Abstract
In order to determine the organic matter redox state in relation to specific sources in mangrove sediments, two 60 cm-long sediment cores were collected from mangrove-covered and mudflat zones within a mangrove forest in Peru. Sediment subsamples from these cores were analyzed to determine δ13C values and C:N ratios, whereas two redox indices, namely, electrochemical (fEAOM) and spectroscopical (A1650/A3400) indices, were taken from a previous study and correlated with the geochemical indices obtained from this work. These indices may provide accurate information on sedimentary organic matter diagenesis by oxidative processes through its redox state. The results show that the electrochemical index (fEAOM) and the spectroscopical index (A1650/A3400) for mangrove-covered sediments exhibited a positive correlation with δ13C values and a negative correlation with C:N molar ratios. These correlations suggest that the more labile sedimentary organic matter derived from non-terrestrial sources is in a more oxidized state than that derived from mangrove vegetation. However, this was not valid for mudflat zones, where non-significant correlations between geochemical indices were observed. Furthermore, the results suggest that the redox state of the organic matter deposited over time is dependent on source mixing influences, being better preserved in the presence of mangrove-derived organic matter.
1. Introduction
The role of mangrove ecosystems in the coastal carbon cycle has been increasingly recognized, as these ecosystems sequester and accumulate large quantities of organic carbon [1,2]. The organic carbon accumulated in these systems is largely influenced by accretion rates [3,4] and geochemical conditions within the sediments [5,6], as well as by the geomorphological and hydrological characteristics [7,8]. As a result, mangrove sediments are estimated to contribute between 15 and 25% of the coastal blue carbon burial [1,9], derived from autochthonous and allochthonous organic matter production [7,10]. These autochthonous and allochthonous organic matter sources are frequently assessed through well-established techniques, including elemental and stable isotope measurements in coastal sediments [3,10].
Alternatively, electrochemical techniques are often used to determine the oxidized and non-oxidized fractions or the redox state of organic matter compounds [11,12]. In addition, spectroscopical techniques allow the determination of the organic matter composition in terms of aliphatic groups and carboxylic acids, providing information on the oxidized state of organic matter [13,14]. The redox state of sedimentary organic matter plays an essential role in geochemical processes, where compounds containing quinones, hydroquinones, aromatic groups and others functional groups are involved in the preservation of organic carbon [15,16,17]. The proposed methodology is based on the capability of electrochemistry to detect oxidizable organic matter compounds and estimate the antioxidant capability of sediments based on monitoring the reaction of antioxidant compounds with reactive oxygen species, which has only previously been applied to vegetal-derived organic matter [18,19]. However, there have been no previous initiatives to determine the influence of sedimentary organic matter sources on the redox state of organic matter using electrochemical and spectroscopic techniques on mangrove-covered and mudflat sediments. An initial attempt using these techniques was based on the relationship between electrochemical and spectroscopical proxies of the redox state and total organic matter content in mangrove sediments [12].
The Peruvian mangrove ecosystems are located in the mangrove distribution boundary of the eastern South Pacific. This geographical position makes this ecosystem very important and sensitive to climate change and environmental impacts [8,20]. The high accumulation of organic matter in this area is controlled by daily and seasonal hydrological regimes (e.g., tidal forces, seasonal rainfall and freshwater intrusion), as well as by the enclosed geomorphology [8,21]. It is worth noting that during extreme hydrological periods (e.g., ENSO events), this ecosystem may be impacted due to the significant increase in flow of the Zarumilla River [22,23]. These factors may influence the sediment distribution as well as the organic matter accumulation and degradation in the sedimentary environments [4,8,21].
This study aimed to determine the influence of specific organic matter sources on the redox state in mangrove sediments. To accomplish this aim, electrochemical and spectroscopical properties [12], and data on the sources of the sedimentary organic matter [4], were taken from a tropical Peruvian mangrove ecosystem. These analyses were used to test the hypothesis that the redox state of the sedimentary organic matter within mangrove ecosystems may be influenced by the organic matter sources and mixing effects during its deposition in sediments, which may directly impact the blue carbon accumulation capacity of these sedimentary environments.
