Genotoxicity of Polystyrene (PS) Microspheres in Short-Term Exposure to Gametes of the Sand Dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s Oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, 111913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, M.J.; Watson, W.; Bowlin, N.M.; Sheavly, S.B. Plastic particles in coastal pelagic ecosystems of the Northeast Pacific ocean. Mar. Environ. Res. 2011, 71, 41–52. [Google Scholar] [CrossRef]
- Thomas, P.J.; Oral, R.; Pagano, G.; Tez, S.; Toscanesi, M.; Ranieri, P.; Trifuoggi, M.; Lyons, D.M. Mild toxicity of polystyrene and polymethylmethacrylate microplastics in Paracentrotus lividus early life stages. Mar. Environ. Res. 2020, 161, 105132. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson ADave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, J.; Zhao, X.; Feng, J.; Teng, Y.; Chen, B.; Sun, X.; Zhu, L.; Sun, X.; Qu, K. Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ. Pollut. 2020, 258, 113657. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Gagné, F.; André, C.; Della Torre, C.; Auclair, J.; Hanana, H.; Parenti, C.C.; Bonasoro, F.; Binelli, A. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total Environ. 2018, 631–632, 778–788. [Google Scholar] [CrossRef]
- Hamlin, H.J.; Marciano, K.; Downs, C.A. Migration of nonylphenol from food-grade plastic is toxic to the coralreef fish species Pseudochromis fridmani. Chemosphere 2015, 139, 223–228. [Google Scholar] [CrossRef]
- Martinez-Gomez, C.; León, V.M.; Calles, S.; Gomáriz-Olcina, M.; Vethaak, A.D. The adverse effects of virgin microplastics on the fertilization and larval development of sea urchins. Mar. Environ. Res. 2017, 130, 69–76. [Google Scholar] [CrossRef]
