Ecological Constraints of Plankton Bio-Indicators for Water Column Stratification and Productivity: A Case Study of the Holocene North Aegean Sedimentary Record
Abstract
:1. Introduction
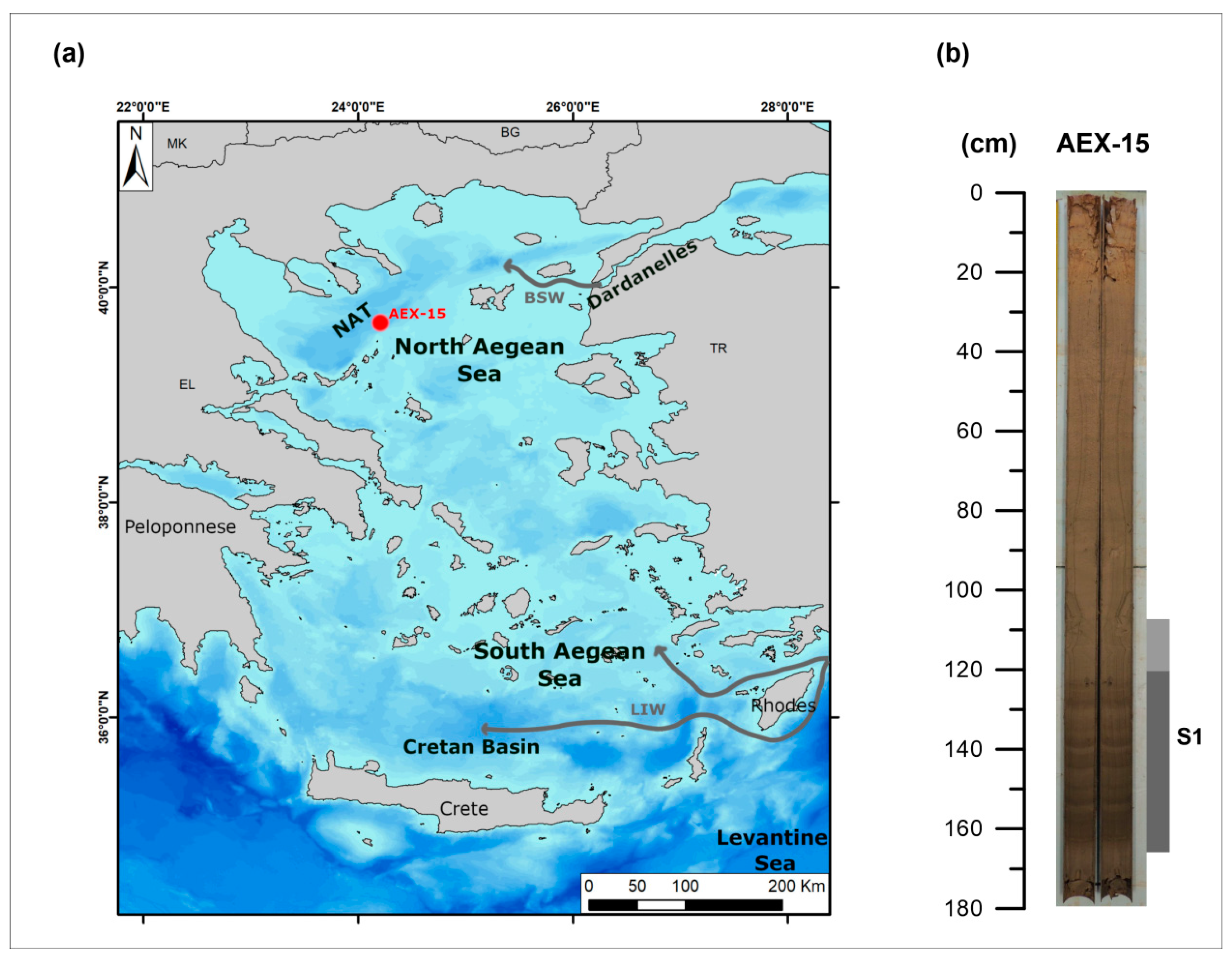
2. Oceanographic and Geologic Setting
3. Materials and Methods
3.1. Material
3.2. Multivariate Statistical Analyses
4. Results
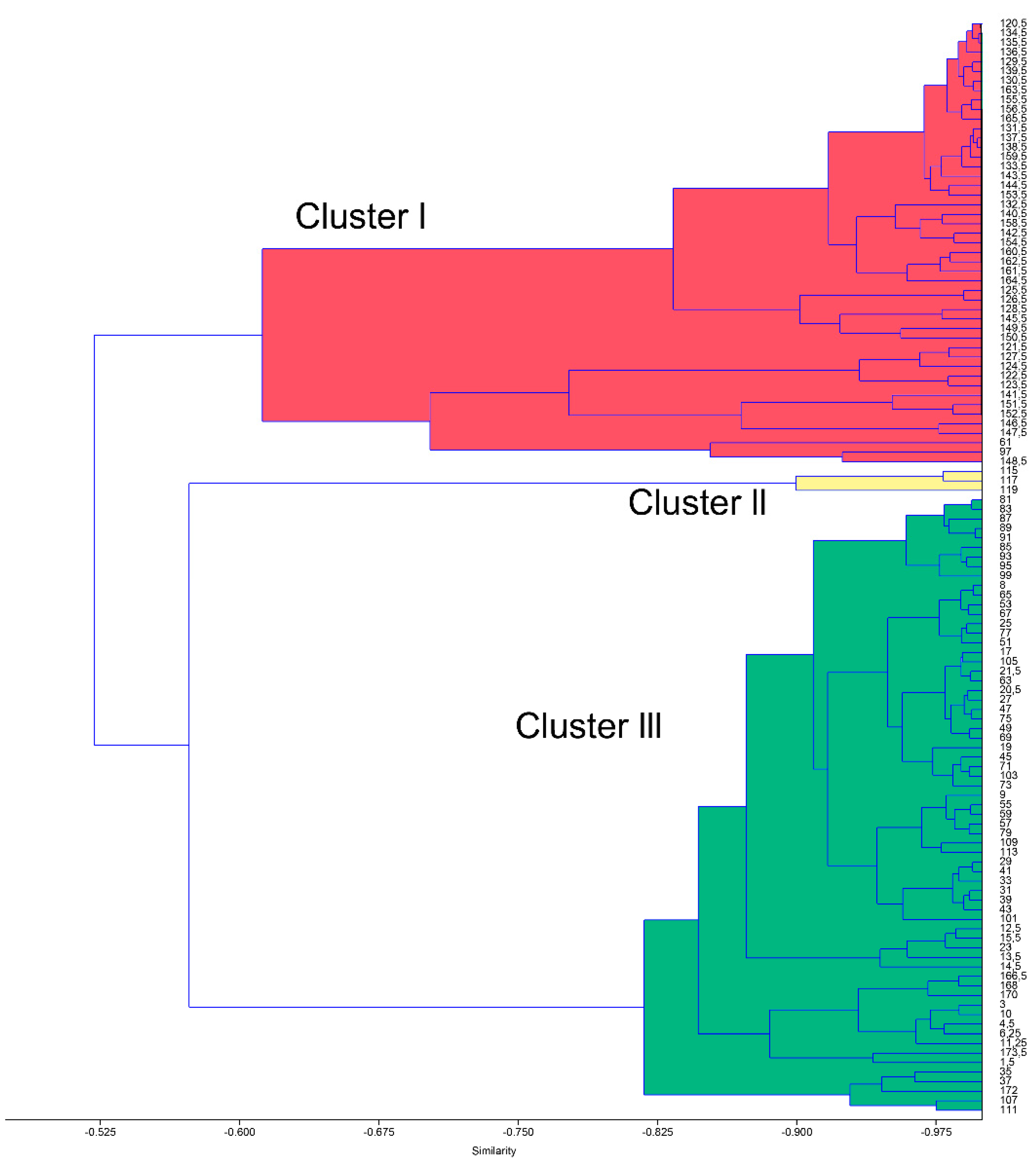
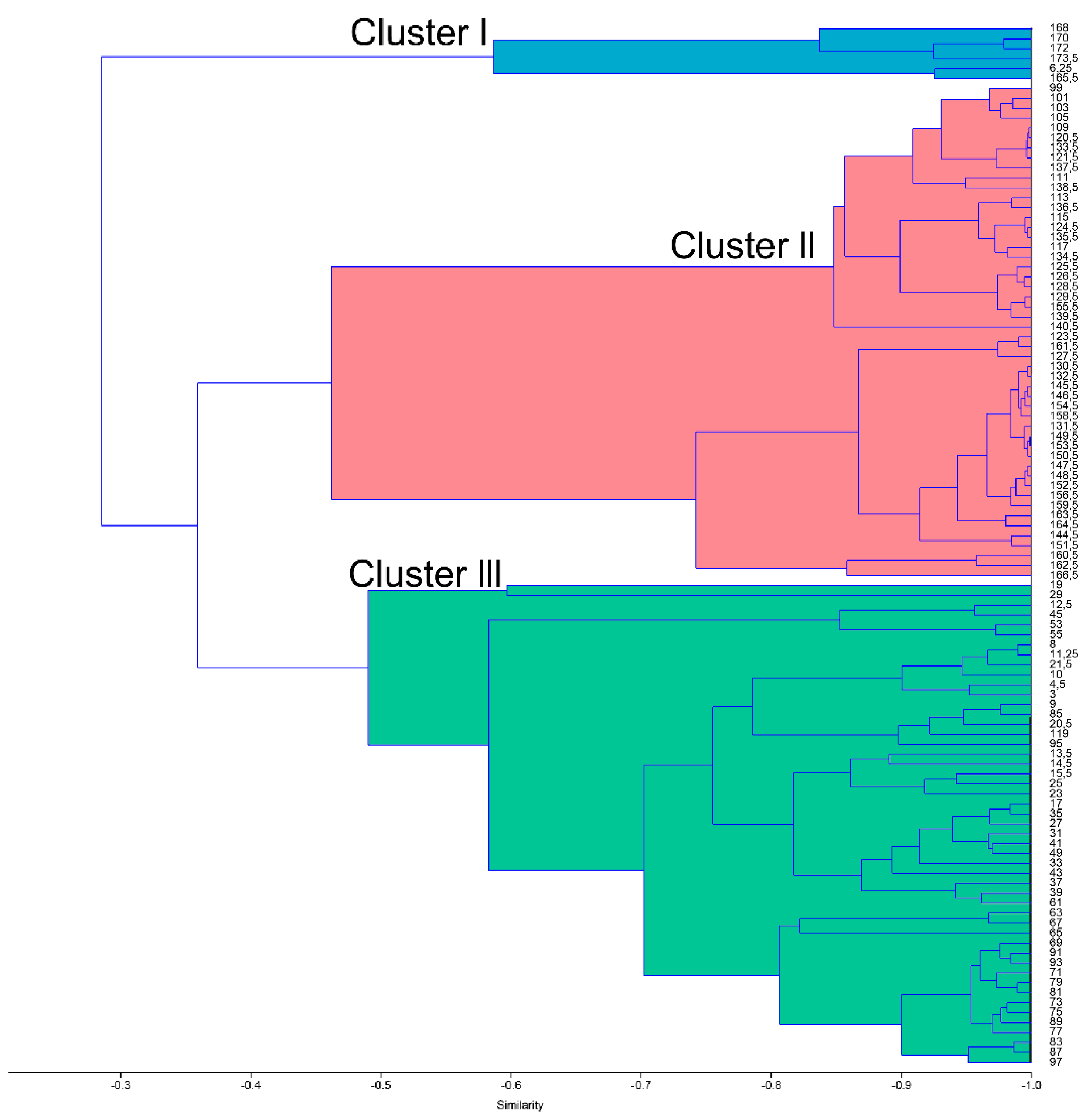
4.1. Q-Mode Cluster Analysis
4.1.1. Planktonic Foraminifera
4.1.2. Pteropods
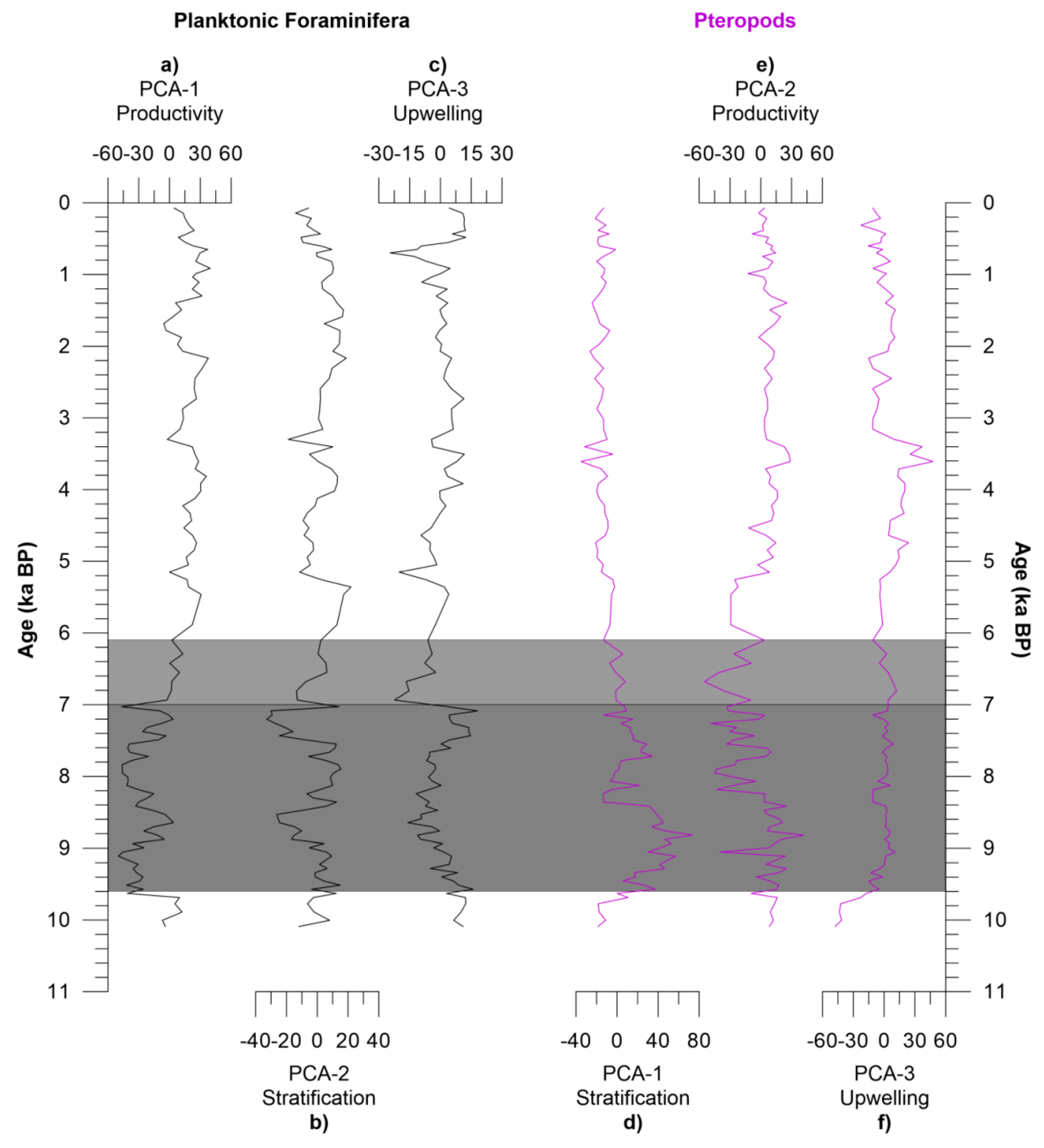
4.2. Principal Component Analysis
4.2.1. Planktonic Foraminifera
4.2.2. Pteropods
5. Discussion
5.1. Biotopes of the North Aegean
5.2. Factors Controlling Planktonic Fauna Distribution
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zervakis, V.; Georgopoulos, D.; Karageorgis, A.P.; Theocharis, A. On the response of the Aegean Sea to climatic variability: A review. Int. J. Climatol. 2004, 24, 1845–1858. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Global Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Rohling, E.J.; Grant, K.; Bolshaw, M.; Roberts, A.; Siddall, M.; Hemleben, C.; Kucera, M. Antarctic temperature and global sea level closely coupled over the past five glacial cycles. Nat. Geosci. 2009, 2, 500–504. [Google Scholar] [CrossRef]
- Aksu, A.E.; Yaşar, D.; Mudie, P.J. Paleoclimatic and paleoceanographic conditions leading to development of sapropel layer S1 in the Aegean Sea. Palaeogeogr. Palaeoclim. Palaeoecol. 1995, 116, 71–101. [Google Scholar] [CrossRef]
- Rousakis, G.; Karageorgis, A.; Conispoliatis, N.; Lykousis, V. Last glacial-Holocene sediment sequences in N. Aegean basins: Structure, accumulation rates and clay mineral distribution. Geo-Mar. Lett. 2004, 24, 97–111. [Google Scholar] [CrossRef]
- Kontakiotis, G. Late Quaternary paleoenvironmental reconstruction and paleoclimatic implications of the Aegean Sea (eastern Mediterranean) based on paleoceanographic indexes and stable isotopes. Quatern. Int. 2016, 401, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Poulos, S.E. The Mediterranean and Black Sea Marine System: An overview of its physico-geographic and oceanographic characteristics. Earth-Sci. Rev. 2020, 200, 103004. [Google Scholar] [CrossRef]
- De Lange, G.J.; Thomson, J.; Reitz, A.; Slomp, C.P.; Principato, M.S.; Erba, E.; Corselli, C. Synchronous basin-wide formation and redox-controlled preservation of a Mediterranean sapropel. Nat. Geosci. 2008, 1, 606–610. [Google Scholar] [CrossRef]
- Triantaphyllou, M.V.; Gogou, A.; Dimiza, M.D.; Kostopoulou, S.; Parinos, C.; Roussakis, G.; Geraga, M.; Bouloubassi, I.; Fleitmann, D.; Zervakis, V. Holocene climatic optimum centennial-scale paleoceanography in the NE Aegean (Mediterranean Sea). Geo-Mar. Lett. 2016, 36, 51–66. [Google Scholar] [CrossRef]
- Kuhnt, T.; Schmiedl, G.; Ehrmann, W.; Hamann, Y.; Hemleben, C. Deep-sea ecosystem variability of the Aegean Sea during the past 22 kyr as revealed by Benthic Foraminifera. Mar. Micropaleontol. 2007, 64, 141–162. [Google Scholar] [CrossRef]
- Schmiedl, G.; Kuhnt, T.; Ehrmann, W.; Emeis, K.-C.; Hamann, Y.; Kotthoff, U.; Dulski, P.; Pross, J. Climatic forcing of eastern Mediterranean deep-water formation and benthic ecosystems during the past 22,000 years. Quat. Sci. Rev. 2010, 29, 3006–3020. [Google Scholar] [CrossRef]
- Louvari, M.A.; Drinia, H.; Kontakiotis, G.; Di Bella, L.; Antonarakou, A.; Anastasakis, G. Impact of latest-glacial to Holocene sea-level oscillations on central Aegean shelf ecosystems: A benthic foraminiferal palaeoenvironmental assessment of South Evoikos Gulf, Greece. J. Mar. Syst. 2019, 199, 103181. [Google Scholar] [CrossRef]
