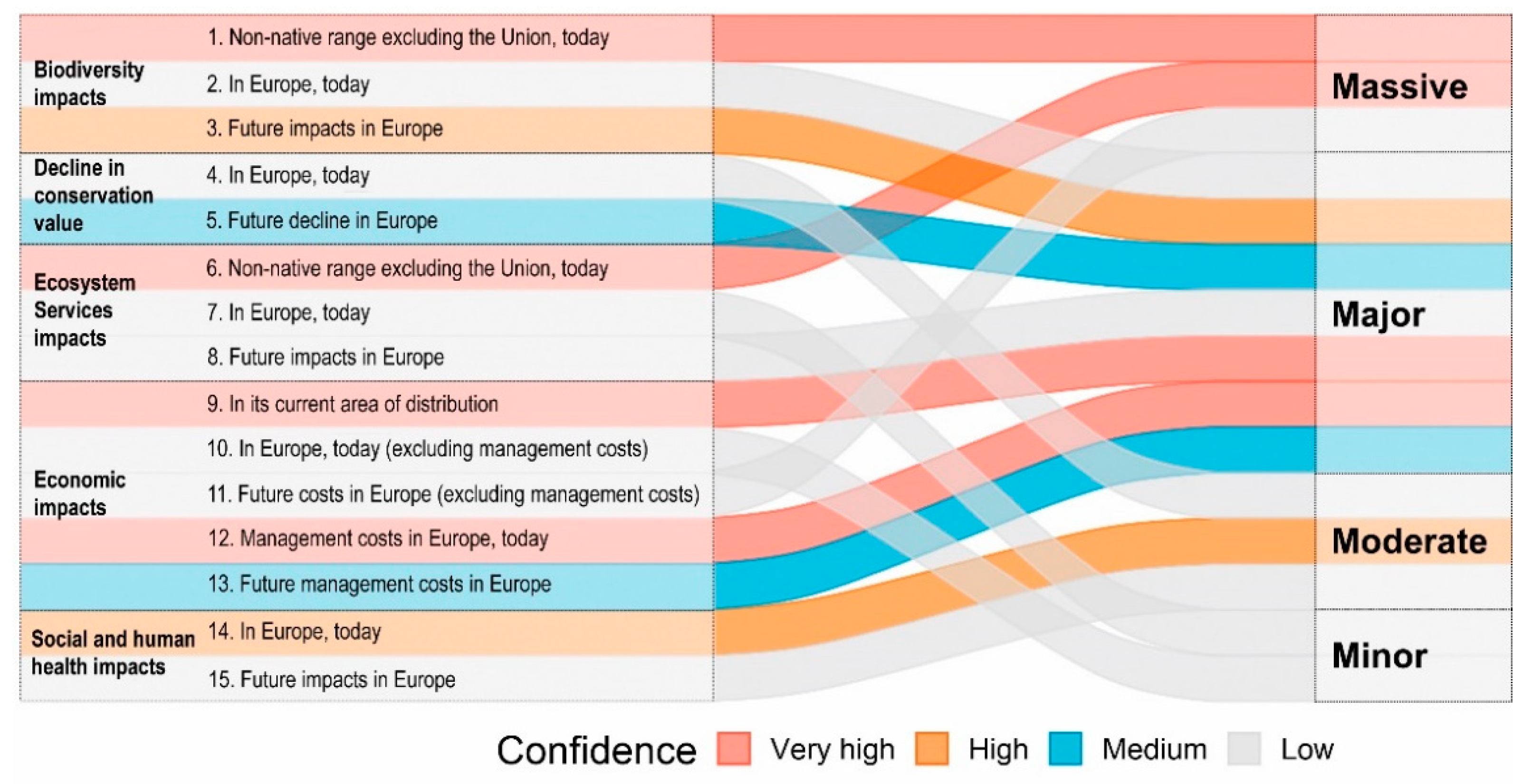
3.2. Results of the Lionfish Risk and Management Assessments
3.3. Insights and Recommendations for the IAS Regulation
3.3.1. Specificities of the Marine Environment, Contradictory Priorities, and Insufficient Proactive Action
3.3.2. Lack of Information and Absence of Effective Surveillance Systems
3.3.3. Inadequate Involvement of Non-EU Member States in Prevention and Control Measures
3.3.4. Adaptive Management Measures Are Needed
3.3.5. Faster Evaluation Processes Are Needed
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- IUCN. Guidelines for the prevention of biodiversity loss caused by alien invasive species. In Secondary Guidelines for the Prevention of Biodiversity Loss Caused by Alien Invasive Species; IUCN: Gland, Switzerland, 2000. [Google Scholar]
- Millenium Ecosystem Assessment. Ecosystems & Human Well-Being; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Brondizio, E.S.; Settele, J.; Díaz, S.; Ngo, H.T. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- EASIN. Available online: https://easin.jrc.ec.europa.eu/ (accessed on 23 January 2021).
