In-Culture Selection and the Potential Effects of Changing Sex Ratios on the Reproductive Success of Multiannual Delayed Gametophytes of Saccharina latissima and Alaria esculenta
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture and Maintenance
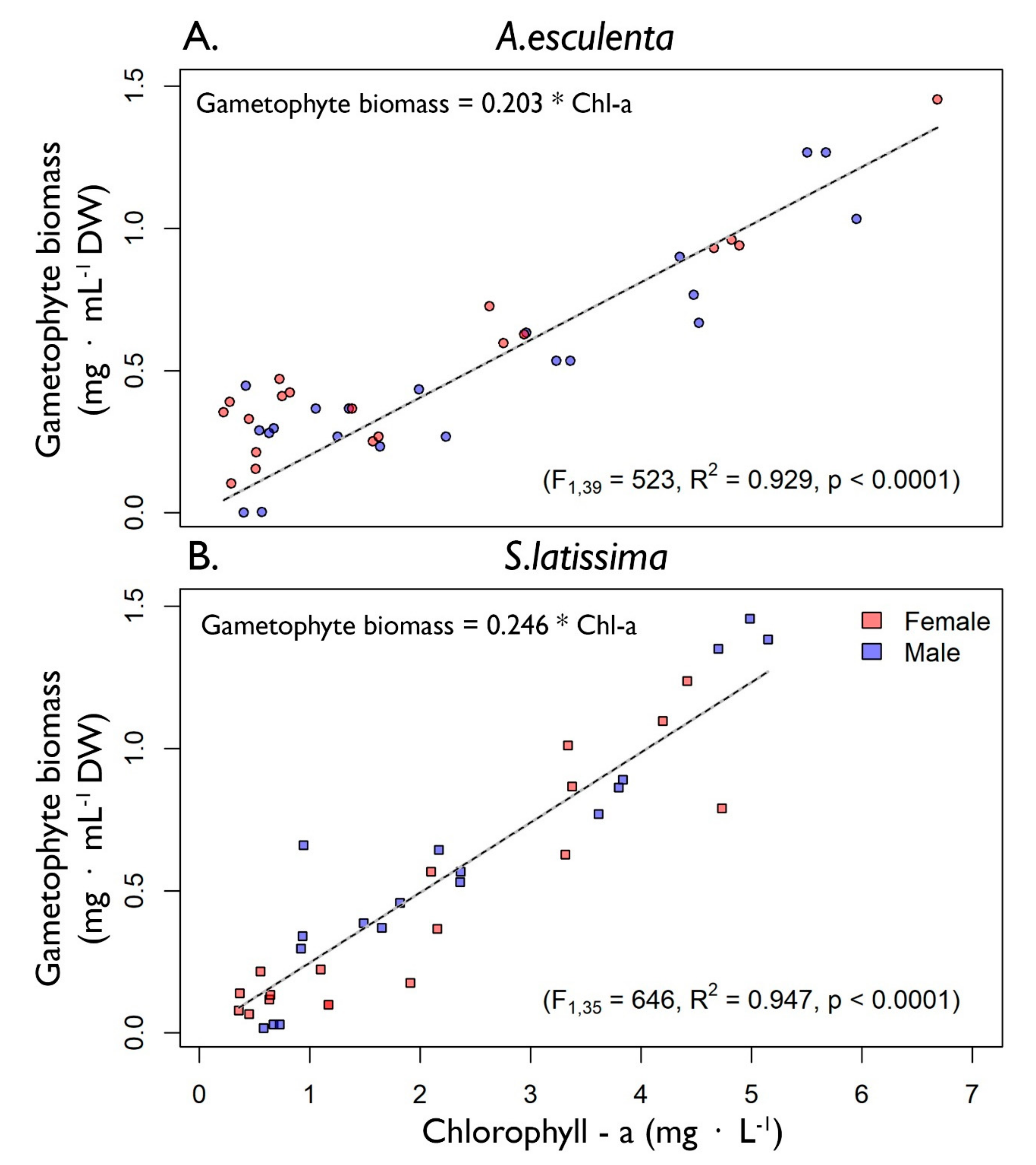
2.2. Gametophyte Biomass Calibration
2.3. The Vegetative Growth of Clonal Gametophyte Biomass
2.4. Gametophyte Reproduction
2.5. Statistical Analysis
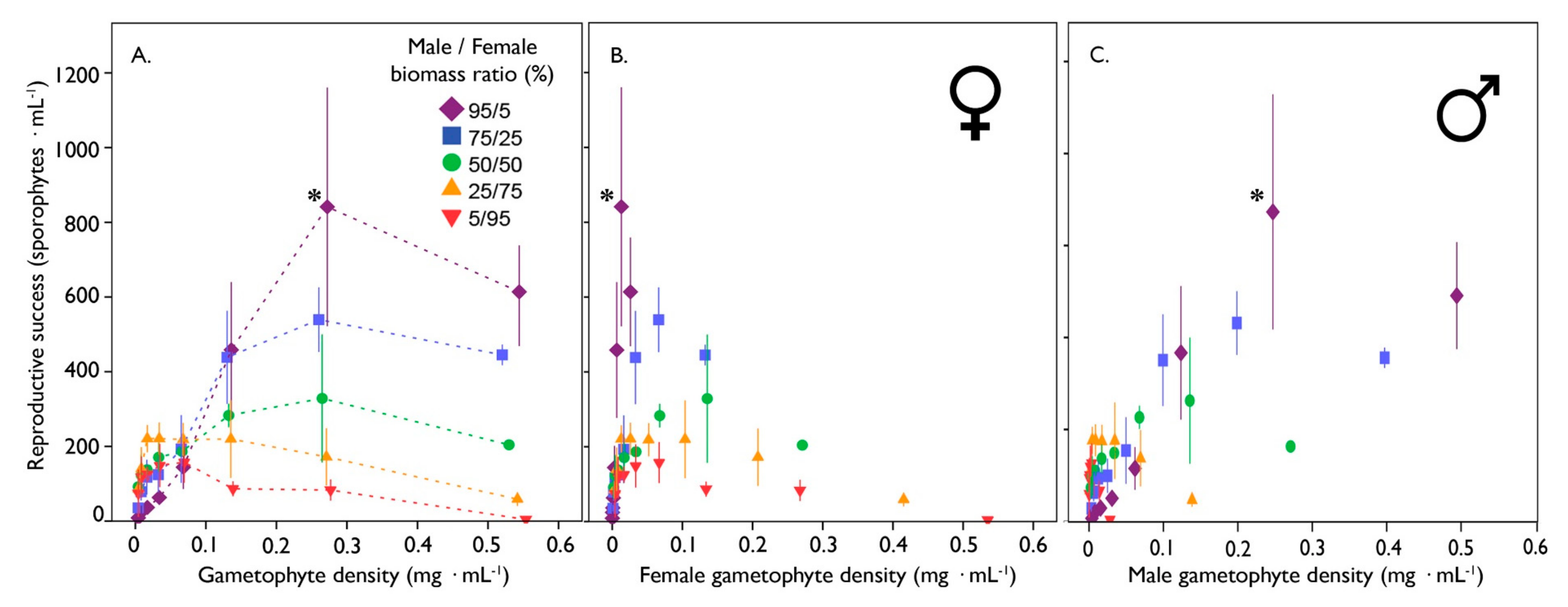
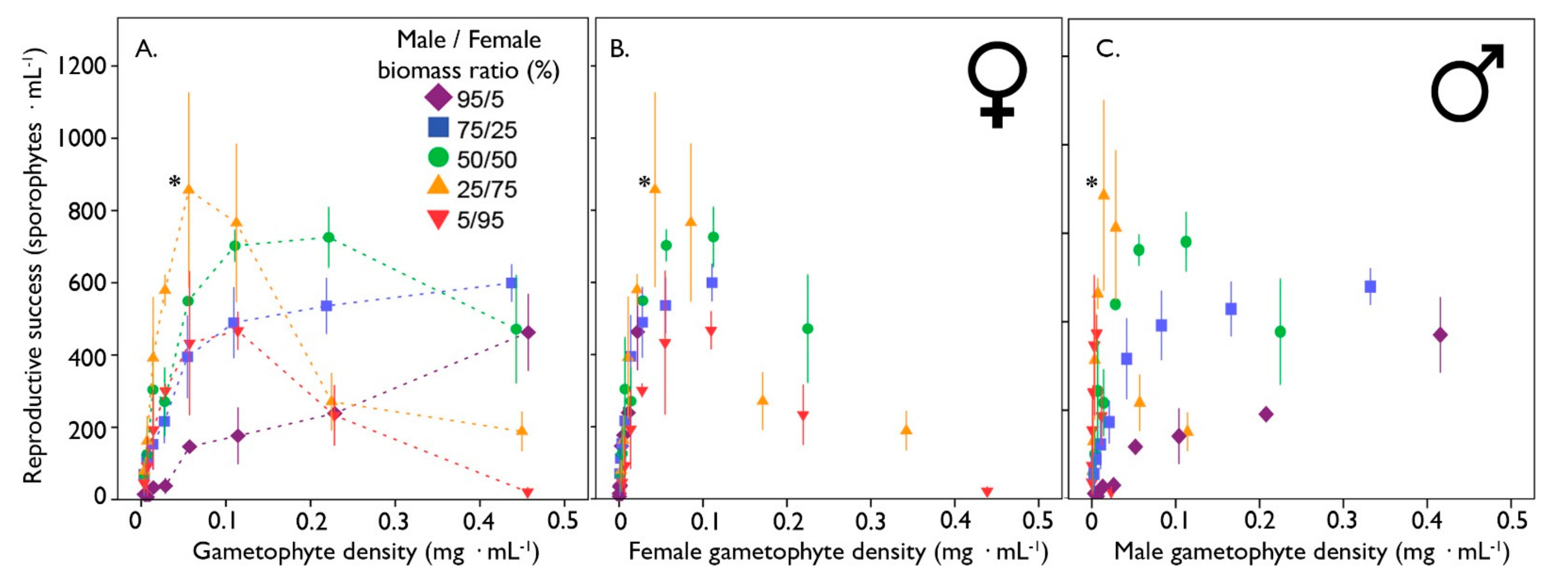
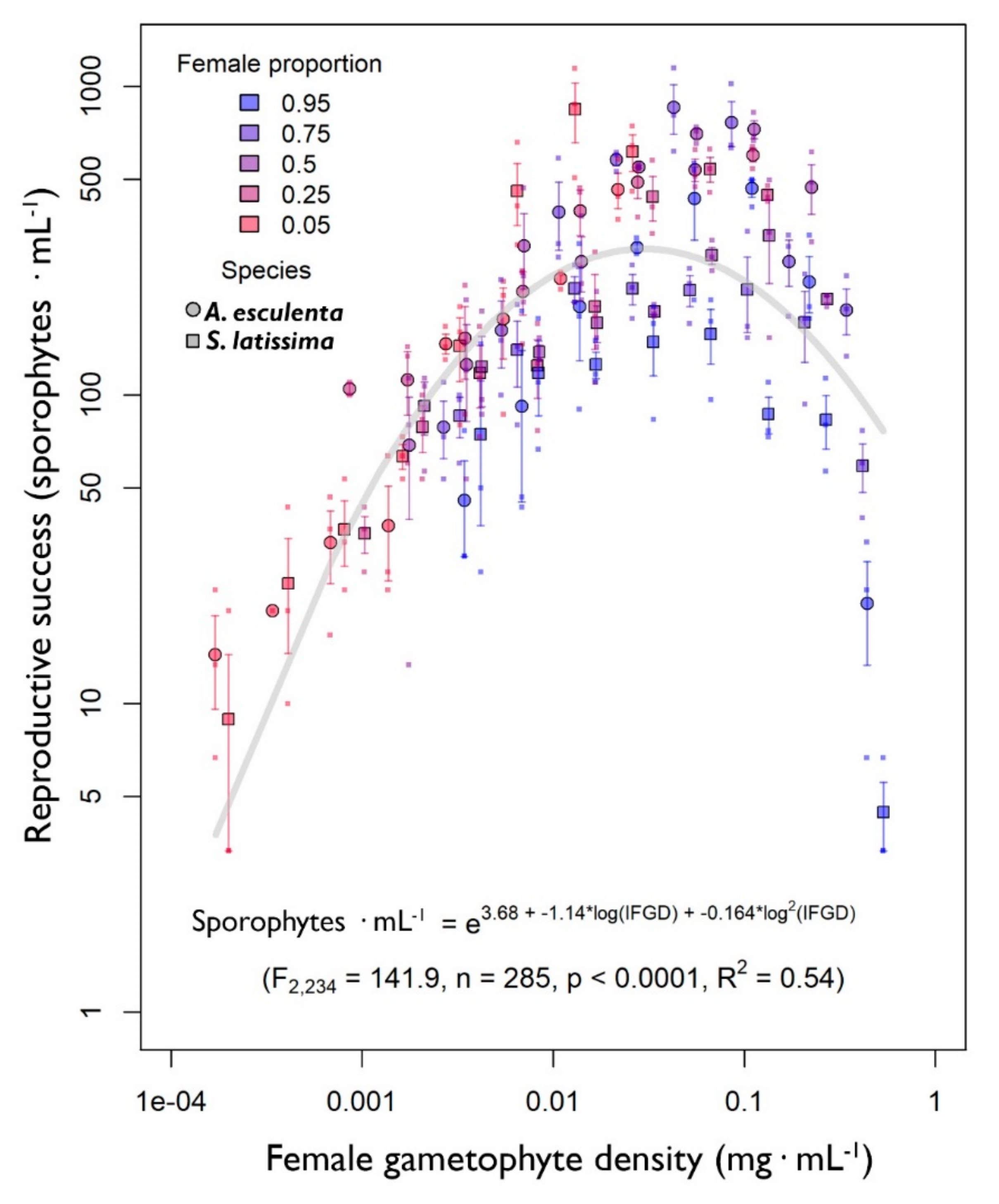
3. Results
3.1. Gametophyte Biomass Calibrations
3.2. The Effect of Sex Ratio on Gametophyte Reproduction of S. latissima
3.3. The Effect of Sex Ratio on Gametophyte Reproduction of A. Esculenta
3.4. Gametophyte Growth over Time
3.5. The Effect of Female Gametophyte Biomass Density on Gametophyte Reproduction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goecke, F.; Klemetsdal, G. Ergon, Åshild Cultivar Development of Kelps for Commercial Cultivation—Past Lessons and Future Prospects. Front. Mar. Sci. 2020, 8, 110. [Google Scholar] [CrossRef]
- Barrento, S.; Camus, C.; Pinto, I.S.; Buschmann, A. Germplasm banking of the giant kelp: Our biological insurance in a changing environment. Algal Res. 2016, 13, 134–140. [Google Scholar] [CrossRef]