- Dinnel, P.A.; Stober, Q.J.; Crumley, S.C.; Nakatani, R.E. Development of a sperm cell toxicity test for marine water. Aquat. Toxicol. Haz. Asses. 1982, 1, 82–98. [Google Scholar] [CrossRef]
- Kobayashi, N. Marine ecotoxicological testing with echinoderms. Ecotoxicological Test. Mar. Environ. 1984, 1, 341–356. [Google Scholar]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.; Turra, A. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef]
- Mazur, A.A.; Zhuravel, E.V.; Slobodskova, V.V.; Mazur, M.A.; Kukla, S.P.; Chelomin, V.P. Waterborne exposure of adult sand dollar, Scaphechinus mirabilis (Agassiz, 1864), to zinc ions and zinc oxide nanoparticles affects early development of its offspring. Water Air Soil Pollut. 2020, 231, 115. [Google Scholar] [CrossRef]
- Trifuoggi, M.; Pagano, G.; Oral, R.; Pavičić-Hamer, D.; Burić, P.; Kovačić, I.; Siciliano, A.; Toscanesi, M.; Thomas, P.J.; Paduano, L.; et al. Microplastic-induced damage in early embryonal development of sea urchin Sphaerechinus granularis. Environ. Res. 2019, 179, 108815. [Google Scholar] [CrossRef] [PubMed]
- Buznikov, G.N.; Podmarev, V.K. The sea urchins Strongylocentrotus droebachiensis, S. nudus, and S. intermedius. In Ob’ekty biologii razvitiya (Objects of Developmental Biology); Nauka: Moscow, Russia, 1975; pp. 188–216. [Google Scholar]
- Bekova, N.V.; Zhuravel, E.V.; Khristoforova, N.K. Effects of desalination and the detergent sodium dodecylsulphate on the early development of the sand dollar Scaphechinus mirabilis. Russ. J. Mar. Biol. 2004, 30, 175–182. [Google Scholar] [CrossRef]