- Katsanevakis, S.; Deriu, I.; D’amico, F.; Nunes, A.L.; Sanchez, S.P.; Crocetta, F.; Arianoutsou, M.; Bazos, I.; Christopoulou, A.; Curto, G. European alien species information network (EASIN): Supporting European policies and scientific research. Manag. Biol. Invasions 2015, 6, 147–157. [Google Scholar] [CrossRef]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; van Kleunen, M.; Kühn, I. Projecting the continental accumulation of alien species through to 2050. Glob. Chang. Biol. 2020, 27, 968–969. [Google Scholar] [CrossRef]
- Essl, F.; Lenzner, B.; Bacher, S.; Bailey, S.; Capinha, C.; Daehler, C.; Dullinger, S.; Genovesi, P.; Hui, C.; Hulme, P.E.; et al. Drivers of future alien species impacts: An expert-based assessment. Glob. Chang. Biol. 2020, 26, 4880–4893. [Google Scholar] [CrossRef]
- EC. Regulation No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. In Secondary Regulation No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species; European Commission: Strasbourg, France, 2014. [Google Scholar]
- EC. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions—EU Biodiversity Strategy for 2030 Bringing nature back into our lives. In Secondary Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions—EU Biodiversity Strategy for 2030 Bringing Nature Back into Our Lives; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Justo-Hanani, R.; Dayan, T. Risk regulation and precaution in Europe and the United States: The case of bioinvasion. Policy Sci. 2020, 54, 3–20. [Google Scholar] [CrossRef]
- Tsiamis, K.; Azzurro, E.; Bariche, M.; Çinar, M.E.; Crocetta, F.; De Clerck, O.; Galil, B.; Gómez, F.; Hoffman, R.; Jensen, K.R. Prioritizing marine invasive alien species in the European Union through horizon scanning. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2020, 30, 794–845. [Google Scholar] [CrossRef]
- Tsiamis, K.; Zenetos, A.; Deriu, I.; Gervasini, E.; Cardoso, A.C. The native distribution range of the European marine non-indigenous species. Aquat. Invasions 2018, 13, 187–198. [Google Scholar] [CrossRef]
- Azzurro, E.; Sbragaglia, V.; Cerri, J.; Bariche, M.; Bolognini, L.; Souissi, J.B.; Busoni, G.; Coco, S.; Chryssanthi, A.; Garrabou, J. The shifting distribution of Mediterranean fishes: A spatio-temporal assessment based on Local Ecological Knowledge. Glob. Chang. Biol. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Edelist, D.; Rilov, G.; Golani, D.; Carlton, J.T.; Spanier, E. Restructuring the Sea: Profound shifts in the world’s most invaded marine ecosystem. Divers. Distrib. 2013, 19, 69–77. [Google Scholar] [CrossRef]
- Corrales, X.; Coll, M.; Ofir, E.; Heymans, J.J.; Steenbeek, J.; Goren, M.; Edelist, D.; Gal, G. Future scenarios of marine resources and ecosystem conditions in the Eastern Mediterranean under the impacts of fishing, alien species and sea warming. Sci. Rep. 2018, 8, 14284. [Google Scholar] [CrossRef]
- Zenetos, A.; Çinar, M.E.; Crocetta, F.; Golani, D.; Rosso, A.; Servello, G.; Shenkar, N.; Turon, X.; Verlaque, M. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuar. Coast. Shelf Sci. 2017, 191, 171–187. [Google Scholar] [CrossRef]
- Albano, P.G.; Steger, J.; Bošnjak, M.; Dunne, B.; Guifarro, Z.; Turapova, E.; Hua, Q.; Kaufman, D.S.; Rilov, G.; Zuschin, M. Native biodiversity collapse in the eastern Mediterranean. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202469. [Google Scholar] [CrossRef]
- Bariche, M.; Torres, M.; Azzurro, E. The presence of the invasive Lionfish Pterois miles in the Mediterranean Sea. Mediterr. Mar. Sci. 2013, 14, 292–294. [Google Scholar] [CrossRef]
- Golani, D.; Sonin, O. New records of the Red Sea fishes, Pterois miles (Scorpaenidae) and Pteragogus pelycus (Labridae) from the eastern Mediterranean Sea. Jap. J. Ichthyol. 1992, 39, 167–169. [Google Scholar]
- Kletou, D.; Hall-Spencer, J.M.; Kleitou, P. A lionfish (Pterois miles) invasion has begun in the Mediterranean Sea. Mar. Biodivers. Rec. 2016, 9, 46. [Google Scholar] [CrossRef]