2. Materials and Methods
The mangrove ecosystem studied in this research is located in Tumbes, which is situated on the northern coast of Peru and is associated with the National Sanctuary “Manglares de Tumbes” (Figure 1a). This estuarine system is influenced by the Zarumilla River and its secondary creeks, which provide fresh water to it and facilitate the continuous transport and deposition of high quantities of particulate organic matter [8,21]. The dominant mangrove species near the creeks are Rhizophora mangle, Rhizophora harrisonii and Avicennia germinans, which are together considered one of the main components of the sedimentary organic matter in this estuarine ecosystem [8].
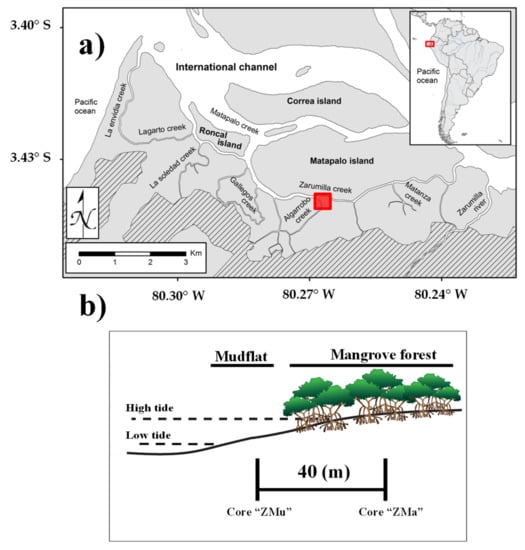
Figure 1.
(a) Map of the study area within the mangrove sanctuary. The red square indicates the sampling site. (b) The spatial location of the sampling sites within the two sedimentary environments. Sediment cores were sampled within the mangrove-covered (ZMa) and mudflat (ZMu) zones.
The fieldwork for this study was carried out in 2014, when two 60 cm-long sediment cores were sampled from a representative area dominated by Rhizophora mangle. The first core (Core “ZMu”) was collected in a mudflat zone, whereas the second core (Core “ZMa”) was collected in a mangrove-covered zone (Figure 1b). The sediment cores were obtained using PVC tubes by means of percussion and rotation to minimize compression [4]. While collecting the sediment columns, the presence of mangrove roots was avoided in order to obtain intact sediments that lacked mixing [3,4]. Each sediment column was sectioned on site in 1 cm intervals from the top of the core to 10 cm depth, and then at 2 cm intervals to the base of the core. Subsamples were bagged, placed on ice, returned to the laboratory and kept frozen at −20 °C, then subsequently lyophilized before analysis. Further subsamples of each core were acidified with HCl 1M to remove carbonate material [3,4]. Subsamples were analyzed for carbon (C), nitrogen (N) and δ13C using an isotope ratio mass spectrometer, Thermo Finnigan Model Delta Plus XP, with an analytical precision of C = 0.1%, N = 0.1% and δ13C = 0.1‰ [3,4]. It is important to mention that non-decarbonated sediment was used to measure nitrogen content [24]. Data for the redox state indices in sedimentary organic matter (fEAOM and A1650/A3400) were taken from Cebrian-Torrejón et al. (2019) [12]. Higher values of the electrochemically active organic matter index (fEAOM) in the sediment core indicate the occurrence of electrochemically active organic matter resulting from the degradation of vegetal components in a more reducing environment [12,14]. Higher values of the spectroscopical index (A1650/A3400) in the sediment core indicate the presence of more oxidized organic matter [12,14]. The data were first normalized and tested by the Shapiro–Wilk test (α = 0.05) [4,8]. After that, analysis of variance (ANOVA) (α = 0.05) was used to determine differences between sedimentary environments (mangrove-covered and mudflat zones).
3. Results and Discussion
3.1. Sedimentary Organic Matter Source
Several studies on wetland ecosystems have reported that sedimentary organic matter containing a high proportion of mangrove litterfall may present δ13C values between −26 and −30‰, whereas values between −17 and −24‰ may reflect a high proportion of non-mangrove-derived organic matter in sediments (e.g., phytoplankton, microphytobenthos, saltmarshes and seagrasses) [3,4,6,25]. In this study, the mudflat zone exhibited a higher proportion of organic matter derived from non-terrestrial vegetation than the mangrove-covered zone (Figure 2), which was likely derived from planktonic and benthic microalgae deposition [3,4]. As expected, the lighter δ13C values were found within the mangrove forest (Table 1), with lighter δ13C values towards the top of the sediment column (Figure 2), suggesting the dominance of terrestrial or mangrove-derived organic matter in the younger sediments [3,4,6]. These differences were observed within the first 15 cm depth of the sediment profile in the mangrove-covered zone (Figure 2). The results agree with the isotopic δ13C values observed in fresh mangrove leaves from the sampling site (Table S1), suggesting that the main proportion of organic matter is derived from mangrove plants.