- Vadsaria, T.; Ramstein, G.; Dutay, J.C.; Li, L.; Ayache, M.; Richon, C. Simulating the occurrence of the last sapropel event (S1): Mediterranean basin ocean dynamics simulations using Nd isotopic composition modeling. Paleoceanogr. Paleocl. 2019, 34, 237–251. [Google Scholar] [CrossRef]
- Le Houedec, S.; Mojtahid, M.; Bicchi, E.; de Lange, G.J.; Hennekam, R. Suborbital Hydrological Variability Inferred From Coupled Benthic and Planktic Foraminiferal-Based Proxies in the Southeastern Mediterranean During the Last 19 ka. Paleoceanogr. Paleocl. 2020, 35, e2019PA003827. [Google Scholar] [CrossRef]
- Rohling, E.J.; Marino, G.; Grant, K.M. Mediterranean climate and oceanography, and the periodic development of anoxic events (sapropels). Earth-Sci. Rev. 2015, 143, 62–97. [Google Scholar] [CrossRef]
- Zirks, E.; Krom, M.D.; Zhu, D.; Schmiedl, G.; Goodman-Tchernov, B.N. Evidence for the Presence of Oxygen-Depleted Sapropel Intermediate Water across the Eastern Mediterranean during Sapropel S1. ACS Earth Space Chem. 2019, 3, 2287–2297. [Google Scholar] [CrossRef]
- Tachikawa, K.; Vidal, L.; Cornuault, M.; Garcia, M.; Pothin, A.; Sonzogni, C.; Bard, E.; Menot, G.; Revel, M. Eastern Mediterranean Sea circulation inferred from the conditions of S1 sapropel deposition. Clim. Past 2015, 11, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Grimm, R.; Maier-Reimer, E.; Mikolajewicz, U.; Schmiedl, G.; Müller-Navarra, K.; Adloff, F.; Grant, K.M.; Ziegler, M.; Lourens, L.J.; Emeis, K.-C. Late glacial initiation of Holocene eastern Mediterranean sapropel formation. Nat. Commun. 2015, 6, 7099. [Google Scholar] [CrossRef] [Green Version]
- Antonarakou, A.; Kontakiotis, G.; Zarkogiannis, S.; Mortyn, P.G.; Drinia, H.; Koskeridou, E.; Anastasakis, G. Planktonic foraminiferal abnormalities in coastal and open marine eastern Mediterranean environments: A natural stress monitoring approach in recent and early Holocene marine systems. J. Mar. Syst. 2018, 181, 63–78. [Google Scholar] [CrossRef]
- Pallacks, S.; Ziveri, P.; Martrat, B.; Mortyn, P.G.; Grelaud, M.; Schiebel, R.; Incarbona, A.; Garcia-Orellana, J.; Anglada-Ortiz, G. Planktic foraminiferal changes in the western Mediterranean Anthropocene. Global Planet. Change 2021, 204, 103549. [Google Scholar] [CrossRef]
- Avnaim-Katav, S.; Herut, B.; Rahav, E.; Katz, T.; Weinstein, Y.; Alkalay, R.; Berman-Frank, I.; Zlatkin, O.; Almogi-Labin, A. Sediment trap and deep sea coretop sediments as tracers of recent changes in planktonic foraminifera assemblages in the southeastern ultra-oligotrophic Levantine Basin. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 171, 104669. [Google Scholar] [CrossRef]
- Zarkogiannis, S.; Kontakiotis, G.; Antonarakou, A. Recent planktonic foraminifera population and size response to Eastern Mediterranean hudrography. Rev. De Micropaleontol. 2020, 69, 100450. [Google Scholar] [CrossRef]
- Kontakiotis, G.; Efstathiou, E.; Zarkogiannis, S.D.; Besiou, E.; Antonarakou, A. Latitudinal Differentiation among Modern Planktonic Foraminiferal Populations of Central Mediterranean: Species–Specific Distribution Patterns and Size Variability. J. Mar. Sci. Eng. 2021, 9, 551. [Google Scholar] [CrossRef]
- Ofstad, S.; Zamelczyk, K.; Kimoto, K.; Chierici, M.; Fransson, A.; Rasmussen, T.L. Shell density of planktonic foraminifera and pteropod species Limacina helicina in the Barents Sea: Relation to ontogeny and water chemistry. PLoS ONE 2021, 16, e0249178. [Google Scholar] [CrossRef]
- Manno, C.; Bednaršek, N.; Tarling, G.A.; Peck, V.L.; Comeau, S.; Adhikari, D.; Bakker, D.C.E.; Bauerfeind, E.; Bergan, A.J.; Berning, M.I.; et al. Shelled pteropods in peril: Assessing vulnerability in a high CO2 ocean. Earth-Sci. Rev. 2017, 169, 132–145. [Google Scholar] [CrossRef]