- Jimenez, C.; Petrou, A.; Andreou, V.; Hadjioannou, L.; Wolf, W.; Koutsoloukas, N.; Alhaija, R.A.; QDivers, A.N.; Aquarium, O. Veni, vidi, vici: The successful establishment of the lionfish Pterois miles in Cyprus (Levantine Sea). Rapp. Comm. Int. Mer. Mediterr. 2016, 41, 417. [Google Scholar]
- Azzurro, E.; Stancanelli, B.; Di Martino, V.; Bariche, M. Range expansion of the common lionfish Pterois miles (Bennett, 1828) in the Mediterranean Sea: An unwanted new guest for Italian waters. Bioinvasions Rec. 2017, 6, 95–98. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Galanidi, M.; Zenetos, A.; Corsini-Foka, M.; Giovos, I.; Karachle, P.K.; Fournari-Konstantinidou, I.; Kytino, E.; Issaris, Y.; Azzurro, E.; et al. Updating the occurrences of Pterois miles in the Mediterranean Sea, with considerations on thermal boundaries and future range expansion. Mediterr. Mar. Sci. 2020, 21, 62–69. [Google Scholar] [CrossRef]
- Hixon, M.A.; Green, S.J.; Albins, M.A.; Akins, J.L.; Morris, J.A., Jr. Lionfish: A major marine invasion. Mar. Ecol. Prog. Ser. 2016, 558, 161–165. [Google Scholar] [CrossRef]
- Roy, H.E.; Adriaens, T.; Aldridge, D.; Bacher, S.; Bishop, J.; Blackburn, T.M.; Branquart, E.; Brodie, J.; Carboneras, C.; Cook, E.J. Invasive Alien Species-Prioritising prevention efforts through horizon scanning: ENV. B. 2/ETU/2014/0016. In Secondary Invasive Alien Species-Prioritising Prevention Efforts through Horizon Scanning: ENVIRON. B. 2/ETU/2014/0016; European Commission: Luxembourg, 2015. [Google Scholar]
- Kleitou, P.; Hall-Spencer, J.; Rees, S.; Sfenthourakis, S.; Demetriou, A.; Chartosia, N.; Jimenez, C.; Hadjioannou, L.; Petrou, A.; Christodoulides, Y. Tackling the lionfish invasion in the Mediterranean. The EU-LIFE RELIONMED Project: Progress and results. In 1st Mediterranean Symp Non-Indigenous Species; Langar, H., Ouerghi, A., Eds.; SPA/RAC: Antalya, Turkey, 2019; pp. 65–70. [Google Scholar]
- Kulbicki, M.; Beets, J.; Chabanet, P.; Cure, K.; Darling, E.; Floeter, S.R.; Galzin, R.; Green, A.; Harmelin-Vivien, M.; Hixon, M. Distributions of Indo-Pacific lionfishes Pterois spp. in their native ranges: Implications for the Atlantic invasion. Mar. Ecol. Prog. Ser. 2012, 446, 189–205. [Google Scholar] [CrossRef]
- Morris, J.A.; Whitfield, P.E. Biology, Ecology, Control and Management of the Invasive Indo-Pacific Lionfish: An Updated Integrated Assessment; NOAA Technical Memorandum NOS NCCOS 99: Beaufort, NC, USA, 2009. [Google Scholar]
- Goodbody-Gringley, G.; Eddy, C.; Pitt, J.M.; Chequer, A.D.; Smith, S.R. Ecological drivers of invasive lionfish (Pterois volitans and Pterois miles) distribution across mesophotic reefs in Bermuda. Front. Mar. Sci. 2019, 6, 258. [Google Scholar] [CrossRef]
- Eddy, C.; Pitt, J.; Morris, J.A.; Smith, S.; Goodbody-Gringley, G.; Bernal, D. Diet of invasive lionfish (Pterois volitans and P. miles) in Bermuda. Mar. Ecol. Prog. Ser. 2016, 558, 193–206. [Google Scholar] [CrossRef]
- Peake, J.; Bogdanoff, A.K.; Layman, C.A.; Castillo, B.; Reale-Munroe, K.; Chapman, J.; Dahl, K.; Patterson, W.F., III; Eddy, C.; Ellis, R.D. Feeding ecology of invasive lionfish (Pterois volitans and Pterois miles) in the temperate and tropical western Atlantic. Biol. Invasions 2018, 20, 2567–2597. [Google Scholar] [CrossRef]
- Rojas-Vélez, S.; Tavera, J.; Acero, A. Unraveling lionfish invasion: Is Pterois volitans truly a morphologically novel predator in the Caribbean? Biol. Invasions 2019, 21, 1921–1931. [Google Scholar] [CrossRef]
- Green, S.J.; Dilley, E.R.; Benkwitt, C.E.; Davis, A.C.; Ingeman, K.E.; Kindinger, T.L.; Tuttle, L.J.; Hixon, M.A. Trait-mediated foraging drives patterns of selective predation by native and invasive coral-reef fishes. Ecosphere 2019, 10, e02752. [Google Scholar] [CrossRef]
- Galloway, K.A.; Porter, M.E. Mechanical properties of the venomous spines of Pterois volitans and morphology among lionfish species. J. Exp. Biol. 2019, 222, jeb197905. [Google Scholar] [CrossRef]
- Fogg, A.Q.; Brown-Peterson, N.J.; Peterson, M.S. Reproductive life history characteristics of invasive red lionfish (Pterois volitans) in the northern Gulf of Mexico. Bull. Mar. Sci. 2017, 93, 791–813. [Google Scholar] [CrossRef]
