Demographics of Scomberomorus commerson in the Central Taiwan Strait
Abstract
:1. Introduction
2. Materials and Methods
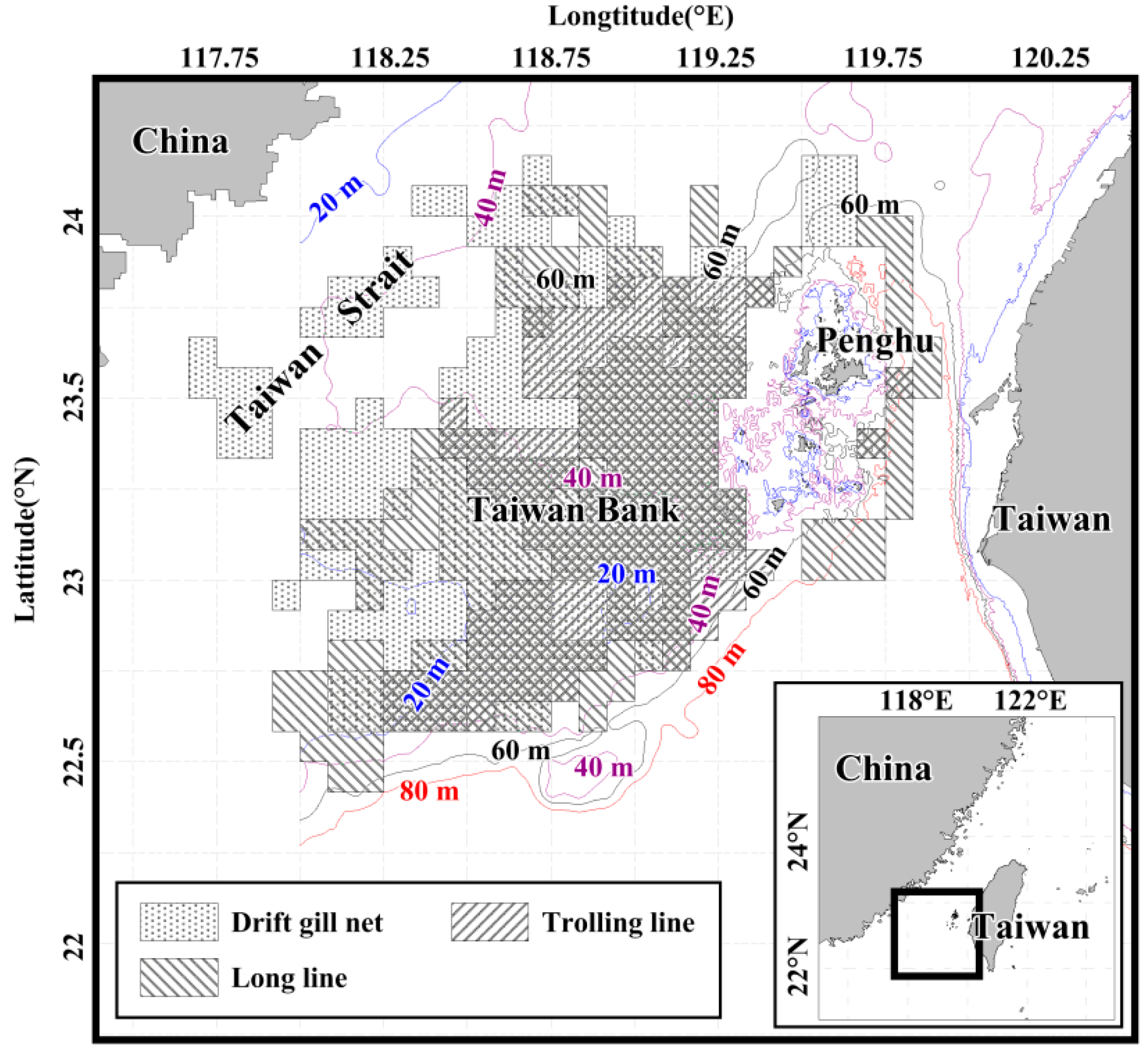
2.1. Sampling
2.2. Otolith Preparation for Age Determination
2.3. Validation of Otolith Opaque Zone Formation
2.4. Growth and Sex Ratio Analysis
2.5. Age Composition Estimation by Using an Age–Length Key
2.6. Analysis of Mortality and Exploitation Rate
3. Results
3.1. Regression of Otolith Size and FL
3.2. Structure of Otolith Sections
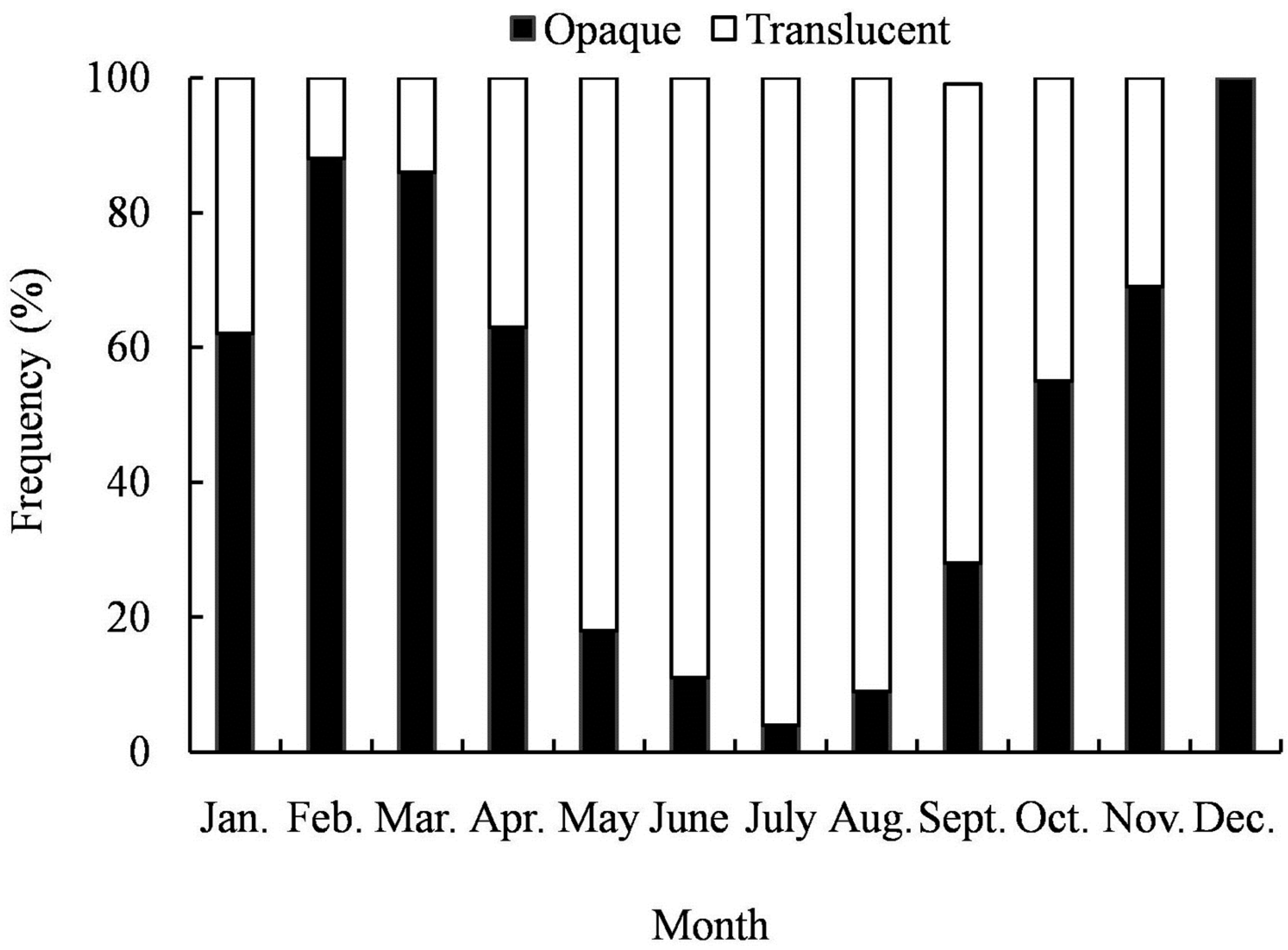
3.3. Formation of Opaque Zones
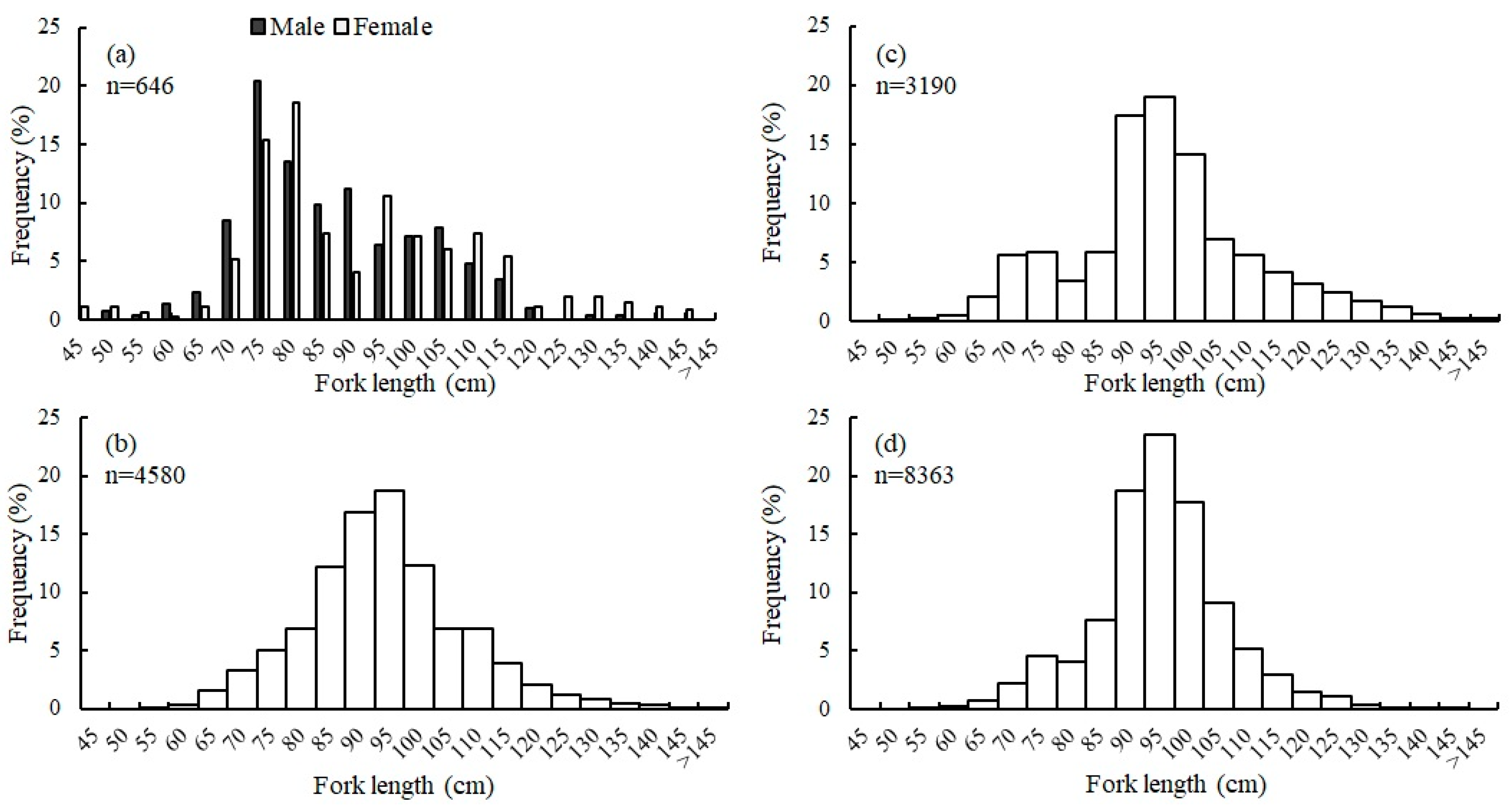
3.4. Size and Age Composition
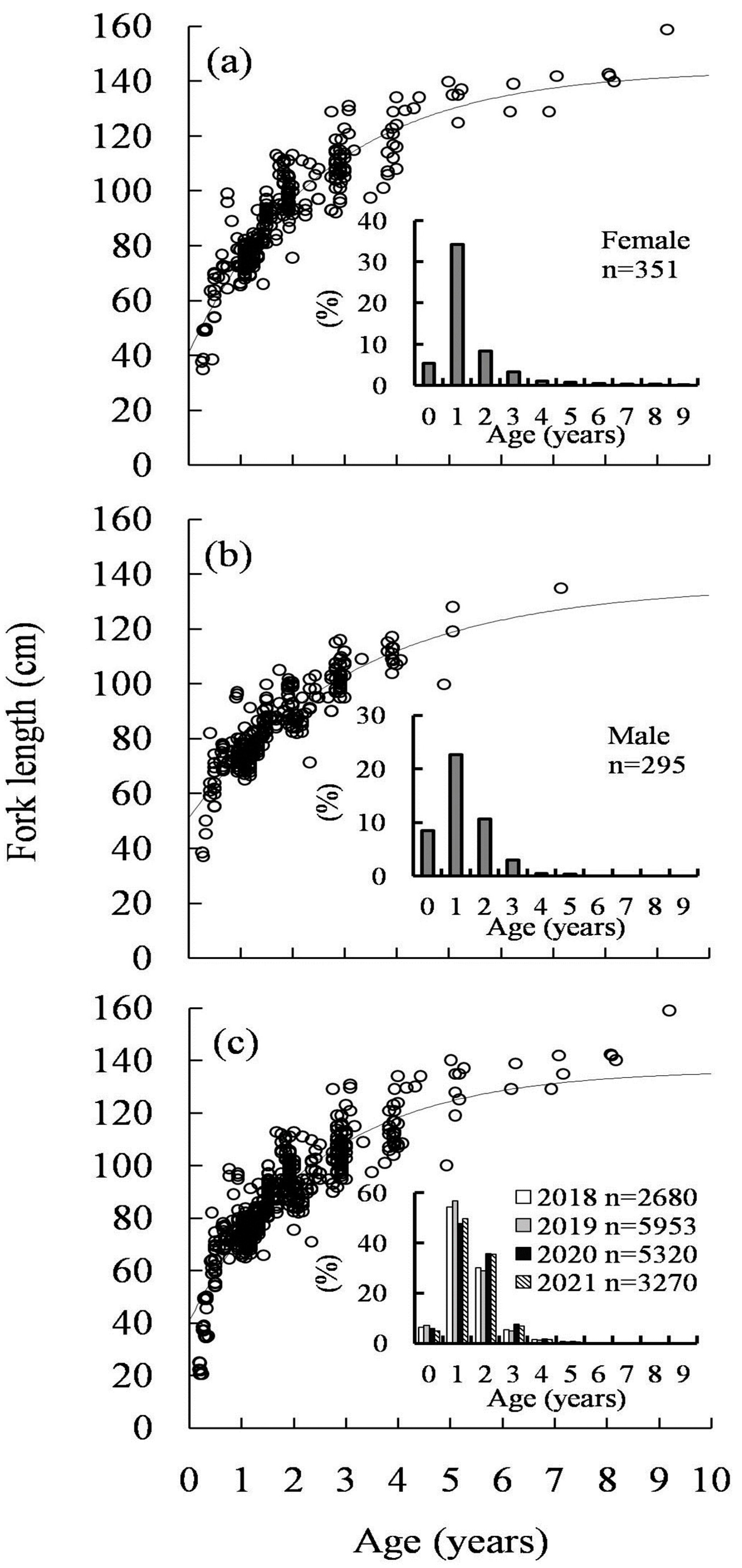
3.5. Growth Model Analysis
3.6. Mortality and Exploitation Rate
3.7. Sex Ratio
4. Discussion
4.1. Age Validation
4.2. Verification of Otolith Direct Aging
4.3. Fish Longevity and Age Composition of the Catch
4.4. Fish Growth among Regions and between Sexes
4.5. Mortality, Sex Ratio, and Suggestions for Fishery Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| FL (cm) | Age (Years) | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| <45 | 14 | - | - | - | - | - | - | - | - | - | 14 |
| 50 | 6 | - | - | - | - | - | - | - | - | - | 6 |
| 55 | 3 | - | - | - | - | - | - | - | - | - | 3 |
| 60 | 5 | - | - | - | - | - | - | - | - | - | 5 |
| 65 | 10 | 1 | - | - | - | - | - | - | - | - | 11 |
| 70 | 17 | 26 | - | - | - | - | - | - | - | - | 43 |
| 75 | 24 | 89 | 1 | - | - | - | - | - | - | - | 114 |
| 80 | 11 | 93 | 1 | - | - | - | - | - | - | - | 105 |
| 85 | 2 | 46 | 7 | - | - | - | - | - | - | - | 55 |
| 90 | 1 | 33 | 13 | - | - | - | - | - | - | - | 47 |
| 95 | 1 | 36 | 18 | 1 | - | - | - | - | - | - | 56 |
| 100 | 4 | 21 | 19 | 4 | 1 | - | - | - | - | - | 46 |
| 105 | - | 13 | 27 | 10 | - | - | - | - | - | - | 44 |
| 110 | - | 5 | 22 | 14 | 3 | - | - | - | - | - | 40 |