- Casford, J.S.L.; Rohling, E.J.; Abu-Zied, R.H.; Fontanier, C.; Jorissen, F.J.; Leng, M.J.; Schmiedl, G.; Thomson, J. A dynamic concept for eastern Mediterranean circulation and oxygenation during sapropel formation. Palaeogeogr. Palaeoclim. Palaeoecol. 2003, 190, 103–119. [Google Scholar] [CrossRef]
- Kontakiotis, G.; Antonarakou, A.; Zachariasse, W.J. Late Quaternary palaeoenvironmental changes in the Aegean Sea: Interrelations and interactions between north and south Aegean Sea. Bull. Geol. Soc. Gr. 2013, 47, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Wall-Palmer, D.; Smart, C.W.; Hart, M.B.; Leng, M.J.; Borghini, M.; Manini, E.; Aliani, S.; Conversi, A. Late Pleistocene pteropods, heteropods and planktonic foraminifera from the Caribbean Sea, Mediterranean Sea and Indian Ocean. Micropaleontology 2014, 60, 557–578. [Google Scholar]
- Giamali, C.; Kontakiotis, G.; Koskeridou, E.; Ioakim, C.; Antonarakou, A. Key Environmental Factors Controlling Planktonic Foraminiferal and Pteropod Community’s Response to Late Quaternary Hydroclimate Changes in the South Aegean Sea (Eastern Mediterranean). J. Mar. Sci. Eng. 2020, 8, 709. [Google Scholar] [CrossRef]
- Giamali, C.; Koskeridou, E.; Antonarakou, A.; Ioakim, C.; Kontakiotis, G.; Karageorgis, A.P.; Roussakis, G.; Karakitsios, V. Multiproxy ecosystem response of abrupt Holocene climatic changes in the northeastern Mediterranean sedimentary archive and hydrologic regime. Quat. Res. 2019, 92, 665–685. [Google Scholar] [CrossRef]
- Checa, H.; Margaritelli, G.; Pena, L.D.; Frigola, J.; Cacho, I.; Rettori, R.; Lirer, F. High resolution paleo-environmental changes during the Sapropel 1 in the North Ionian Sea, central Mediterranean. Holocene 2020, 30, 1504–1515. [Google Scholar] [CrossRef]
- Casford, J.S.L.; Abu-Zied, R.; Rohling, E.J.; Cooke, S.; Fontanier, C.; Leng, M.; Millard, A.; Thomson, J. A stratigraphically controlled multiproxy chronostratigraphy for the eastern Mediterranean. Paleoceanography 2007, 22, PA4215. [Google Scholar] [CrossRef]
- Siani, G.; Paterne, M.; Colin, C. Late glacial to Holocene planktic foraminifera bioevents and climatic record in the South Adriatic Sea. J. Quat. Sci. 2010, 25, 808–821. [Google Scholar] [CrossRef]
- Drinia, H.; Antonarakou, A.; Tsourou, T.; Kontakiotis, G.; Psychogiou, M.; Anastasakis, G. Foraminifera eco-biostratigraphy of the southern Evoikos outer shelf, central Aegean Sea, during MIS 5 to present. Cont. Shelf Res. 2016, 126, 36–49. [Google Scholar] [CrossRef]
- Antonarakou, A.; Kontakiotis, G.; Karageorgis, A.P.; Besiou, E.; Zarkogiannis, S.; Drinia, H.; Mortyn, G.P.; Tripsanas, E. Eco-biostratigraphic advances on late Quaternary geochronology and palaeoclimate: The marginal Gulf of Mexico analogue. Geol. Q. 2019, 63, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Zarkogiannis, S.D.; Antonarakou, A.; Tripati, A.; Kontakiotis, G.; Mortyn, P.G.; Drinia, H.; Greaves, M. Influence of surface ocean density on planktonic foraminifera calcification. Sci. Rep. 2019, 9, 533. [Google Scholar] [CrossRef]
- Mojtahid, M.; Manceau, R.; Schiebel, R.; Hennekam, R.; de Lange, G.J. Thirteen thousand years of southeastern Mediterranean climate variability inferred from an integrative planktic foraminiferal-based approach. Paleoceanography 2015, 30, 402–422. [Google Scholar] [CrossRef]
- Zachariasse, W.J.; Kontakiotis, G.; Lourens, L.J.; Antonarakou, A. The Messinian of Agios Myron (Crete, Greece): A key to better understanding of diatomite formation on Gavdos (south of Crete). Palaeogeogr. Palaeoclim. Palaeoecol. 2021, 581, 110633. [Google Scholar] [CrossRef]