- Slobodskova, V.V.; Kukla, S.P.; Chelomin, V.P. An analysis of the quality of the marine environment based on determination of the genotoxicity of DNA in the gill cells of the Yesso Scallop Mizuhopecten yessoensis (Jay, 1856). Russ. J. Mar. Biol. 2015, 41, 495–498. [Google Scholar] [CrossRef]
- Collins, A.R.; Kumar, A.; Dhawam, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. Review of recent in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2013, 52, 1–70. [Google Scholar] [CrossRef]
- Cavas, T.; Konen, S. In vivo genotoxicity testing of the amnesic shellfish poison (domonic acid) in piscine erythrocytes using the micronucleus test and the comet assay. Aquat. Toxicol. 2008, 90, 154–159. [Google Scholar] [CrossRef]
- Balbi, T.; Camisassi, G.; Montagna, M.; Fabbri, R.; Franzellitti, S.; Carbone, C. Impact of cationic polystyrene nanoparticles (PS-NH2) on early embryo development of Mytilus galloprovincialis: Effects on shell formation. Chemosphere 2017, 186, 1–9. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Tallec, K.; Gonzalez-Fernandez, C.; Lambert, C.; Vincent, D.; Mazurais, D.; Zambonino-Infante, J.L.; Brotons, G.; Lagarde, F.; Fabioux, C.; et al. Constraints and priorities for conducting experimental exposures of marine organisms to microplastics. Front. Mar. Sci. 2018, 252, 1–22. [Google Scholar] [CrossRef]
- Jha, A.N. Ecotoxicological applications and significance of the comet assay. Mutagenesis 2008, 23, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istomina, A.; Chelomin, V.; Kukla, S.; Zvyagintsev, A.; Karpenko, A.; Slinko, E.; Dovzhenko, N.; Slobodskova, V.; Kolosova, L. Copper effect on the biomarker state of the Mizuhopecten yessoensis tissues in the prespawning period. Environ. Toxicol. Pharmacol. 