- Ahrenholz, D.W.; Morris, J.A. Larval duration of the lionfish, Pterois volitans along the Bahamian Archipelago. Environ. Biol. Fishes 2010, 88, 305–309. [Google Scholar] [CrossRef]
- DeRoy, E.M.; Scott, R.; Hussey, N.E.; MacIsaac, H.J. Density dependence mediates the ecological impact of an invasive fish. Divers. Distrib. 2020, 26, 867–880. [Google Scholar] [CrossRef]
- Green, S.J.; Akins, J.L.; Maljković, A.; Côté, I.M. Invasive lionfish drive Atlantic coral reef fish declines. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Côté, I.M.; Green, S.J.; Hixon, M.A. Predatory fish invaders: Insights from Indo-Pacific lionfish in the western Atlantic and Caribbean. Biol. Conserv. 2013, 164, 50–61. [Google Scholar] [CrossRef]
- Albins, M.A.; Hixon, M.A. Invasive Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Mar. Ecol. Prog. Ser. 2008, 367, 233–238. [Google Scholar] [CrossRef]
- Benkwitt, C.E. Non-linear effects of invasive lionfish density on native coral-reef fish communities. Biol. Invasions 2015, 17, 1383–1395. [Google Scholar] [CrossRef]
- Ingeman, K.E. Lionfish cause increased mortality rates and drive local extirpation of native prey. Mar. Ecol. Prog. Ser. 2016, 558, 235–245. [Google Scholar] [CrossRef]
- Ballew, N.G.; Bacheler, N.M.; Kellison, G.T.; Schueller, A.M. Invasive lionfish reduce native fish abundance on a regional scale. Sci. Rep. 2016, 6, 32169. [Google Scholar] [CrossRef]
- Layman, C.; Jud, Z.; Nichols, P. Lionfish alter benthic invertebrate assemblages in patch habitats of a subtropical estuary. Mar. Biol. 2014, 161, 2179–2182. [Google Scholar] [CrossRef]
- Kindinger, T.L.; Albins, M.A. Consumptive and non-consumptive effects of an invasive marine predator on native coral-reef herbivores. Biol. Invasions 2017, 19, 131–146. [Google Scholar] [CrossRef]
- Lesser, M.P.; Slattery, M. Phase shift to algal dominated communities at mesophotic depths associated with lionfish (Pterois volitans) invasion on a Bahamian coral reef. Biol. Invasions 2011, 13, 1855–1868. [Google Scholar] [CrossRef]
- Slattery, M.; Lesser, M.P. Allelopathy in the tropical alga L obophora variegata (P haeophyceae): Mechanistic basis for a phase shift on mesophotic coral reefs? J. Phycol. 2014, 50, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Green, S.J.; Dulvy, N.K.; Brooks, A.M.; Akins, J.L.; Cooper, A.B.; Miller, S.; Côté, I.M. Linking removal targets to the ecological effects of invaders: A predictive model and field test. Ecol. Appl. 2014, 24, 1311–1322. [Google Scholar] [CrossRef]
- Savva, I.; Chartosia, N.; Antoniou, C.; Kleitou, P.; Georgiou, A.; Stern, N.; Hadjioannou, L.; Jimenez, C.; Andreou, V.; Hall-Spencer, J.M. They are here to stay: The biology and ecology of lionfish (Pterois miles) in the Mediterranean Sea. J. Fish Biol. 2020, 97, 148–162. [Google Scholar] [CrossRef]
- Dimitriou, A.C.; Chartosia, N.; Hall-Spencer, J.M.; Kleitou, P.; Jimenez, C.; Antoniou, C.; Hadjioannou, L.; Kletou, D.; Sfenthourakis, S. Genetic Data Suggest Multiple Introductions of the Lionfish (Pterois miles) into the Mediterranean Sea. Diversity 2019, 11, 149. [Google Scholar] [CrossRef]
- Kleitou, P.; Savva, I.; Kletou, D.; Hall-Spencer, J.M.; Antoniou, C.; Christodoulides, Y.; Chartosia, N.; Hadjioannou, L.; Dimitriou, A.C.; Jimenez, C. Invasive lionfish in the Mediterranean: Low public awareness yet high stakeholder concerns. Mar. Policy 2019, 104, 66–74. [Google Scholar] [CrossRef]
- Giovos, I.; Kleitou, P.; Paravas, V.; Marmara, D.; Romanidis-Kyriakidis, G.; Poursanidis, D. Citizen scientists monitoring the establishment and expansion of Pterois miles (Bennett, 1828) in the Aegean Sea, Greece. Cah. Biol. Mar. 2018, 59, 359–365. [Google Scholar]
- Poursanidis, D.; Kalogirou, S.; Azzurro, E.; Parravicini, V.; Bariche, M.; zu Dohna, H. Habitat suitability, niche unfilling and the potential spread of Pterois miles in the Mediterranean Sea. Mar. Pollut. Bull. 2020, 154, 111054. [Google Scholar] [CrossRef]