- Antonarakou, A.; Kontakiotis, G.; Mortyn, P.G.; Drinia, H.; Sprovieri, M.; Besiou, E.; Tripsanas, E. Biotic and geochemical (δ18O, δ13C, Mg/Ca, Ba/Ca) responses of Globigerinoides ruber morphotypes to upper water column variations during the last deglaciation, Gulf of Mexico. Geochim. Cosmochim. Acta 2015, 170, 69–93. [Google Scholar] [CrossRef]
- Vasiliev, I.; Karakitsios, V.; Bouloubassi, I.; Agiadi, K.; Kontakiotis, G.; Antonarakou, A.; Triantaphyllou, M.; Gogou, A.; Kafousia, N.; de Rafélis, M.; et al. Large Sea Surface Temperature, Salinity, and Productivity-Preservation Changes Preceding the Onset of the Messinian Salinity Crisis in the Eastern Mediterranean Sea. Paleoceanogr. Paleocl. 2019, 34, 182–202. [Google Scholar] [CrossRef]
- Kontakiotis, G.; Karakitsios, V.; Mortyn, P.G.; Antonarakou, A.; Drinia, H.; Anastasakis, G.; Agiadi, K.; Kafousia, N.; De Rafelis, M. New insights into the early Pliocene hydrographic dynamics and their relationship to the climatic evolution of the Mediterranean Sea. Palaeogeogr. Palaeoclim. Palaeoecol. 2016, 459, 348–364. [Google Scholar] [CrossRef]
- Kontakiotis, G.; Besiou, E.; Antonarakou, A.; Zarkogiannis, S.D.; Kostis, A.; Mortyn, P.G.; Moissette, P.; Cornée, J.J.; Schulbert, C.; Drinia, H.; et al. Decoding sea surface and paleoclimate conditions in the eastern Mediterranean over the Tortonian-Messinian Transition. Palaeogeogr. Palaeoclim. Palaeoecol. 2019, 534, 109312. [Google Scholar] [CrossRef]
- Margaritelli, G.; Cisneros, M.; Cacho, I.; Capotondi, L.; Vallefuoco, M.; Rettori, R.; Lirer, F. Climatic variability over the last 3000 years in the central-western Mediterranean Sea (Menorca Basin) detected by planktonic foraminifera and stable isotope records. Global Planet. Chang. 2018, 169, 179–187. [Google Scholar] [CrossRef]
- Margaritelli, G.; Cacho, I.; Català, A.; Barra, M.; Bellucci, L.G.; Lubritto, C.; Rettori, R.; Lirer, F. Persistent warm Mediterranean surface waters during the Roman period. Sci. Rep. 2020, 10, 10431. [Google Scholar] [CrossRef]
- Margaritelli, G.; Vallefuoco, M.; Di Rita, F.; Capotondi, L.; Bellucci, L.G.; Insinga, D.D.; Petrosino, P.; Bonomo, S.; Cacho, I.; Cascella, A. Marine response to climate changes during the last five millennia in the central Mediterranean Sea. Global Planet. Chang. 2016, 142, 53–72. [Google Scholar] [CrossRef]
- Thirumalai, K.; Richey, J.N.; Quinn, T.M. Holocene evolution of sea-surface temperature and salinity in the Gulf of Mexico. Paleoceanogr. Paleocl. 2021, 36, e2021PA004221. [Google Scholar] [CrossRef]
- Hemleben, C.; Spindler, M.; Anderson, O. Modern Planktic Foraminifera; Springer-Verlag: New York, NY, USA, 1989; Volume 22. [Google Scholar]
- Kucera, M. Chapter six planktonic foraminifera as tracers of past oceanic environments. Dev. Mar. Geol. 2007, 1, 213–262. [Google Scholar]
- Lalli, C.M.; Gilmer, R.W. Pelagic Snails: The Biology of Holoplanktonic Gastropod Mollusks; Stanford University Press: Redwood City, CA, USA, 1989. [Google Scholar]
- Pierrot-Bultsa, A.C.; Peijnenburga, K.T.C.A. Pteropods. In Encyclopedia of Marine Geosciences; Harff, J., Meschede, M., Petersen, S., Thiede, J., Eds.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Hunt, B.; Pakhomov, E.; Hosie, G.W.; Siegel, V.; Ward, P.; Bernard, K. Pteropods in Southern Ocean ecosystems. Prog. Oceanogr. 2008, 78, 193–221. [Google Scholar] [CrossRef]
- Berner, R.A.; Honjo, S. Pelagic sedimentation of aragonite: Its geochemical significance. Science 1981, 211, 940–942. [Google Scholar] [CrossRef]
- Herman, Y. Evidence of climatic changes in Red Sea cores. In Proceedings of the VII Congress International Association for Quaternary Research; University of Utah Press: Salt Lake City, UT, USA, 1968; pp. 325–348. [Google Scholar]