2019, 70, 103189. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Fernandez-Triana, J.; Roughley, R.; Hebert, D.N. DNA barcode accumulation curves for understudied taxa and areas. Mol. Ecol. Resour. 2009, 9, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kazama, M.; Hino, A. Sea urchin spermatozoa generate at least two reactive oxygen species; the type of reactive oxygen species changes under different conditions. Mol. Reprod. Dev. 2012, 79, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Soto, N.; Hatfield, J.; Katsumiti, A.; Duroudier, N.; Lacave, J.M.; Bilbao, E.; Orbea, A.; Navarro, E.; Cajaraville, M.P. Impacts of dietary exposure to different sized polystyrene microplastics alone and with sorbed benzo[a]pyrene on biomarkers and whole organism responses in mussels Mytilus galloprovincialis. Sci Total Environ. 2019, 684, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Berber, A.A. Genotoxic evaluation of polystyrene microplastic. SAUJS. 2019, 23, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.; Galloway, T.S. Genotoxic damage in Polychaetes: A study of species and cell-type sensitivities. Mutat. Res. Genet. Toxicol. Environ. Mutat. 2008, 654, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacaze, E.; Geffard, O.; Goyet, D.; Bony, S.; Devaux, A. Linking genotoxic responses in Gammarus fossarum germ cells with reproduction impairment, using the Comet assay. Environ. Res. 2011, 111, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Dautov, S.S.; Kashenko, S.D. Development of the sand dollar Scaphechinus mirabilis. Russ. J. Mar. Biol. 2008, 34, 415–420. [Google Scholar] [CrossRef]