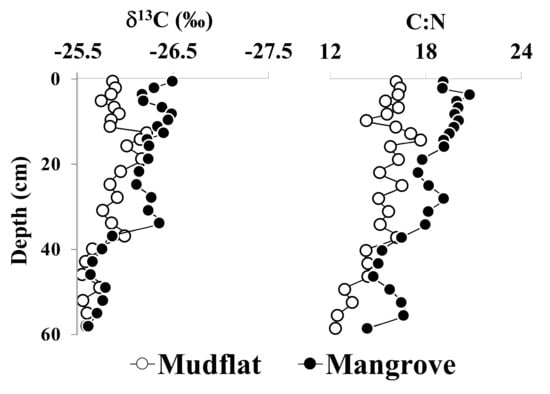
Figure 2.
Vertical distribution of isotopic values of carbon (δ13C) and molar ratios of carbon and nitrogen (C:N) obtained from cores from the mudflat (ZMu) and mangrove-covered (ZMa) sediment zones.

Table 1.
Analysis of variance (ANOVA) results for the geochemical proxies in each sediment core. ZMa: mangrove-covered zone. ZMu: mudflat zone. p < 0.05; Significant correlation (*); N.S.: non-significant. n: number of subsamples of each sediment core.
In addition, the C:N molar ratios observed during this research exhibited non-significant differences between sedimentary environments (Table 1). However, lower than average C:N ratios were noted from the mudflat zone in comparison with the mangrove-covered zone (Table 1), whereas the sediment profiles that showed lower C:N molar ratios were observed towards the top of the sediment core (Figure 2). These results suggest there was a higher proportion of non-terrestrial organic matter (e.g., phytoplankton- and microphytobenthos-derived organic matter) in the mudflat zone throughout the sediment column [3,4]. It is worth noting that these C:N molar ratios were negatively correlated with the obtained δ13C values during this study (Figure 3), suggesting that in both sedimentary zones, lighter isotopic values are associated with more refractory organic matter (e.g., terrestrial- or mangrove-derived), which may present less nitrogen content [3,6,25]. It is also important to mention that several reports have found an inverse relationship between the C:N molar ratios and the δ13C values derived from mangrove litterfall, indicating that the more refractory mangrove litter degradation is strongly dependent on the availability of nitrogen [6,25]. Our results are consistent with other studies that showed the sedimentary organic matter in mangrove systems is mainly composed of mangrove litterfall and roots (~70%), which is efficiently accumulated within mangrove-covered zones due to their highly productive root systems [6,26]. Finally, it is important to consider that the occurrence of organic matter mixing in surficial sediments is also likely in these dynamic mangrove ecosystems, exhibiting values between the intervals described above [27,28].
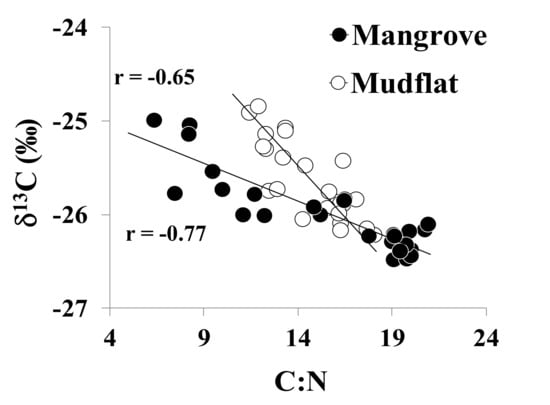
Figure 3.
Isotopic values of carbon (δ13C) plotted against C:N molar ratios for sediment cores within the mangrove-covered (ZMa) and mudflat (ZMu) zones.