- Carney, L.T. A MULTISPECIES LABORATORY ASSESSMENT OF RAPID SPOROPHYTE RECRUITMENT FROM DELAYED KELP GAMETOPHYTES1. J. Phycol. 2011, 47, 244–251. [Google Scholar] [CrossRef]
- Wade, R.; Augyte, S.; Harden, M.; Nuzhdin, S.; Yarish, C.; Alberto, F. Macroalgal germplasm banking for conservation, food security, and industry. PLoS Biol. 2020, 18, e3000641. [Google Scholar] [CrossRef]
- Visch, W.; Menéndez, C.R.; Nylund, G.M.; Pavia, H.; Ryan, M.; Day, J. Underpinning the Development of Seaweed Biotechnology: Cryopreservation of Brown Algae (Saccharina latissima) Gametophytes. Biopreserv. Biobank. 2019, 17, 378–386. [Google Scholar] [CrossRef]
- Reddy, C.R.K.; Jha, B.; Fujita, Y.; Ohno, M. Seaweed micropropagation techniques and their potentials: An overview. Environ. Boil. Fishes 2007, 20, 609–617. [Google Scholar] [CrossRef]
- Shan, T.F.; Pang, S.J.; Li, J.; Gao, S.Q. Breeding of an elite cultivar Haibao No. 1 of Undaria pinnatifida (Phaeophyceae) through gametophyte clone crossing and consecutive selection. Environ. Boil. Fishes 2016, 28, 2419–2426. [Google Scholar] [CrossRef]
- Zhao, X.B.; Pang, S.J.; Liu, F.; Shan, T.F.; Li, J.; Gao, S.Q.; Kim, H.G. Intraspecific crossing of Saccharina japonica using distantly related unialgal gametophytes benefits kelp farming by improving blade quality and productivity at Sanggou Bay, China. Environ. Boil. Fishes 2016, 28, 449–455. [Google Scholar] [CrossRef]