| 115 | - | 4 | 11 | 2 | - | - | - | - | - | - | 29 |
| 120 | - | - | 3 | 1 | 1 | 1 | - | - | - | - | 7 |
| 125 | - | - | - | 5 | 1 | 1 | - | - | - | - | 7 |
| 130 | - | - | 1 | 2 | 2 | 1 | 2 | - | - | - | 8 |
| 135 | - | - | - | 1 | 2 | 2 | - | 1 | - | - | 6 |
| 140 | - | - | - | - | - | 2 | 1 | - | 1 | - | 4 |
| 145 | - | - | - | - | - | - | - | 1 | 2 | - | 3 |
| >145 | - | - | - | - | - | - | - | - | - | 1 | 1 |
| Total | 98 | 367 | 123 | 40 | 10 | 7 | 3 | 2 | 3 | 1 | 654 |
| FL (cm) | Age (Years) | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Percentage (%) | |||||||||||
| <45 | 0.00 | - | - | - | - | - | - | - | - | - | 0 |
| 50 | 100.00 | - | - | - | - | - | - | - | - | - | 4 |
| 55 | 100.00 | - | - | - | - | - | - | - | - | - | 15 |
| 60 | 100.0 | - | - | - | - | - | - | - | - | - | 44 |
| 65 | 90.80 | 9.20 | - | - | - | - | - | - | - | - | 174 |
| 70 | 39.54 | 60.46 | - | - | - | - | - | - | - | - | 435 |
| 75 | 20.98 | 78.13 | 0.89 | - | - | - | - | - | - | - | 567 |
| 80 | 10.56 | 88.49 | 0.95 | - | - | - | - | - | - | - | 426 |
| 85 | 3.64 | 80.03 | 16.33 | - | - | - | - | - | - | - | 1182 |
| 90 | 2.11 | 70.22 | 27.67 | - | - | - | - | - | - | - | 2794 |
| 95 | 1.79 | 64.25 | 32.16 | 1.80 | - | - | - | - | - | - | 3734 |
| 100 | 8.70 | 45.65 | 41.30 | 2.16 | 2.19 | - | - | - | - | - | 2966 |
| 105 | - | 29.57 | 61.33 | 9.10 | - | - | - | - | - | - | 1593 |
| 110 | - | 12.51 | 54.99 | 25.03 | 7.47 | - | - | - | - | - | 951 |
| 115 | - | 13.88 | 37.90 | 48.22 | - | - | - | - | - | - | 533 |
| 120 | - | - | 42.81 | 28.43 | 14.38 | 14.38 | - | - | - | - | 299 |
| 125 | - | - | - | 71.28 | 14.36 | 14.36 | - | - | - | - | 188 |
| 130 | - | - | 12.84 | 24.77 | 24.78 | 12.84 | 24.77 | - | - | - | 109 |
| 135 | - | - | - | 16.13 | 33.87 | 33.87 | - | 16.13 | - | - | 62 |
| 140 | - | - | - | - | - | 48.48 | 24.24 | - | 27.28 | - | 33 |