3.2. Redox State of Sedimentary Organic Matter
The fEAOM index in mangrove ecosystems exhibited higher values below 20 cm depth in the mangrove-covered and mudflat zones (Figure S1). This reflects that the sedimentary organic matter in the deeper layers is electrochemically active due to oxidative degradation and natural bioturbation [12]. In the mangrove-covered zone, this degradation may occur through hydrolysis or bacterial respiration, which may be enhanced by oxygenation produced by the mangrove rhizosphere [29,30,31]. In the mudflat zone, the organic matter degradation may be facilitated by oxygenation driven by tidal and hydrological regime energies in this study area [8,21]. It should be noted that within the mangrove-covered zone, the fEAOM values exhibited a negative correlation with the C:N molar ratios, and a positive correlation with the δ13C values (Figure 4a). This indicates less oxidative degradation of mangrove- or terrestrial-derived organic matter than non-terrestrial organic matter, which is explained by the more refractory composition of the mangrove vegetation in comparison with the non-terrestrial material [6]. In addition, higher fEAOM values were associated with heavier δ13C values mainly within the mangrove-covered zone (Figure 4a), suggesting that the presence of terrestrial organic matter may trigger lower oxidative degradation rates [12,29]. In addition, fEAOM values and C:N molar ratios were not correlated in the mudflat zone (Figure 4a) because this environment is more exposed to creek water flow and tidal forces and, therefore, more prone to organic matter suspension, mixing and subsequent transport [8,21]. Under this context, a higher proportion of organic matter remains efficiently sequestered within the mangrove-covered zone, facilitating the accumulation of more refractory organic matter as compared to that within the more dynamic mudflat zone.

Figure 4.
(a) fEAOM vs. C:N molar ratios and δ13C values. (b) A1650/A3440 vs. C:N molar ratios and δ13C values. The trend lines and correlation coefficients refer to the cores from the mangrove-covered (ZMa) and mudflat (ZMu) zones.
The A1650/A3400 index exhibited significantly higher values in the mangrove-covered zone than those in the mudflat zone (Table 1). These results indicate that the organic matter in the mangrove-covered zone may contain higher carbonyl/hydroxyl ratios, as expected in the presence of more degraded organic matter [12,14]. It should be mentioned that the vertical distribution of the A1650/A3400 index presented higher values towards the bottom of the sediment column for both sedimentary zones (Figure S1). This agrees with the vertical distribution of fEAOM values (Figure S1), indicating that the sedimentary organic matter in deeper intervals suffered more oxidative degradation [6,29]. Finally, the A1650/A3400 index within mangrove-covered sediments was negatively correlated with C:N molar ratios and positively correlated with δ13C values (Figure 4b). These correlations indicate lower organic matter degradation in the intervals with higher proportions of terrestrial-derived organic matter [4,12]. These correlations were not validated in the mudflat zone, indicating that, in spite of the hydrological and textural differences between the zones [4], the organic matter transported and deposited within the mudflat zone has a lower residence time than the organic matter deposited within the mangrove-vegetated zone, being partially transported by the tidal forces and creek water outflow [8,21].
4. Conclusions
The assessment of the influence of organic matter sources on the redox state in mangrove sediment ecosystems was made possible through the coupled applications of electrochemical and spectroscopical indices, along with C:N molar ratios and isotopic δ13C analyses. The results show that the sedimentary organic matter derived from mangrove and terrestrial sources was associated with a less oxidized state than the non-terrestrial organic matter. However, this association was not valid for mudflat sediments, where the non-significant correlations indicate the more dynamic conditions in these sedimentary zones. Stable carbon isotope ratios combined with C:N molar ratios identified the susceptibility of sedimentary organic matter to oxidation, wherein both mangrove and algal organic matter deposition may occur. Further research is recommended for other coastal vegetated systems (such as mangrove, salt marsh and seagrass habitats) to better understand the carbon accumulation capacity of blue carbon systems.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jmse9121438/s1: Figure S1: Vertical distribution of the electrochemical and the spectroscopical indices obtained from cores in the mudflat and man-grove-covered sediment zones. Table S1: The isotopic values ± SD for carbon (δ13C) from fresh Rhizophora mangle leaves. The samples were collected in duplicate from trees in the interior and at the border of the mangrove-covered zone (30 m distance) in the sampling site.
Author Contributions
A.P.: conceptualization, writing—original draft preparation. G.C.-T.: conceptualization, review and editing. N.M. and J.P.-C.: methodology and validation. C.J.S.: conceptualization, methodology, review and editing. A.D.-C.: methodology, review and editing. W.M.: conceptualization, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from this study are available from the corresponding author upon request.