- Herman, Y. Vertical and horizontal distribution of pteropods in Quaternary sequences. In The Micropalaeontology of Oceans; Funnell, B.M., Reidel, W.R., Eds.; Cambridge University Press: Cambridge, UK, 1971; pp. 463–486. [Google Scholar]
- Chen, C. Pteropods in the Hot Brine Sediments of the Red Sea; Springer: Berlin/Heidelberg, Germany, 1969. [Google Scholar] [CrossRef]
- Almogi-Labin, A.; Edelman-Furstenberg, Y.; Hemleben, C. Variations in the biodiversity of thecosomatous pteropods during the Late Quaternary as a response to environmental changes in the Gulf of Aden-Red Sea-Gulf of Aqaba ecosystem. In The Improbable Gulf. Environment, Biodiversity and Preservation; Por, D.e.A.-E., Ed.; The Hebrew University Magnes Press: Jerusalem, Israel, 2008; pp. 31–48. [Google Scholar]
- Singh, A.; Nisha, N.R.; Joydas, T.; Joydas, V. Distribution patterns of Recent pteropods in surface sediments of the western continental shelf of India. J. Micropalaeontol. 2005, 24, 39. [Google Scholar] [CrossRef] [Green Version]
- Wall-Palmer, D.; Smart, C.W.; Hart, M.B. In-life pteropod shell dissolution as an indicator of past ocean carbonate saturation. Quat. Sci. Rev. 2013, 81, 29–34. [Google Scholar] [CrossRef]
- Wall-Palmer, D.; Jones, M.T.; Hart, M.B.; Fisher, J.K.; Smart, C.W.; Hembury, D.J.; Palmer, M.R.; Fones, G.R. Explosive volcanism as a cause for mass mortality of pteropods. Mar. Geol. 2011, 282, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Lykousis, V.; Chronis, G.; Tselepides, A.; Price, N.B.; Theocharis, A.; Siokou-Frangou, I.; Van Wambeke, F.; Danovaro, R.; Stavrakakis, S.; Duineveld, G.; et al. Major outputs of the recent multidisciplinary biogeochemical researches undertaken in the Aegean Sea. J. Mar. Syst. 2002, 33, 313–334. [Google Scholar] [CrossRef]
- Papanikolaou, D.; Alexandri, M.; Nomikou, P.; Ballas, D. Morphotectonic structure of the western part of the North Aegean Basin based on swath bathymetry. Mar. Geol. 2002, 190, 465–492. [Google Scholar] [CrossRef]
- Poulos, S.E.; Drakopoulos, P.G.; Collins, M.B. Seasonal variability in sea surface oceanographic conditions in the Aegean Sea (Eastern Mediterranean): An overview. J. Mar. Syst. 1997, 13, 225–244. [Google Scholar] [CrossRef]
- Theocharis, A.; Georgopoulos, D. Dense water formation over the Samothraki and Limnos Plateaux in the north Aegean Sea (Eastern Mediterranean Sea). Cont. Shelf Res. 1993, 13, 919–939. [Google Scholar] [CrossRef]
- Velaoras, D.; Lascaratos, A. Deep water mass characteristics and interannual variability in the North and Central Aegean Sea. J. Mar. Syst. 2005, 53, 59–85. [Google Scholar] [CrossRef]
- Karageorgis, A.P.; Kaberi, H.G.; Tengberg, A.; Zervakis, V.; Hall, P.O.J.; Anagnostou, C.L. Comparison of particulate matter distribution, in relation to hydrography, in the mesotrophic Skagerrak and the oligotrophic northeastern Aegean Sea. Cont. Shelf Res. 2003, 23, 1787–1809. [Google Scholar] [CrossRef]
- Androulidakis, Y.S.; Krestenitis, Y.N.; Psarra, S. Coastal upwelling over the North Aegean Sea: Observations and simulations. Cont. Shelf Res. 2017, 149, 32–51. [Google Scholar] [CrossRef]
- Krom, M.D.; Kress, N.; Brenner, S.; Gordon, L.I. Phosphorus limitation of primary productivity in the eastern Mediterranean Sea. Limnol. Oceanogr. 1991, 36, 424–432. [Google Scholar] [CrossRef]
- Stergiou, K.I.; Christou, E.D.; Georgopoulos, D.; Zenetos, A.; Souvermezoglou, C. The Hellenic Seas: Physics, chemistry, biology and fisheries. Oceanogr. Mar. Biol. 1997, 35, 415–538. [Google Scholar]