| 145 | - | - | - | - | - | - | - | 33.33 | 66.67 | - | 15 |
| >145 | - | - | - | - | - | - | - | - | - | 100 | 9 |
| Total | 6.09 | 52.21 | 32.51 | 6.36 | 1.57 | 0.75 | 0.23 | 0.10 | 0.12 | 0.06 | 16,133 |
References
- Randall, J.E.; Hoover, J.P. Coastal Fishes of Oman; Crawford House Publishing Pty Limited: Goolwa, NSW, Australia, 1995. [Google Scholar]
- Tobin, A.; Mapleston, A. Exploitation Dynamics and Biological Characteristics of the Queensland East Coast Spanish Mackerel (Scomberomorus Commerson) Fishery; CRC Reef Research Centre, James Cook University: Townsville, QLD, Australia, 2004. [Google Scholar]
- McPherson, G.R. Age and growth of the narrow-barred Spanish Mackerel (Scomberomorus commerson Lacepede, 1880) in North-eastern Queensland waters. Aust. J. Mar. Fresh. Res. 1992, 43, 1269–1282. [Google Scholar] [CrossRef]
- McIlwain, J.; Claereboudt, M.; Al-Oufi, H.; Zaki, S.; Goddard, J.S. Spatial variation in age and growth of the kingfish (Scomberomorus commerson) in the coastal waters of the Sultanate of Oman. Fish. Res. 2005, 73, 283–298. [Google Scholar] [CrossRef]
- Carpenter, K.E. Food and Agriculture Organization of the United Nations. Living Marine Resources of Kuwait, Eastern Saudi Arabia, Bahrain, Qatar, and the United Arab Emirates; Food and Agriculture Organization of the United Nations: Rome, Italy, 1997. [Google Scholar]
- Fauvelot, C.; Borsa, P. Patterns of genetic isolation in a widely distributed pelagic fish, the narrow-barred Spanish mackerel (Scomberomorus commerson). Biol. J. Linnean Soc. 2011, 104, 886–902. [Google Scholar] [CrossRef] [Green Version]
- Claereboudt, M.R.; McIlwain, J.L.; Al-Oufi, H.S.; Ambu-Ali, A.A. Patterns of reproduction and spawning of the kingfish (Scomberomorus commerson, Lacepede) in the coastal waters of the Sultanate of Oman. Fish. Res. 2005, 73, 273–282. [Google Scholar] [CrossRef]