- Martins, N.; Pearson, G.A.; Gouveia, L.; Tavares, A.I.; Serrão, E.A.; Bartsch, I. Hybrid vigour for thermal tolerance in hybrids between the allopatric kelps Laminaria digitata and L. pallida (Laminariales, Phaeophyceae) with contrasting thermal affinities. Eur. J. Phycol. 2019, 54, 548–561. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Qu, S.C.; Cong, Y.Z.; Luo, S.J.; Tang, X.X. High throughput culture and gametogenesis induction of Laminaria japonica gametophyte clones. Environ. Boil. Fishes 2008, 20, 205–211. [Google Scholar] [CrossRef]
- Ebbing, A.P.J.; Pierik, R.; Fivash, G.S.; van de Loosdrecht, N.C.; Bouma, T.J.; Kromkamp, J.C.; Timmermans, K. The role of seasonality in reproduction of multiannual delayed gametophytes of Saccharina latissima. J. Phycol. 2021, 57, 1580–1589. [Google Scholar] [CrossRef]
- Alsuwaiyan, N.A.; Mohring, M.B.; Cambridge, M.; Coleman, M.A.; Kendrick, G.A.; Wernberg, T. A review of protocols for the experimental release of kelp (Laminariales) zoospores. Ecol. Evol. 2019, 9, 8387–8398. [Google Scholar] [CrossRef]
- Gil Choi, H.; Kim, Y.S.; Lee, S.J.; Park, E.J.; Nam, K.W. Effects of daylength, irradiance and settlement density on the growth and reproduction of Undaria pinnatifida gametophytes. Environ. Boil. Fishes 2005, 17, 423–430. [Google Scholar] [CrossRef]