Acknowledgments
The fieldwork campaign was carried out within the framework of the project “Impacto de la Variabilidad y Cambio Climático en el Ecosistema de Manglares de Tumbes”. The project was supported by the International Development Research Centre (IRDC) of Canada managed by the Instituto Geofísico del Perú (IGP). A.P. was supported by the “Fondo Nacional de Desarrollo Científico Tecnológico y de Innovación Tecnológica” (FONDECYT—PERU) through the Magnet program (Grant no. 007-2017-FONDECYT) and the “Incorporación de Investigadores” program (Grant no. E038-2019-02-FONDECYT-BM). W.M. acknowledges support from CAPES (Financial Code 001 and Feedbacks-Print-UFF grant 88887.310301/2018-00). C.J.S. was supported by the Australian Research Council (DE160100443, DP150103286 and LE140100083).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Breithaupt, J.L.; Smoak, J.M.; Smith, T.J.; Sanders, C.J.; Hoare, A. Organic carbon burial rates in mangrove sediments: Strengthening the global budget. Glob. Biogeochem. Cycles 2012, 26, GB3011. [Google Scholar] [CrossRef]
- Pérez, A.; Libardoni, B.G.; Sanders, C.J. Factors influencing organic carbon accumulation in mangrove ecosystems. Biol. Lett. 2018, 14, 20180237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, C.J.; Eyre, B.D.; Santos, I.R.; Machado, W.; Luiz-Silva, W.; Smoak, J.M.; Breithaupt, J.L.; Ketterer, M.E.; Sanders, L.; Marotta, H.; et al. Elevated rates of organic carbon, nitrogen, and phosphorus accumulation in a highly impacted mangrove wetland. Geophys. Res. Lett. 2014, 41, 2475–2480. [Google Scholar] [CrossRef]
- Pérez, A.; Machado, W.; Gutierrez, D.; Saldarriaga, M.S. Shrimp farming influence on carbon and nutrient accumulation within Peruvian mangroves sediments. Estuar. Coast. Shelf Sci. 2020, 243, 106879. [Google Scholar] [CrossRef]
- Ferreira, T.O.; Otero, X.L.; Vidal-Torrado, F. Redox Processes in Mangrove Soils under Rhizophora mangle in Relation to Different Environmental Conditions. Soil. Sci. Soc. Am. J. 2007, 71, 484–491. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef] [Green Version]
- Sanders, J.C.; Smoak, J.M.; Sanders, L.; Naidu, A.S.; Patchineelam, S.R. Organic carbon accumulation in Brazilian mangal sediments. J. S. Am. Earth Sci. 2010, 30, 189–192. [Google Scholar] [CrossRef]
- Pérez, A.; Gutiérrez, D.; Saldarriaga, M.; Sanders, C.J. Hydrological controls on the biogeochemical dynamics of a Peruvian mangrove system. Hydrobiologia 2017, 803, 69–86. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Pérez, A.; Machado, W.; Gutierrez, D.; Stokes, D.; Sanders, L.; Smoak, J.M.; Santos, I.; Sanders, C.J. Changes in organic carbon accumulation driven by mangrove expansion and deforestation in a New Zealand estuary. Estuar. Coast. Shelf Sci. 2017, 192, 108–116. [Google Scholar] [CrossRef]
- Scholz, F.; Schröder, U.; Gulabowski, R.; Doménech-Carbó, A. Electrochemistry of Immobilized Particles and Droplets, 2nd ed.; Scholz, F., Ed.; Monographs in Electrochemistry Series; Springer: Berlin/Heidelberg, Germany, 2014; p. 327. [Google Scholar]
- Cebrián-Torrejón, G.; Pérez, A.; Montoya, N.; Piquero-Cilla, J.; Saldarriaga, M.S.; Gutiérrez, D.; Sanders, C.J.; Machado, W.; Doménech-Carbó, A. Electrochemical characterization of mangrove sediments: A proposal of new proxies for organic matter oxidation. Appl. Geochem. 2019, 101, 42–49. [Google Scholar] [CrossRef]
- Pascaud, G.; Soubrand, M.; Lemee, L.; Laduranty, J.; El-Mufleh, A.; Rabiet, M.; Joussein, E. Molecular fingerprint of soil organic matter as an indicator of pedogenesis processes in Technosols. J. Soil. Sediment. 2017, 17, 340–351. [Google Scholar] [CrossRef]