- Mackie, M.C.; Lewis, P.D.; Gaughan, D.J.; Newman, S.J. Variability in spawning frequency and reproductive development of the narrow-barred Spanish mackerel (Scomberomorus commerson) along the west coast of Australia. Fish. Bull. 2005, 103, 344–354. [Google Scholar]
- Niamaimandi, N.; Kaymaram, F.; Hoolihan, J.P.; Hossien, G.; Seyed, M.; Fatemi, R. Conservation. Population dynamics parameters of narrow-barred Spanish mackerel, Scomberomorus commerson (Lacèpéde, 1800), from commercial catch in the northern Persian Gulf. Glob. Ecol. 2015, 4, 666–672. [Google Scholar]
- Govender, A.; Al-Oufi, H.; McIlwain, J.; Claereboudt, M.C. A per-recruit assessment of the kingfish ( Scomberomorus commerson) resource of Oman with an evaluation of the effectiveness of some management regulations. Fish. Res. 2006, 77, 239–247. [Google Scholar] [CrossRef]
- Shojaei, M.G.; Motlagh, S.A.T.; Seyfabadi, J.; Abtahi, B.; Dehghani, R. Age, growth and mortality rate of the narrow-barred Spanish Mackerel (Scomberomerus commerson Lacepede, 1800) in coastal waters of Iran from length frequency data. Turk. J. Fish. Aqu. Sci. 2007, 7, 115–221. [Google Scholar]
- O’Neill, M.F.; Tobin, A. Torres Strait Spanish Mackerel; Department of Agriculture and Fisheries: Brisbane, QLD, Australia, 2015. [Google Scholar]
- Fisheries Agency. Fisheries Statistical Yearbook Taiwan, Kinmen and Matsu; Fisheries Agency, Council of Agriculture, Executive Yuan: Taipei, Taiwan, 2020. [Google Scholar]
- Hüssy, K.; Radtke, K.; Plikshs, M.; Oeberst, R.; Baranova, T.; Krumme, U.; Sjöberg, R.; Walther, Y.; Mosegaard, H. Challenging ICES age estimation protocols: Lessons learned from the eastern Baltic cod stock. ICES. J. Mar. Sci. 2016, 73, 2138–2149. [Google Scholar] [CrossRef]
- Ballagh, A.C.; Welch, D.; Williams, A.J.; Mapleston, A.; Tobin, A.; Marton, N. Integrating methods for determining length-at-age to improve growth estimates for two large scombrids. Fish. Bull. 2011, 109, 90–100. [Google Scholar]