- Wu, C.; Li, D.; Liu, H.; Peng, G.; Liu, J. Mass culture of Undaria gametophyte clones and their use in sporeling culture. Hydrobiologia 2004, 512, 153–156. [Google Scholar] [CrossRef]
- Li, D.; Zhou, Z.-G.; Liu, H.; Wu, C. A new method of Laminaria japonica strain selection and sporeling raising by the use of gametophyte clones. Hydrobiologia 1999, 398/399, 473–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, M.; Li, L.; Tang, X.; Gao, J. Homogenization significantly enhances growth of macroalga Saccharina japonica female gametophytes. Algal Res. 2019, 43, 101640. [Google Scholar] [CrossRef]
- Lakeman, M.B.; von Dassow, P.; Cattolico, R.A. The strain concept in phytoplankton ecology. Harmful Algae 2009, 8, 746–758. [Google Scholar] [CrossRef]
- Destombe, C.; Oppliger, L.V. Male gametophyte fragmentation in laminaria digitata: A life history strategy to enhance reproductive success. Cah. De Biol. Mar. 2011, 52, 385–394. [Google Scholar]
- Oppliger, L.V.; Correa, J.A.; Engelen, A.; Tellier, F.; Vieira, V.; Faugeron, S.; Valero, M.; Gomez, G.; Destombe, C. Temperature Effects on Gametophyte Life-History Traits and Geographic Distribution of Two Cryptic Kelp Species. PLoS ONE 2012, 7, e39289. [Google Scholar] [CrossRef]