- Kontakiotis, G.; Antonarakou, A.; Mortyn, P.G.; Drinia, H.; Anastasakis, G.; Zarkogiannis, S.; Möbius, J. Morphological recognition of Globigerinoides ruber morphotypes and their susceptibility to diagenetic alteration in the eastern Mediterranean Sea. J. Mar. Syst. 2017, 174, 12–24. [Google Scholar] [CrossRef]
- Zarkogiannis, S.D.; Kontakiotis, G.; Gkaniatsa, G.; Kuppili, V.S.C.; Marathe, S.; Wanelik, K.; Lianou, V.; Besiou, E.; Makri, P.; Antonarakou, A. An Improved Cleaning Protocol for Foraminiferal Calcite from Unconsolidated Core Sediments: HyPerCal—A New Practice for Micropaleontological and Paleoclimatic Proxies. J. Mar. Sci. Eng. 2020, 8, 998. [Google Scholar] [CrossRef]
- Patterson, R.T.; Fishbein, E. Re-examination of the statistical methods used to determine the number of point counts needed for micropaleontological quantitative research. J. Paleontol. 1989, 63, 245–248. [Google Scholar] [CrossRef]
- Davis, J.C.; Sampson, R.J. Statistics and Data Analysis in Geology; Wiley New York: New York, NY, USA, 1986; Volume 646. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Rohling, E.; Jorissen, F.; Stigter, H. 200 Year interruption of Holocene sapropel formation in the Adriatic Sea. J. Micropalaeontol. 1997, 16, 97–108. [Google Scholar] [CrossRef]
- Fairbanks, R.; Wiebe, P. Foraminifera and Chlorophyll Maximum: Vertical Distribution, Seasonal Succession, and Paleoceanographic Significance. Science 1980, 209, 1524–1526. [Google Scholar] [CrossRef]
- Lourens, L.; Hilgen, F.; Gudjonsson, L.; Zachariasse, W.J. Late Pliocene to early Pleistocene astonomically forced sea surface productivity and temperature variations in the Mediterranean. Mar. Micropaleontol. 1992, 19, 49–78. [Google Scholar] [CrossRef]
- Thunell, R. Distribution of recent planktonic foraminifera in surface sediments of the Mediterranean Sea. Mar. Micropaleontol. 1978, 3, 147–173. [Google Scholar] [CrossRef]
- Pujol, C.; Vergnaud-Grazzini, C. Distribution patterns of live planktic foraminifers as related to regional hydrology and productive systems of the Mediterranean Sea. Mar. Micropaleontol. 1995, 25, 187–217. [Google Scholar] [CrossRef]
- Rohling, E.J.; Jorissen, F.; Grazzini, C.V.; Zachariasse, W.J. Northern Levantine and Adriatic Quaternary planktic foraminifera; Reconstruction of paleoenvironmental gradients. Mar. Micropaleontol. 1993, 21, 191–218. [Google Scholar] [CrossRef]
- Jonkers, L.; Kučera, M. Global analysis of seasonality in the shell flux of extant planktonic Foraminifera. Biogeosciences 2015, 12, 2207–2226. [Google Scholar] [CrossRef] [Green Version]
- Weinkauf, M.F.G.; Kunze, J.G.; Waniek, J.J.; Kučera, M. Seasonal variation in shell calcification of planktonic foraminifera in the NE Atlantic reveals species-specific response to temperature, productivity, and optimum growth conditions. PLoS ONE 2016, 11, e0148363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiebel, R.; Hemleben, C. Planktic Foraminifers in the Modern Ocean; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Naidu, P.D. Distribution patterns of Recent planktonic foraminifera in surface sediments of the western continental margin of India. Mar. Geol. 1993, 110, 403–418. [Google Scholar] [CrossRef]
- Peterson, L.C.; Overpeck, J.T.; Kipp, N.G.; Imbrie, J. A high-resolution Late Quaternary upwelling record from the anoxic Cariaco Basin, Venezuela. Paleoceanography 1991, 6, 99–119. [Google Scholar] [CrossRef]
- Prell, W.L.; Curry, W.B. Faunal and isotopic indices of monsoonal upwelling-western arabian sea. Oceanol. Acta 1981, 4, 91–98. [Google Scholar]
- Cullen, J.L.; Prell, W.L. Planktonic foraminifera of the northern Indian Ocean: Distribution and preservation in surface sediments. Mar. Micropaleontol. 1984, 9, 1–52. [Google Scholar] [CrossRef]