- Jimenez, C.; Patsalou, P.; Andreou, V.; Huseyinoglu, M.; Çiçek, B.; Hadjioannou, L.; Petrou, A. Out of sight, out of reach, out of mind: Invasive lionfish Pterois miles in Cyprus at depths beyond recreational diving limits. In 1st Mediterranean Symp Non-Indigenous Species; Langar, H., Ouerghi, A., Eds.; SPA/RAC: Antalya, Turkey, 2019; pp. 17–18. [Google Scholar]
- Orejas, C.; Jiménez, C.; Gori, A.; Rivera, J.; Iacono, C.L.; Aurelle, D.; Hadjioannou, L.; Petrou, A.; Achilleos, K. Corals of Aphrodite: Dendrophyllia ramea Populations of Cyprus. In Mediterranean Cold-Water Corals: Past, Present and Future; Orejas, C., Jiménez, C., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; Volume 9, pp. 257–260. ISBN 978-3-319-91607-1. [Google Scholar]
- Zannaki, K.; Corsini-Foka, M.; Kampouris, T.E.; Batjakas, I.E. First results on the diet of the invasive Pterois miles (Actinopterygii: Scorpaeniformes: Scorpaenidae) in the Hellenic waters. Acta Ichthyol. Piscat. 2019, 49, 311–317. [Google Scholar] [CrossRef]
- Agostino, D.D.; Jimenez, C.; Reader, T.; Hadjioannou, L.; Heyworth, S.; Aplikioti, M.; Argyrou, M.; Feary, D.A. Behavioural traits and feeding ecology of Mediterranean lionfish and naiveté of native species to lionfish predation. Mar. Ecol. Prog. Ser. 2020, 638, 123–135. [Google Scholar] [CrossRef]
- Crocetta, F.; Shokouros-Oskarsson, M.; Doumpas, N.; Giovos, I.; Kalogirou, S.; Langeneck, J.; Tanduo, V.; Tiralongo, F.; Virgili, R.; Kleitou, P. Protect the natives to combat the aliens: Octopus vulgaris Cuvier, 1797 as a natural agent for the control of the lionfish invasion in the Mediterranean Sea? J. Mar. Sci. Eng. 2021, 9, 308. [Google Scholar] [CrossRef]
- Bariche, M.; Kleitou, P.; Kalogirou, S.; Bernardi, G. Genetics reveal the identity and origin of the lionfish invasion in the Mediterranean Sea. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Stern, N.; Jimenez, C.; Huseyinoglu, M.F.; Andreou, V.; Hadjioannou, L.; Petrou, A.; Öztürk, B.; Golani, D.; Rothman, S.B. Constructing the genetic population demography of the invasive lionfish Pterois miles in the Levant Basin, Eastern Mediterranean. Mitochondrial DNA Part A 2019, 30, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Poursanidis, D. Ecological Niche Modeling of the the invasive lionfish Pterois miles (Bennett, 1828) in the Mediterranean Sea. In Proceedings of the 11th Panhellenic Symposium of Oceanography & Fisheries, Mytiline, Greece, 13–17 May 2015. [Google Scholar]
- Parravicini, V.; Azzurro, E.; Kulbicki, M.; Belmaker, J. Niche shift can impair the ability to predict invasion risk in the marine realm: An illustration using Mediterranean fish invaders. Ecol. Lett. 2015, 18, 246–253. [Google Scholar] [CrossRef] [PubMed]
- D’Amen, M.; Azzurro, E. Lessepsian fish invasion in Mediterranean marine protected areas: A risk assessment under climate change scenarios. ICES J. Mar. Sci. 2020, 77, 388–397. [Google Scholar] [CrossRef]
- Whitfield, P.E.; Muñoz, R.C.; Buckel, C.A.; Degan, B.P.; Freshwater, D.W.; Hare, J.A. Native fish community structure and Indo-Pacific lionfish Pterois volitans densities along a depth-temperature gradient in Onslow Bay, North Carolina, USA. Mar. Ecol. Prog. Ser. 2014, 509, 241–254. [Google Scholar] [CrossRef]
- Harris, H.E.; Fogg, A.Q.; Gittings, S.R.; Ahrens, R.N.; Allen, M.S.; Patterson, W.F. Testing the efficacy of lionfish traps in the northern Gulf of Mexico. PLoS ONE 2020, 15, e0230985. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, W.J.; Barnard, P.; Broad, S.; Clout, M.; Connor, B.; Côté, I.M.; Dicks, L.V.; Doran, H.; Entwistle, A.C.; Fleishman, E. A 2017 horizon scan of emerging issues for global conservation and biological diversity. Trends Ecol. Evol. 2017, 32, 31–40. [Google Scholar] [CrossRef]
- Kleitou, P.; Crocetta, F.; Giakoumi, S.; Giovos, I.; Hall-Spencer, J.M.; Kalogirou, S.; Kletou, D.; Moutopoulos, D.K.; Rees, S. Fishery reforms for the management of non-indigenous species. J. Environ. Manag. 2021, 280, 111690. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.H.; Neigel, J.E.; Estes, J.A.; Andelman, S.; Warner, R.R.; Largier, J.L. Comparing marine and terrestrial ecosystems: Implications for the design of coastal marine reserves. Ecol. Appl. 2003, 13, 90–107. [Google Scholar] [CrossRef]