- Akcha, F.; Spagnol, C.; Rouxel, J. Genotoxicity of diuron and glyphosate in oyster spermatozoa and embryos. Aquat. Toxicol. 2012, 106–107, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.; Palos-Ladeiro, M.; Besnard, A.; Porcher, J.M.; Bony, S.; Sanchez, W.; Devaux, A. Relationship between DNA damage in sperm after ex vivo exposure and abnormal embryo development in the progeny of the three-spined stickleback. Reprod. Toxicol. 2013, 36, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Devaux, Y.; Zangrando, J.; Schroen, B.; Creemers, E.E.; Pedrazzini, T.; Chang, C.P.; Dorn, G.W.; Thum, T.; Heymans, S. Cardiolinc Network. Long noncoding RNAs in cardiac development and ageing. Nat. Rev. Cardiol. 2015, 12, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Galloway, T.S. Ingestion of nanoplastics and microplastics by pacific oyster larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Imhof, H.K.; Rusek, J.; Thiel, M.; Wolinska, J.; Laforsch, C. Do microplastic particles affect Daphnia magna at the morphological, life history and molecular level? PLoS ONE 2017, 12, 0187590. [Google Scholar] [CrossRef] [Green Version]
- Chatel, A.; Bruneau, M.; Lièvre, C.; Goupil, A.; Mouneyrac, C. Spermatozoa: A relevant biological target for genotoxicity assessment of contaminants in the estuarine bivalve Scrobicularia plana. Mar. Pollut. Bull. 2017, 116, 488–490. [Google Scholar] [CrossRef] [PubMed]
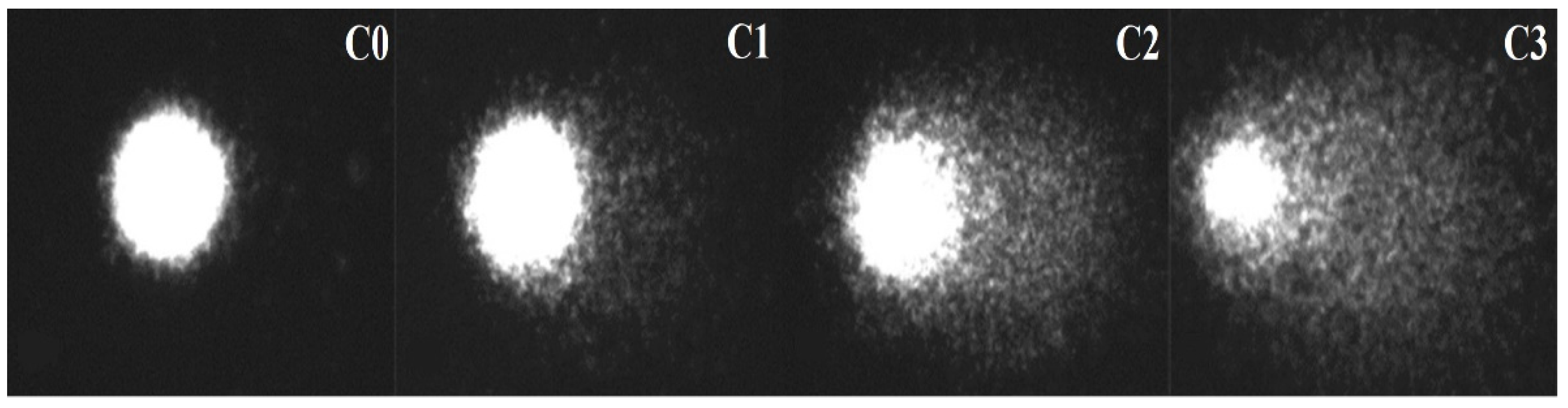
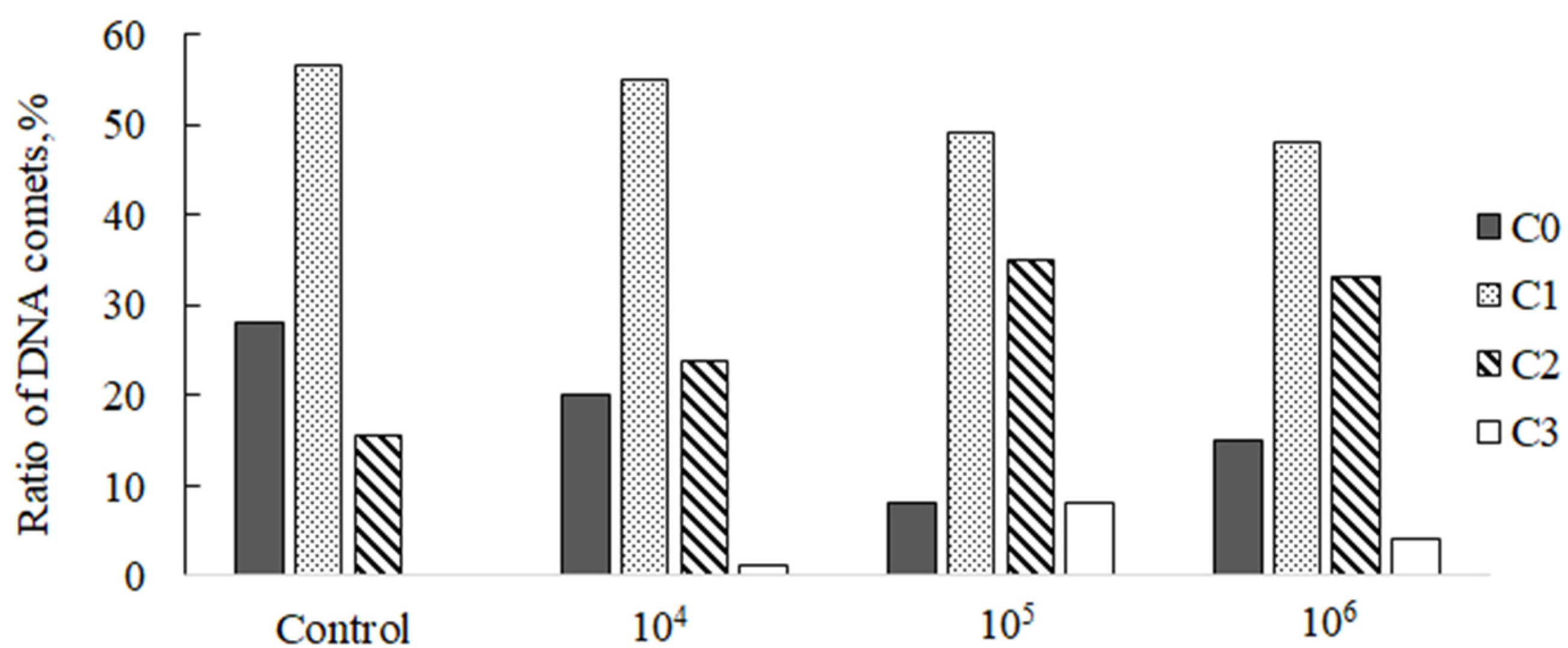
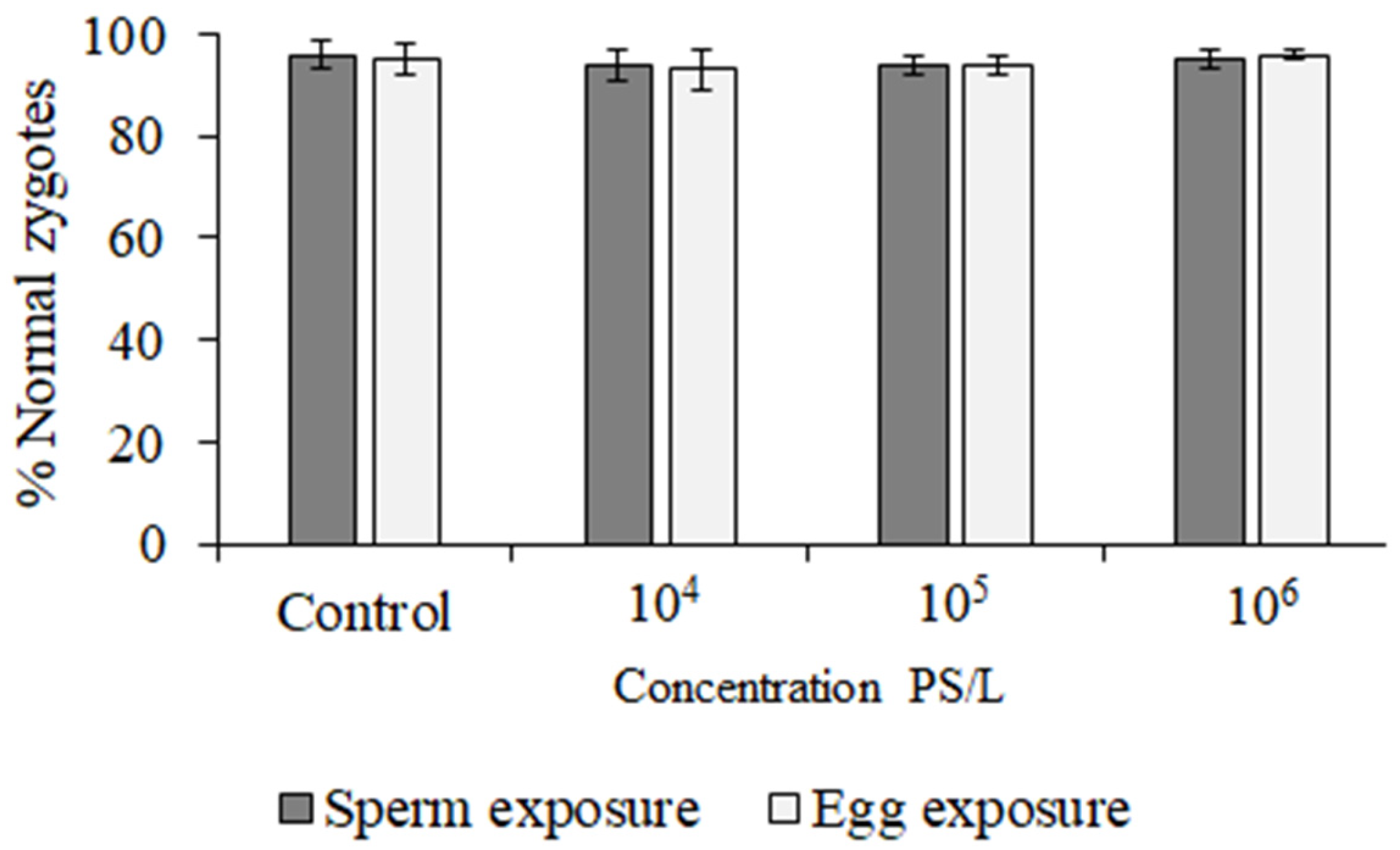
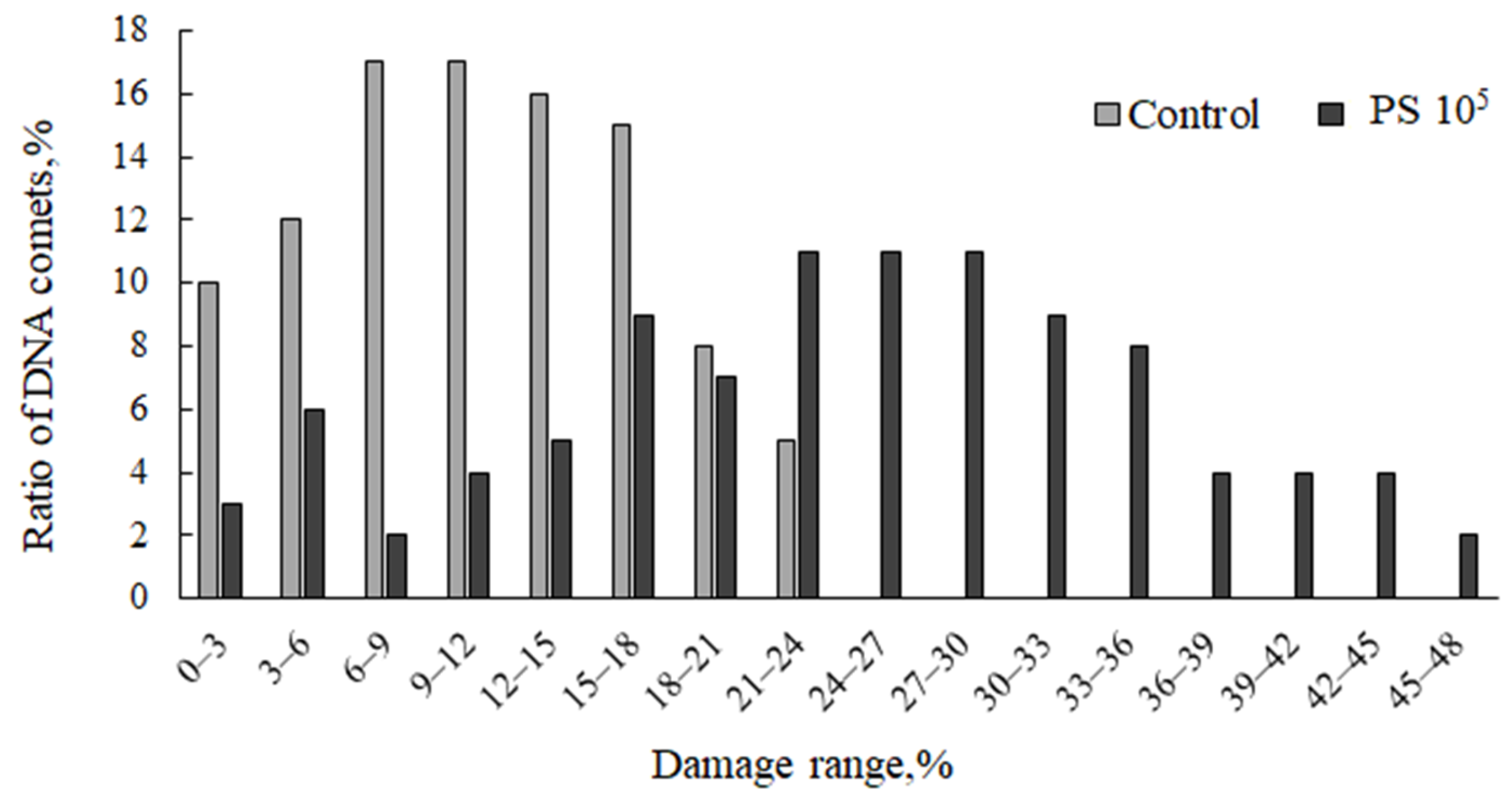
| Concentration (Microspheres/L) | %DNA in tail | GDI | ||
|---|---|---|---|---|
| Sperm Cells | Egg Cells | Sperm Cells | Egg Cells | |