- Bé, A.W.H.; Gilmer, R.W. A zoogeographic and taxonomic review of euthecosomatous Pteropoda. Oceanic Micropaleontol. 1977, 1, 733–808. [Google Scholar]
- Almogi-Labin, A.; Hemleben, C.; Deuser, W.G. Seasonal variation in the flux of euthecosomatous pteropods collected in a deep sediment trap in the Sargasso Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 1988, 35, 441–464. [Google Scholar] [CrossRef]
- Geraga, M.; Ioakim, C.; Lykousis, V.; Tsaila-Monopolis, S.; Mylona, G. The high-resolution palaeoclimatic and palaeoceanographic history of the last 24,000years in the central Aegean Sea, Greece. Palaeogeogr. Palaeoclim. Palaeoecol. 2010, 287, 101–115. [Google Scholar] [CrossRef]
- Zachariasse, W.; Jorissen, F.; Perissoratis, C.; Rohling, E.; Tsapralis, V. Late quaternary foraminiferal changes and the nature of sapropel S1 in Skopelos Basin. In Proceedings of the 5th Hellenic Symposium of Oceanography and Fisheries, Kavala, Greece, 15–18 April 1997; pp. 391–394. [Google Scholar]
- Almogi-Labin, A.; Hemleben, C.; Meischner, D.; Erlenkeuser, H. Paleoenvironmental events during the last 13,000 years in the central Red Sea as recorded by pteropoda. Paleoceanography 1991, 6, 83–98. [Google Scholar] [CrossRef]
- Rottman, M.L. Net tow and surface sediment distributions of pteropods in the South China Sea region: Comparison and oceanographic implications. Mar. Micropaleontol. 1980, 5, 71–110. [Google Scholar] [CrossRef]
- Sijinkumar, A.V.; Bejugam, N.; Guptha, M.V.S. Late Quaternary record of pteropod preservation from the Andaman Sea. Mar. Geol. 2010, 275, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Buccheri, G.; Capretto, G.; Di Donato, V.; Esposito, P.; Ferruzza, G.; Pescatore, T.; Russo Ermolli, E.; Senatore, M.R.; Sprovieri, M.; Bertoldo, M.; et al. A high resolution record of the last deglaciation in the southern Tyrrhenian Sea: Environmental and climatic evolution. Mar. Geol. 2002, 186, 447–470. [Google Scholar] [CrossRef]
- Ehrmann, W.; Schmiedl, G.; Hamann, Y.; Kuhnt, T.; Hemleben, C.; Siebel, W. Clay minerals in late glacial and Holocene sediments of the northern and southern Aegean Sea. Palaeogeogr. Palaeoclim. Palaeoecol. 2007, 249, 36–57. [Google Scholar] [CrossRef]
- Sperling, M.; Schmiedl, G.; Hemleben, C.; Emeis, K.C.; Erlenkeuser, H.; Grootes, P.M. Black Sea impact on the formation of eastern Mediterranean sapropel S1? Evidence from the Marmara Sea. Palaeogeogr. Palaeoclim. Palaeoecol. 2003, 190, 9–21. [Google Scholar] [CrossRef]
- Casford, J.S.L.; Rohling, E.J.; Abu-Zied, R.; Cooke, S.; Fontanier, C.; Leng, M.; Lykousis, V. Circulation changes and nutrient concentrations in the late Quaternary Aegean Sea: A nonsteady state concept for sapropel formation. Paleoceanography 2002, 17, 1024–1034. [Google Scholar] [CrossRef] [Green Version]
- Rampal, J. Biodiversité et biogéographie chez les Cavoliniidae (Mollusca, Gastropoda, Opisthobranchia, Euthecosomata). Régions faunistiques marines. Zoosystema 2002, 24, 209–258. [Google Scholar]
- Johnson, R.; Manno, C.; Ziveri, P. Spring distribution of shelled pteropods across the Mediterranean Sea. Biogeosci. Discuss. 2020, 2020, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Almogi-Labin, A.; Hemleben, C.; Meischner, D. Carbonate preservation and climatic changes in the central Red Sea during the last 380 kyr as recorded by pteropods. Mar. Micropaleontol. 1998, 33, 87–107. [Google Scholar] [CrossRef]
- Thunell, R.; Reynolds, L.S. Planktonic Foraminifera faunal and stable isotopic indices of upwelling: A sediment trap study in the San Pedro Basin, Southern California Bight. In Upwelling Systems: Evolution Since the Early Miocene; Summerhayes, C.P., Prell, W.L., Emeis, K.C., Eds.; Geological Society London: London, UK, 1992; Volume 64, pp. 77–91. [Google Scholar]