- Kinlan, B.P.; Gaines, S.D. Propagule dispersal in marine and terrestrial environments: A community perspective. Ecology 2003, 84, 2007–2020. [Google Scholar] [CrossRef]
- Burgess, S.C.; Baskett, M.L.; Grosberg, R.K.; Morgan, S.G.; Strathmann, R.R. When is dispersal for dispersal? Unifying marine and terrestrial perspectives. Biol. Rev. 2016, 91, 867–882. [Google Scholar] [CrossRef]
- Anderson, L.W. California’s reaction to Caulerpa taxifolia: A model for invasive species rapid response. Biol. Invasions 2005, 7, 1003–1016. [Google Scholar] [CrossRef]
- Willan, R.C.; Russell, B.C.; Murfet, N.B.; Moore, K.L.; McEnnulty, F.R.; Horner, S.K.; Hewitt, C.L.; Dally, G.M.; Campbell, M.L.; Bourke, S.T. Outbreak of Mytilopsis sallei (Recluz, 1849)(Bivalvia: Dreissenidae) in Australia. Molluscan Res. 2000, 20, 25–30. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Coll, M.; Fraschetti, S.; Giakoumi, S.; Goldsborough, D.; Mačić, V.; Mackelworth, P.; Rilov, G.; Stelzenmüller, V.; Albano, P.G. Twelve recommendations for advancing marine conservation in European and contiguous seas. Front. Mar. Sci. 2020, 7, 879. [Google Scholar] [CrossRef]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Booy, O.; Robertson, P.; Moore, N.; Ward, J.; Roy, H.; Adriaens, T.; Shaw, R.; Valkenburg, J.; Wyn, G.; Bertolino, S.; et al. Using structured eradication feasibility assessment to prioritise the management of new and emerging invasive alien species in Europe. Glob. Chang. Biol. 2020, 26, 6235–6250. [Google Scholar] [CrossRef] [PubMed]
- Mannino, A.M.; Borfecchia, F.; Micheli, C. Tracking Marine Alien Macroalgae in the Mediterranean Sea: The Contribution of Citizen Science and Remote Sensing. J. Mar. Sci. Eng. 2021, 9, 288. [Google Scholar]
- Peyton, J.; Martinou, A.F.; Pescott, O.L.; Demetriou, M.; Adriaens, T.; Arianoutsou, M.; Bazos, I.; Bean, C.W.; Booy, O.; Botham, M. Horizon scanning for invasive alien species with the potential to threaten biodiversity and human health on a Mediterranean island. Biol. Invasions 2019, 21, 2107–2125. [Google Scholar] [CrossRef]
- Peyton, J.M.; Martinou, A.F.; Adriaens, T.; Chartosia, N.; Karachle, P.K.; Rabitsch, W.; Tricarico, E.; Arianoutsou, M.; Bacher, S.; Bazos, I. Horizon scanning to predict and prioritize invasive alien species with the potential to threaten human health and economies on Cyprus. Front. Ecol. Evol. 2020, 8, 284. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef]
- Boon, P.J.; Clarke, S.A.; Copp, G.H. Alien species and the EU Water Framework Directive: A comparative assessment of European approaches. Biol. Invasions 2020, 22, 1497–1512. [Google Scholar] [CrossRef]
- Roy, H.E.; Bacher, S.; Essl, F.; Adriaens, T.; Aldridge, D.C.; Bishop, J.D.; Blackburn, T.M.; Branquart, E.; Brodie, J.; Carboneras, C. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Chang. Biol. 2019, 25, 1032–1048. [Google Scholar] [CrossRef]
- Rilov, G.; Mazaris, A.D.; Stelzenmüller, V.; Helmuth, B.; Wahl, M.; Guy-Haim, T.; Mieszkowska, N.; Ledoux, J.-B.; Katsanevakis, S. Adaptive marine conservation planning in the face of climate change: What can we learn from physiological, ecological and genetic studies? Glob. Ecol. Conserv. 2019, 17, e00566. [Google Scholar] [CrossRef]
- Bonanno, G.; Orlando-Bonaca, M. Non-indigenous marine species in the Mediterranean Sea—Myth and reality. Environ. Sci. Policy 2019, 96, 123–131. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Bogucarskis, K.; Gatto, F.; Vandekerkhove, J.; Deriu, I.; Cardoso, A.C. Building the European Alien Species Information Network (EASIN): A novel approach for the exploration of distributed alien species data. Bioinvasions Rec. 2012, 1, 235–245. [Google Scholar] [CrossRef]