- Murúa, P.; Patiño, D.J.; Müller, D.G.; Westermeier, R. Sexual compatibility in giant kelp gametophytes: Inter-cultivar hybridization is average between parents but excels under harsher conditions. Environ. Boil. Fishes 2021, 33, 3261–3275. [Google Scholar] [CrossRef]
- Ebbing, A.; Pierik, R.; Bouma, T.; Kromkamp, J.C.; Timmermans, K. How light and biomass density influence the reproduction of delayed Saccharina latissima gametophytes (Phaeophyceae). J. Phycol. 2020, 56, 709–718. [Google Scholar] [CrossRef]
- Redmond, S.; Green, L.; Yarish, C.; Kim, J.; Neefus, C. New England Seaweed Culture Handbook. Seaweed Cultiv. 2014. [Google Scholar] [CrossRef]
- Bartsch, I. Derivation of clonal stock cultures and hybridization of kelps: A tool for strain preservation and breeding programs. In Protocols for Macroalgae Research; Charrier, B., Wichard, T., Reddy, C.R.K., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2018. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotellanana Hustedt and Detonula Confervacea (cleve). Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Huot, Y.; Babin, M.; Bruyant, F.; Grob, C.; Twardowski, T.H.; Claustre, H. Does chlorophyll a provide the best index of phytoplankton biomass for primary productivity studies? Biogeosci. Discuss. 2007, 4, 707–745. [Google Scholar] [CrossRef]
- Team R Core. R: A Language and Environment for Statistical Computing; Team R Core: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 4 March 2021).