| PCA Factors | Eigenvalue | % Variance | Cumulative % of the Total Variance |
|---|---|---|---|
| 1 | 622.458 | 67.949 | 67.949 |
| 2 | 142.717 | 15.579 | 83.528 |
| 3 | 67.9386 | 7.416 | 90.944 |
| 4 | 27.039 | 2.952 | 93.896 |
| 5 | 22.7885 | 2.488 | 96.384 |
| 6 | 17.852 | 1.949 | 98.333 |
| 7 | 6.21708 | 0.679 | 99.011 |
| 8 | 3.39922 | 0.371 | 99.382 |
| 9 | 2.74832 | 0.300 | 99.682 |
| 10 | 1.76144 | 0.192 | 99.875 |
| 11 | 0.784001 | 0.086 | 99.960 |
| 12 | 0.342134 | 0.037 | 99.998 |
| 13 | 0.0172397 | 0.002 | 99.999 |
| PCA Factors | Eigenvalue | % Variance | Cumulative % of the Total Variance |
|---|---|---|---|
| 1 | 494.862 | 37.764 | 37.764 |
| 2 | 362.476 | 27.662 | 65.426 |
| 3 | 183.956 | 14.038 | 79.464 |
| 4 | 95.0715 | 7.255 | 86.719 |
| 5 | 78.8895 | 6.020 | 92.739 |
| 6 | 35.6337 | 2.719 | 95.459 |
| 7 | 29.4056 | 2.244 | 97.703 |
| 8 | 24.0161 | 1.833 | 99.536 |
| 9 | 3.7509 | 0.286 | 99.822 |
| 10 | 2.33109 | 0.178 | 99.999 |
| Species | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Orbulina universa | −0.084 | −0.170 | −0.137 |
| Globigerinoides ruber alba | 0.670 | 0.569 | 0.256 |
| Globigerinoides ruber rosea | 0.077 | −0.209 | −0.515 |
| Globigerinoides sacculifer | 0.027 | 0.033 | −0.027 |
| Globorotalia inflata | 0.002 | −0.044 | −0.183 |
| Globigerina bulloides | −0.024 | −0.536 | 0.734 |
| Globoturborotalita rubescens | 0.011 | −0.158 | −0.155 |
| Globigerinella siphonifera gr. | 0.004 | 0.034 | −0.023 |
| Neogloboquadrina pachyderma | 0.050 | −0.004 | −0.155 |
| Neogloboquadrina dutertrei | −0.003 | −0.006 | −0.001 |
| Turborotalita quinqueloba | −0.731 | 0.536 | 0.159 |
| Globigerinita glutinata | 0.008 | −0.005 | 0.063 |
| Species | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Heliconoides inflatus | 0.866 | 0.380 | 0.162 |
| Limacina retroversa | −0.006 | 0.006 | −0.011 |
| Limacina trochiformis | −0.364 | 0.300 | 0.733 |
| Boasia chierchiae | 0.218 | −0.842 | 0.244 |
| Creseis acicula | −0.133 | 0.071 | −0.448 |
| Styliola subula | −0.101 | 0.062 | −0.017 |
| Clio cuspidata | −0.015 | 0.010 | 0.028 |
| Clio pyramidata | −0.190 | 0.166 | −0.088 |
| Diacria trispinosa | −0.011 | 0.035 | −0.394 |
| Cavolinia spp. | −0.079 | −0.137 | 0.109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giamali, C.; Kontakiotis, G.; Antonarakou, A.; Koskeridou, E. Ecological Constraints of Plankton Bio-Indicators for Water Column Stratification and Productivity: A Case Study of the Holocene North Aegean Sedimentary Record. J. Mar. Sci. Eng. 2021, 9, 1249. https://doi.org/10.3390/jmse9111249
Giamali C, Kontakiotis G, Antonarakou A, Koskeridou E. Ecological Constraints of Plankton Bio-Indicators for Water Column Stratification and Productivity: A Case Study of the Holocene North Aegean Sedimentary Record. Journal of Marine Science and Engineering. 2021; 9(11):1249. https://doi.org/10.3390/jmse9111249
Chicago/Turabian StyleGiamali, Christina, George Kontakiotis, Assimina Antonarakou, and Efterpi Koskeridou. 2021. "Ecological Constraints of Plankton Bio-Indicators for Water Column Stratification and Productivity: A Case Study of the Holocene North Aegean Sedimentary Record" Journal of Marine Science and Engineering 9, no. 11: 1249. https://doi.org/10.3390/jmse9111249
APA StyleGiamali, C., Kontakiotis, G., Antonarakou, A., & Koskeridou, E. (2021). Ecological Constraints of Plankton Bio-Indicators for Water Column Stratification and Productivity: A Case Study of the Holocene North Aegean Sedimentary Record. Journal of Marine Science and Engineering, 9(11), 1249. https://doi.org/10.3390/jmse9111249