- Lee, B. The Biology of and Fishery for King Mackerel, Scomberomorus Commerson (Scombridae), along the Southern Mozambique and KwaZulu-Natal Coast; Oceanographic Research Institute: Durban, South Africa, 2013. [Google Scholar]
- Haddon, M. Modelling and Quantitative Methods in Fisheries; Taylor & Francis: New York, NY, USA, 2001. [Google Scholar]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Devaraj, M. Age and growth of three species of seerfishes scomeromorus commersou, S. guttatus and S. lineolatus. Indian J. Fish. 1981, 28, 104–127. [Google Scholar]
- Al-Hosni, A.H.S.; Siddeek, M.S. Growth and mortality of the narrowbarred Spanish Mackerel, Scomberomorus commerson (Lacepede), in Omani waters. Fish. Manag. Ecol. 1999, 6, 145–160. [Google Scholar] [CrossRef]
- Govender, A. Growth of the king mackerel (Scomberomorus commerson) off the coast of Natal, South Africa— from length and age data. Fish. Res. 1994, 20, 63–79. [Google Scholar] [CrossRef]
- Govender, A. Mortality and biological reference points for the king mackerel (Scomberomorus commerson) fishery off Natal, South Africa (based on a per-recruit assessment). Fish. Res. 1995, 23, 195–208. [Google Scholar] [CrossRef]
- Lee, B.; Mann, B.Q. Age and growth of narrow-barred Spanish mackerel Scomberomorus commerson in the coastal waters of southern Mozambique and KwaZulu-Natal South Africa. Afr. J. Mar. Sci. 2017, 39, 397–407. [Google Scholar] [CrossRef]
- Grandcourt, E.M.; Abdessalaam, T.Z.; Francis, F.; Al Shamsi, A.T. Preliminary assessment of the biology and fishery for the narrow-barred Spanish mackerel, Scomberomorus commerson (Lacépède, 1800), in the southern Arabian Gulf. Fish. Res. 2005, 76, 277–290. [Google Scholar] [CrossRef]