- Tsiamis, K.; Palialexis, A.; Stefanova, K.; Gladan, Ž.N.; Skejić, S.; Despalatović, M.; Cvitković, I.; Dragičević, B.; Dulčić, J.; Vidjak, O. Non-indigenous species refined national baseline inventories: A synthesis in the context of the European Union’s Marine Strategy Framework Directive. Mar. Pollut. Bull. 2019, 145, 429–435. [Google Scholar] [CrossRef]
- Murillas-Maza, A.; Uyarra, M.C.; Papadopoulou, K.N.; Smith, C.J.; Gorjanc, S.; Klancnik, K.; Paramana, T.; Chalkiadaki, O.; Dassenakis, M.; Pavicic, M. Programmes of measures of the marine strategy framework directive: Are they contributing to achieving good environmental status in the Mediterranean? Mar. Pollut. Bull. 2020, 161, 111715. [Google Scholar] [CrossRef] [PubMed]
- Azzurro, E.; Bolognini, L.; Dragičević, B.; Drakulović, D.; Dulčić, J.; Fanelli, E.; Grati, F.; Kolitari, J.; Lipej, L.; Magaletti, E. Detecting the occurrence of indigenous and non-indigenous megafauna through fishermen knowledge: A complementary tool to coastal and port surveys. Mar. Pollut. Bull. 2019, 147, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Giovos, I.; Kleitou, P.; Poursanidis, D.; Batjakas, I.; Bernardi, G.; Crocetta, F.; Doumpas, N.; Kalogirou, S.; Kampouris, T.E.; Keramidas, I. Citizen-science for monitoring marine invasions and stimulating public engagement: A case project from the eastern Mediterranean. Biol. Invasions 2019, 21, 3707–3721. [Google Scholar] [CrossRef]
- Kleitou, P.; Giovos, I.; Wolf, W.; Crocetta, F. On the importance of citizen-science: The first record of Goniobranchus obsoletus (Rüppell and Leuckart, 1830) from Cyprus (Mollusca: Gastropoda: Nudibranchia). Bioinvasions Rec. 2019, 8, 252–257. [Google Scholar] [CrossRef]
- Crocetta, F.; Agius, D.; Balistreri, P.; Bariche, M.; Bayhan, Y.K.; Çakir, M.; Ciriaco, S.; Corsini-Foka, M.; Deidun, A.; El Zrelli, R. New mediterranean biodiversity records (October 2015). Mediterr. Mar. Sci. 2015, 16, 682–702. [Google Scholar] [CrossRef]
- Carballo-Cárdenas, E.C.; Tobi, H. Citizen science regarding invasive lionfish in Dutch Caribbean MPAs: Drivers and barriers to participation. Ocean Coast. Manag. 2016, 133, 114–127. [Google Scholar] [CrossRef]
- Encarnação, J.; Morais, P.; Teodosio, M.A. Citizen science and biological invasions: A review. Front. Environ. Sci. 2020, 8, 303. [Google Scholar]
- Katsanevakis, S.; Poursanidis, D.; Hoffman, R.; Rizgalla, J.; Rothman, S.B.-S.; Levitt-Barmats, Y.; Hadjioannou, L.; Trkov, D.; Garmendia, J.M.; Rizzo, M. Unpublished Mediterranean records of marine alien and cryptogenic species. Bioinvasions Rec. 2020, 9, 165–182. [Google Scholar] [CrossRef]
- Kelly, R.P.; Closek, C.J.; O’Donnell, J.L.; Kralj, J.E.; Shelton, A.O.; Samhouri, J.F. Genetic and manual survey methods yield different and complementary views of an ecosystem. Front. Mar. Sci. 2017, 3, 283. [Google Scholar] [CrossRef]
- Aglieri, G.; Baillie, C.; Mariani, S.; Cattano, C.; Calò, A.; Turco, G.; Spatafora, D.; Di Franco, A.; Di Lorenzo, M.; Guidetti, P. Environmental DNA effectively captures functional diversity of coastal fish communities. Mol. Ecol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen science as an ecological research tool: Challenges and benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Ward, R.C.; Loftis, J.C.; McBride, G.B. The “data-rich but information-poor” syndrome in water quality monitoring. Environ. Manag. 1986, 10, 291–297. [Google Scholar] [CrossRef]
- Wilding, T.A.; Gill, A.B.; Boon, A.; Sheehan, E.; Dauvin, J.C.; Pezy, J.-P.; O’beirn, F.; Janas, U.; Rostin, L.; De Mesel, I. Turning off the DRIP (‘Data-rich, information-poor’)–rationalising monitoring with a focus on marine renewable energy developments and the benthos. Renew. Sustain. Energy Rev. 2017, 74, 848–859. [Google Scholar] [CrossRef]
- García-Barón, I.; Giakoumi, S.; Santos, M.B.; Granado, I.; Louzao, M. The value of time-series data for conservation planning. J. Appl. Ecol. 2020, 58, 608–619. [Google Scholar] [CrossRef]