- Ebbing, A.; Fivash, G.; Pierik, R.; Bouma, T.; Kromkamp, J.C.; Timmermans, K. The SeaCoRe system for large scale kelp aquaculture: A plug-and-play, compatible, open-source system for the propagation and transport of clonal gametophyte cultures. J. Appl. Phycol. 2021. [Google Scholar] [CrossRef]
- Reed, D.C. The Effects of Variable Settlement and Early Competition on Patterns of Kelp Recruitment. Ecology 1990, 71, 776–787. [Google Scholar] [CrossRef]
- Carney, L.T.; Edwards, M.S. Role of nutrient fluctuations and delayed development in gametophyte reproduction by Macrocystis pyrifera (Phaeophyceae) in southern California. J. Phycol. 2010, 46, 987–996. [Google Scholar] [CrossRef]
- Reed, D.C.; Neushul, M.; Ebeling, A.W. Role of Settlement Density on Gametophyte Growth and Reproduction in the Kelps Pterygophora Californica and Macrocystis pyrifera (Phaeophyceae). J. Phycol. 1991, 27, 361–366. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebbing, A.P.J.; Fivash, G.S.; Martin, N.B.; Pierik, R.; Bouma, T.J.; Kromkamp, J.C.; Timmermans, K. In-Culture Selection and the Potential Effects of Changing Sex Ratios on the Reproductive Success of Multiannual Delayed Gametophytes of Saccharina latissima and Alaria esculenta. J. Mar. Sci. Eng. 2021, 9, 1250. https://doi.org/10.3390/jmse9111250
Ebbing APJ, Fivash GS, Martin NB, Pierik R, Bouma TJ, Kromkamp JC, Timmermans K. In-Culture Selection and the Potential Effects of Changing Sex Ratios on the Reproductive Success of Multiannual Delayed Gametophytes of Saccharina latissima and Alaria esculenta. Journal of Marine Science and Engineering. 2021; 9(11):1250. https://doi.org/10.3390/jmse9111250
Chicago/Turabian StyleEbbing, Alexander P. J., Gregory S. Fivash, Nuria B. Martin, Ronald Pierik, Tjeerd J. Bouma, Jacco C. Kromkamp, and Klaas Timmermans. 2021. "In-Culture Selection and the Potential Effects of Changing Sex Ratios on the Reproductive Success of Multiannual Delayed Gametophytes of Saccharina latissima and Alaria esculenta" Journal of Marine Science and Engineering 9, no. 11: 1250. https://doi.org/10.3390/jmse9111250
APA StyleEbbing, A. P. J., Fivash, G. S., Martin, N. B., Pierik, R., Bouma, T. J., Kromkamp, J. C., & Timmermans, K. (2021). In-Culture Selection and the Potential Effects of Changing Sex Ratios on the Reproductive Success of Multiannual Delayed Gametophytes of Saccharina latissima and Alaria esculenta. Journal of Marine Science and Engineering, 9(11), 1250. https://doi.org/10.3390/jmse9111250