- Ballagh, A.C.; Begg, G.A.; Mapleston, A.; Tobin, A. Growth trends of Queensland east coast Spanish mackerel (Scomberomorus commerson) from otolith back-calculations. Mar. Fresh. Res. 2006, 57, 383–393. [Google Scholar] [CrossRef]
- Chen, T.S. Studies on the age, growth, maturity and spawning of Spanish mackerel Scomberomorus commersoni (Lacépéde) in Taiwan Strait. Bull. Taiwan Fish. Res. Inst. 1973, 22, 103–108. [Google Scholar]
- Wang, H.Y.; Shen, S.F.; Chen, Y.S.; Kiang, Y.K.; Heino, M. Life histories determine divergent population trends for fishes under climate warming. Nat. Commun. 2020, 11, 4088. [Google Scholar] [CrossRef]
- Weng, J.S.; Yu, S.F.; Lo, Y.S.; Shiao, J.C.; Lee, M.A.; Liu, K.M.; Huang, H.H.; Wang, Y.C.; Wu, L.J. Reproductive biology of the narrow-barred Spanish mackerel (Scomberomorus commerson) in the central Taiwan Strait, western Pacific. Deep Sea Res. Part II 2020, 175, 104755. [Google Scholar] [CrossRef]
- Beamish, R.J.; Fournier, D.A. A Method for Comparing the Precision of a Set of Age Determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- Radebe, P.; Mann, B.; Beckley, L.; Govender, A. Age and growth of Rhabdosargus sarba (Pisces: Sparidae), from KwaZulu-Natal, South Africa. Fish. Res. 2002, 58, 193–201. [Google Scholar] [CrossRef]
- von Bertalanffy, L. A quantitative theory of organic growth. Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Kimura, D. Likelihood methods for the von Bertalanffy growth curve. Fish. Bull. 1980, 77, 765–776. [Google Scholar]
- FriÁriksson, Á. On the Calculation of Age-Distribution Within a Stock of Cod by Means of Relatively Few Age-Determinations as a Key to Measurements on a Large Scale. Rapports et Procbs-Verbaux des R6unions, Conseil International Pour Exploration de la Mer. 1934, Volume 86, pp. 1–14. Available online: https://books.google.co.kr/books/about/On_the_Calculation_of_Age_distribution_W.html?id=uCHXzQEACAAJ&redir_esc=y (accessed on 20 November 2021).