- Sharma, P.; Laor, Y.; Raviv, M.; Medina, S.; Saadi, I.; Krasnovsky, A.; Vager, M.; Levy, G.; Bar-Tal, A.; Borisover, M. Compositional characteristics of organic matter and its water-extractable components across a profile of organically managed soil. Geoderma 2017, 286, 73–82. [Google Scholar] [CrossRef]
- Scott, D.T.; McKnight, D.M.; Blunt-Harris, E.L.; Kolesar, S.E.; Lovley, D.R. Quinone moieties act as electron acceptors in the reduction of humic substances by humicsreducing microorganisms. Environ. Sci. Technol. 1998, 32, 2984–2989. [Google Scholar] [CrossRef]
- Gaberell, M.; Chin, Y.P.; Hug, S.J.; Sulzberger, B. Role of dissolved organic matter composition on the photoreduction of Cr(VI) to Cr(III) in the presence of iron. Environ. Sci. Technol. 2003, 37, 4403–4409. [Google Scholar] [CrossRef]
- Meunier, L.; Laubscher, H.; Hug, S.J.; Sulzberger, B. Effects of size and origin of natural dissolved organic matter compounds on the redox cycling of iron in sunlit surface waters. Aquat. Sci. 2005, 67, 292–307. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Gavara, R.; Hernandez, P.; Domínguez, I. Contact probe voltammetry for in situ monitoring of the reactivity of phenolic tomato (Solanum lycopersicum L.) compounds with ROS. Talanta 2015, 144, 1207–1215. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Cebrián-Torrejón, G.; Montya, N.; Ueberschaar, N.; Scotti, M.T.; Benfodda, Z.; Hetweck, C. Electrochemical monitoring of ROS generation by anticancer agents: The case of chartreusin. RSC Adv. 2017, 7, 45200–45210. [Google Scholar] [CrossRef] [Green Version]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves; Earthscan: London, UK, 2010. [Google Scholar]
- Pérez, A.; Gutiérrez, D.; Saldarriaga, M.S.; Sanders, C.J. Tidally driven sulfidic conditions in Peruvian mangrove sediments. Biol. Lett. 2018, 38, 457–465. [Google Scholar] [CrossRef]
- Lagos, P.; Silva, Y.; Nickl, E.; Mosquera, K. El Niño-related precipitation variability in Peru. Adv. Geosci. 2008, 14, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Lavado, W.; Espinoza, J.C. Impactos de El Niño y La Niña en las lluvias del Peru´ (1965–2007). Rev. Bras. Meteorol. 2014, 29, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, P.; Kennedy, H.; Papadimitriou, S. The effect of acidification on the determination of organic carbon, total nitrogen and their stable isotopic composition in algae and marine sediment. Rapid Commun. Mass Spectrom 2005, 19, 1063–1068. [Google Scholar] [CrossRef]
- Pérez, A.; Machado, W.; Gutierrez, D.; Smoak, J.M.; Breithaupt, J.L.; Saldarriaga, M.S.; Sanders, L.; Marotta, H.; Sanders, C.J. Carbon and nutrient accumulation in mangrove sediments affected by multiple environmental changes. J. Soils Sediments 2020, 20, 2504–2509. [Google Scholar] [CrossRef]
- Alongi, D.M. The Energetics of Mangrove Forests; Springer: Berlin/Heidelberg, Germany, 2009; p. 216. [Google Scholar]
- Fry, B.; Sherr, E.B. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib. Mar. Sci. 1984, 27, 13–47. [Google Scholar]
- Boutton, T.W. Stable Carbon Isotope Ratios of Natural Materials, II: Atmospheric, Terrestrial, Marine, and Freshwater Environments. In Carbon Isotope Techniques; Coleman, D.C., Fry, B., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1991; pp. 177–185. [Google Scholar]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Fimmen, R.L.; Cory, R.M.; Chin, Y.P.; Trouts, T.D.; McKnight, D.M. Probing the oxidation–reduction properties of terrestrially and microbially derived organic matter. Geochim. Cosmochim. Acta 2007, 71, 3003–3015. [Google Scholar] [CrossRef]
- Rivalland, C.; Madhkour, S.; Salvin, P.; Robert, F. Electrochemical and microbial monitoring of multi-generational electroactive biofilms formed from mangrove sediment. Bioelectrochemistry 2015, 106, 125–132. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).