| Control | 10.89 a ± 0.72 | 5.51 ± 0.57 | 0.87 | 0.47 |
| 104 | 14.06 b ± 1.03 | 4.86 ± 0.62 | 1.06 | 0.34 |
| 105 | 20.17 c ± 0.81 | 4.15 ± 0.49 | 1.43 | 0.28 |
| 106 | 19.11 c ± 0.90 | 5.17 ± 0.51 | 1.26 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, A.A.; Chelomin, V.P.; Zhuravel, E.V.; Kukla, S.P.; Slobodskova, V.V.; Dovzhenko, N.V. Genotoxicity of Polystyrene (PS) Microspheres in Short-Term Exposure to Gametes of the Sand Dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea). J. Mar. Sci. Eng. 2021, 9, 1088. https://doi.org/10.3390/jmse9101088
Mazur AA, Chelomin VP, Zhuravel EV, Kukla SP, Slobodskova VV, Dovzhenko NV. Genotoxicity of Polystyrene (PS) Microspheres in Short-Term Exposure to Gametes of the Sand Dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea). Journal of Marine Science and Engineering. 2021; 9(10):1088. https://doi.org/10.3390/jmse9101088
Chicago/Turabian StyleMazur, Andrey Alexandrovich, Viktor Pavlovich Chelomin, Elena Vladimirovna Zhuravel, Sergey Petrovich Kukla, Valentina Vladimirovna Slobodskova, and Nadezda Vladimirovna Dovzhenko. 2021. "Genotoxicity of Polystyrene (PS) Microspheres in Short-Term Exposure to Gametes of the Sand Dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea)" Journal of Marine Science and Engineering 9, no. 10: 1088. https://doi.org/10.3390/jmse9101088
APA StyleMazur, A. A., Chelomin, V. P., Zhuravel, E. V., Kukla, S. P., Slobodskova, V. V., & Dovzhenko, N. V. (2021). Genotoxicity of Polystyrene (PS) Microspheres in Short-Term Exposure to Gametes of the Sand Dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea). Journal of Marine Science and Engineering, 9(10), 1088. https://doi.org/10.3390/jmse9101088