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 1975, 191, 382. [Google Scholar]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- King, M. Fisheries Biology, Assessment and Management, 2nd ed.; Wiley-Blackwell: London, UK, 2007; 400p. [Google Scholar]
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Fowler, A.J. Annulus formation in otoliths of coral reef fish-a review. In Recent Developments in Fish Otolith Research; Secor, D.H., Dean, J.M., Campana, S.E., Eds.; University of South Carolina Press: Columbia, SC, USA, 1995; pp. 45–64. [Google Scholar]
- Beckman, D.W.; Wilson, C.A. Seasonal timing of opaque zone formation in fish otoliths. In Recent Developments in Fish Otolith Research; Secor, D.H., Dean, J.M., Campana, S.E., Miller, A.B., Eds.; University of South Carolina Press: Columbia, SC, USA, 1995; pp. 27–44. [Google Scholar]
- Hsieh, Y.; Shiao, J.C.; Lin, S.W.; Iizuka, Y. Quantitative reconstruction of salinity history by otolith oxygen stable isotopes: An example of a euryhaline fish Lateolabrax japonicus. Rapid Commun. Mass Spectrom. 2019, 33, 1344–1354. [Google Scholar] [CrossRef]
- Newman, S.J.; Mackie, M.C.; Lewis, P.D. Age-based demography and relative fisheries productivity of Spanish mackerel, Scomberomorus commerson (Lacepede) in Western Australia. Fish. Res. 2012, 129, 46–60. [Google Scholar] [CrossRef]
- Lewis, P.; Mackie, M.C. Methods Used in the Collection, Preparation and Interpretation of Narrow-Barred Spanish Mackerel (Scomberomorus Commerson) Otoliths for a Study of Age and Growth in Western Australia; Fisheries Research Report No. 143; Department of Fisheries: Perth, WA, Australia, 2002. [Google Scholar]
- Lester, R.J.G.; Thompson, C.; Moss, H.; Barker, S.C. Movement and stock structure of narrow-barred Spanish mackerel as indicated by parasites. J. Fish Biol. 2001, 59, 833–842. [Google Scholar] [CrossRef]
- Moore, B.R.; Buckworth, R.C.; Moss, H.; Lester, R.J.G. Stock discrimination and movements of narrow-barred Spanish mackerel across northern Australia as indicated by parasites. J. Fish Biol. 2003, 63, 765–779. [Google Scholar] [CrossRef]
- Jan, S.; Wang, J.; Chern, C.S.; Chao, S.Y. Seasonal variation of the circulation in the Taiwan Strait. J. Mar. Syst. 2002, 35, 249–268. [Google Scholar] [CrossRef]
- Wang, S.P.; Sun, C.L.; Punt, A.E.; Yeh, S.Z. Evaluation of a sex-specific age-structured assessment method for the swordfish, Xiphias gladius, in the North Pacific Ocean. Fish. Res. 2005, 73, 79–97. [Google Scholar] [CrossRef]
- Williams, E.H.; Shertzer, K.W. Effects of fishing on growth trait: A simulation analysis. Fish. Bull. 2006, 103, 392–403. [Google Scholar]
- Rose, D.; Bailey, S.; Staunton-Smith, J.; Mackenzie, B. Fisheries Long Term Monitoring Program: Summary of Spanish Mackerel (Scomberomorus commerson) Survey Results: 2004–2005; Department of Primary Industries and Fisheries: Brisbane, QLD, Australia, 2006. [Google Scholar]
- Claereboudt, M.R.; Al-Oufi, H.S.; McIlwain, J.; Goddard, J.S. Relationships between fishing gear, size frequency and reproductive patterns for the kingfish (Scomberomoms commerson Lacépède) fishery in the gulf of oman. In Management of Shared Fish Stocks; Pogue, A.I.L., O’Brien, C.M., Rogers, S.I., Eds.; Blackwell: Oxford, UK, 2004; pp. 56–67. [Google Scholar]