- Rotter, A.; Klun, K.; Francé, J.; Mozetič, P.; Orlando-Bonaca, M. Non-indigenous species in the Mediterranean Sea: Turning from pest to source by developing the 8Rs model, a new paradigm in pollution mitigation. Front. Mar. Sci. 2020, 7, 178. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Belchior, C.; Cardoso, A.C. Invading European Seas: Assessing pathways of introduction of marine aliens. Ocean Coast. Manag. 2013, 76, 64–74. [Google Scholar] [CrossRef]
- Galil, B.; Marchini, A.; Occhipinti-Ambrogi, A.; Ojaveer, H. The enlargement of the Suez Canal—Erythraean introductions and management challenges. Manag. Biol. Invasions 2017, 8, 141–152. [Google Scholar] [CrossRef]
- Galil, B.S.; Boero, F.; Campbell, M.L.; Carlton, J.T.; Cook, E.; Fraschetti, S.; Gollasch, S.; Hewitt, C.L.; Jelmert, A.; Macpherson, E. ‘Double trouble’: The expansion of the Suez Canal and marine bioinvasions in the Mediterranean Sea. Biol. Invasions 2015, 17, 973–976. [Google Scholar] [CrossRef]
- Galil, B.S.; Mienis, H.K.; Hoffman, R.; Goren, M. Non-indigenous species along the Israeli Mediterranean coast: Tally, policy, outlook. Hydrobiologia 2020. [Google Scholar] [CrossRef]

| Challenge | Recommendation(s) |
|---|---|
| Specificities of the marine environment, contradictory priorities, and insufficient proactive action | (i) Horizon scanning exercises (ii) Pre-defined rapid response plans on the basis of species traits and initial spread patterns |
| Lack of information and absence of effective surveillance systems | (i) A more strategic and coherent monitoring in NIS hotspot areas (ii) Stationary monitoring stations and long-term ecological and socioeconomic data collection |
| Inadequate involvement of non-EU Member States in prevention and control measures | (i) Synergies with established regional legally binding instruments (ii) Common strategies, protocols, and management activities |
| Adaptive management measures are needed | (i) Dead specimens of IAS of Union concern to be allowed (and promoted) in the food market in order to incentivize targeted fishery |
| Evaluation processes are slow | (i) All the steps of the invasive species evaluation for inclusion in the Union list need to be shorter in duration (ii) Peer-review to be conducted with strict deadlines or even not required in cases where risk assessments are conducted by more than three authors and at least two independent affiliations (iii) Use of the Article 11 provisions of the IAS Regulation for faster and regional response |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleitou, P.; Hall-Spencer, J.M.; Savva, I.; Kletou, D.; Hadjistylli, M.; Azzurro, E.; Katsanevakis, S.; Antoniou, C.; Hadjioannou, L.; Chartosia, N.; et al. The Case of Lionfish (Pterois miles) in the Mediterranean Sea Demonstrates Limitations in EU Legislation to Address Marine Biological Invasions. J. Mar. Sci. Eng. 2021, 9, 325. https://doi.org/10.3390/jmse9030325
Kleitou P, Hall-Spencer JM, Savva I, Kletou D, Hadjistylli M, Azzurro E, Katsanevakis S, Antoniou C, Hadjioannou L, Chartosia N, et al. The Case of Lionfish (Pterois miles) in the Mediterranean Sea Demonstrates Limitations in EU Legislation to Address Marine Biological Invasions. Journal of Marine Science and Engineering. 2021; 9(3):325. https://doi.org/10.3390/jmse9030325
Chicago/Turabian StyleKleitou, Periklis, Jason M. Hall-Spencer, Ioannis Savva, Demetris Kletou, Margarita Hadjistylli, Ernesto Azzurro, Stelios Katsanevakis, Charalampos Antoniou, Louis Hadjioannou, Niki Chartosia, and et al. 2021. "The Case of Lionfish (Pterois miles) in the Mediterranean Sea Demonstrates Limitations in EU Legislation to Address Marine Biological Invasions" Journal of Marine Science and Engineering 9, no. 3: 325. https://doi.org/10.3390/jmse9030325
APA StyleKleitou, P., Hall-Spencer, J. M., Savva, I., Kletou, D., Hadjistylli, M., Azzurro, E., Katsanevakis, S., Antoniou, C., Hadjioannou, L., Chartosia, N., Christou, M., Christodoulides, Y., Giovos, I., Jimenez, C., Smeraldo, S., & Rees, S. E. (2021). The Case of Lionfish (Pterois miles) in the Mediterranean Sea Demonstrates Limitations in EU Legislation to Address Marine Biological Invasions. Journal of Marine Science and Engineering, 9(3), 325. https://doi.org/10.3390/jmse9030325