| Sex | N | Equation | r2 | p-Value |
|---|---|---|---|---|
| Female | 218 | FL = 7.49 × OL − 2.80 | 0.914 | <0.001 |
| Male | 205 | FL = 8.43 × OL − 17.52 | 0.842 | <0.001 |
| Sexes combined | 423 | FL = 7.64 × OL − 6.10 | 0.88 | <0.001 |
| VBGF Parameter | Φ’ | Age (Year) | Sex | Region | Source | |
|---|---|---|---|---|---|---|
| k | ||||||
| 0.39 | 144.1 | 9.0 | 9.2 | Female | Taiwan Strait | This study |
| 0.31 | 135.9 | 8.7 | 7.2 | Male | Taiwan Strait | This study |
| 0.16 | 130.1 | 7.9 | 6 | Combined | Taiwan Strait | [26] |
| 0.17 | 155.0 | 8.3 | 14 | Female | North-eastern Queensland | [3] |
| 0.25 | 127.0 | 8.3 | 10 | Male | North-eastern Queensland | [3] |
| 0.51 | 124.8 | 9.0 | 15 | Female | East coast of Queensland | [25] |
| 0.75 | 104.7 | 9.0 | 11 | Male | East coast of Queensland | [25] |
| 0.18 | 187.1 | 8.7 | - | Combined | Indian | [19] |
| 0.29 | 134.3 | 8.6 | - | Combined | South Africa | [21] |
| 0.28 | 173.6 | 9.0 | - | Combined | Oman | [20] |
| 0.20 | 151.3 | 8.4 | 20 | Female | Oman | [10] |
| 0.28 | 134.7 | 8.5 | 10 | Male | Oman | [10] |
| 0.31 | 140.4 | 8.7 | 20 | Female | Sultanate of Oman | [4] |
| 0.60 | 118.8 | 9.0 | 10 | Male | Sultanate of Oman | [4] |
| 0.24 | 136.1 | 8.4 | 15.3 | Female | Southern Arabian Gulf | [24] |
| 0.22 | 125.6 | 8.2 | 16.2 | Male | Southern Arabian Gulf | [24] |
| 0.31 | 130.5 | 8.6 | 14.6 | Female | KZN and Mozambique | [23] |
| 0.28 | 119.2 | 8.3 | 13.6 | Male | KZN and Mozambique | [23] |
| 0.50 | 148.0 | 9.3 | - | Combined | Northern Persian Gulf | [9] |
| 0.42 | 140.0 | 9.0 | 10 | Combined | South of Iran | [11] |
| Age | Female | Male | Sex Ratio (%) | χ2 | p |
|---|---|---|---|---|---|
| 0 | 35 | 55 | 38.9 | 4.44 | 0.035 * |
| 1 | 221 | 146 | 60.2 | 15.33 | 0.0001 * |
| 2 | 54 | 69 | 43.9 | 1.83 | 0.176 |
| 3 | 21 | 19 | 52.5 | 0.10 | 0.751 |
| 4 | 7 | 3 | 70.0 | 1.60 | 0.205 |
| 5 | 5 | 2 | 71.4 | - | - |
| 6 | 3 | 0 | - | - | - |
| 7 | 2 | 1 | - | - | - |
| 8 | 2 | 0 | - | - | - |
| 9 | 1 | 0 | - | - | - |
| Total | 351 | 295 | 54.0 | 4.85 | 0.027 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, J.-S.; Cheng, L.-C.; Lo, Y.-S.; Shiao, J.-C.; He, J.-S.; Lee, M.-A.; Liu, K.-M. Demographics of Scomberomorus commerson in the Central Taiwan Strait. J. Mar. Sci. Eng. 2021, 9, 1346. https://doi.org/10.3390/jmse9121346
Weng J-S, Cheng L-C, Lo Y-S, Shiao J-C, He J-S, Lee M-A, Liu K-M. Demographics of Scomberomorus commerson in the Central Taiwan Strait. Journal of Marine Science and Engineering. 2021; 9(12):1346. https://doi.org/10.3390/jmse9121346
Chicago/Turabian StyleWeng, Jinn-Shing, Li-Chi Cheng, Yun-Sin Lo, Jen-Chieh Shiao, Jia-Sin He, Ming-An Lee, and Kwang-Ming Liu. 2021. "Demographics of Scomberomorus commerson in the Central Taiwan Strait" Journal of Marine Science and Engineering 9, no. 12: 1346. https://doi.org/10.3390/jmse9121346
APA StyleWeng, J.-S., Cheng, L.-C., Lo, Y.-S., Shiao, J.-C., He, J.-S., Lee, M.-A., & Liu, K.-M. (2021). Demographics of Scomberomorus commerson in the Central Taiwan Strait. Journal of Marine Science and Engineering, 9(12), 1346. https://doi.org/10.3390/jmse9121346







