Oceanographic Drivers of Cuvier’s (Ziphius cavirostris) and Sowerby’s (Mesoplodon bidens) Beaked Whales Acoustic Occurrence along the Irish Shelf Edge
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Beaked Whale Detections
2.3. Environmental Variables
2.4. Data Analysis
3. Results
3.1. Detector Performance
3.2. Scale Selection
3.3. General Observed Trends
3.4. Modelling Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMAR | Autonomous Multichannel Acoustic Recorders |
| AUC | Area Under the Curve |
| AVHRR | Advanced Very High Resolution Radiometer |
| chla | chlorophyll a |
| EU | European Union |
| GAM | Generalised Additive Model |
| GEE | Generalised Estimating Equation |
| GLM | Generalised Linear Model |
| MODIS | Moderate Resolution Imaging Spectroradiometer |
| MSFD | Marine Strategy Framework Directive |
| NAC | North Atlantic Current |
| PAM | Passive Acoustic Monitoring |
| POES | Polar Operational Environmental Satellites |
| QIC | Quasi-likelihood Independence model Criterion |
| relSST | relative sea surface temperature |
| ROC | Receiver Operating Characteristic |
| sdSST | standard deviation of sea surface temperature |
| SeaDAS | SeaWiFS Data Analysis System |
| SEC | Shelf Edge Current |
| SSH | Sea surface height |
References
- Yamada, T.K.; Kitamura, S.; Abe, S.; Tajima, Y.; Matsuda, A.; Mead, J.G.; Matsuishi, T.F. Description of a new species of beaked whale (Berardius) found in the North Pacific. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hooker, S.K.; De Soto, N.A.; Baird, R.W.; Carroll, E.L.; Claridge, D.; Feyrer, L.; Miller, P.J.; Onoufriou, A.; Schorr, G.; Siegal, E.; et al. Future directions in research on beaked whales. Front. Mar. Sci. 2019, 5, 514. [Google Scholar] [CrossRef]
- Tepsich, P.; Rosso, M.; Halpin, P.N.; Moulins, A. Habitat preferences of two deep-diving cetacean species in the northern Ligurian Sea. Mar. Ecol. Prog. Ser. 2014, 508, 247–260. [Google Scholar] [CrossRef]
- Benoit-Bird, K.; Southall, B.; Moline, M.; Claridge, D.; Dunn, C.; Dolan, K.; Moretti, D. Critical threshold identified in the functional relationship between beaked whales and their prey. Mar. Ecol. Prog. Ser. 2020, 654, 1–16. [Google Scholar] [CrossRef]
- Giorli, G.; Neuheimer, A.; Copeland, A.; Au, W.W.L. Temporal and spatial variation of beaked and sperm whales foraging activity in Hawai’i, as determined with passive acoustics. J. Acoust. Soc. Am. 2016, 140, 2333–2343. [Google Scholar] [CrossRef]
- Quick, N.J.; Cioffi, W.R.; Shearer, J.M.; Fahlman, A.; Read, A.J. Extreme diving in mammals: First estimates of behavioural aerobic dive limits in Cuvier’s beaked whales. J. Exp. Biol. 2020, 223, 1–6. [Google Scholar] [CrossRef]
- Tyack, P.L.; Johnson, M.; Soto, N.A.; Sturlese, A.; Madsen, P.T. Extreme diving of beaked whales. J. Exp. Biol. 2006, 209, 4238–4253. [Google Scholar] [CrossRef]
- Rogan, E.; Cañadas, A.; Macleod, K.; Santos, M.B.; Mikkelsen, B.; Uriarte, A.; Van Canneyt, O.; Vázquez, J.A.; Hammond, P.S. Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 8–19. [Google Scholar] [CrossRef]
- Mellinger, D.K.; Stafford, K.M.; Moore, S.E.; Dziak, R.P.; Matsumoto, H. An overview of fixed passive acoustic observation methods for Cetaceans. Oceanography 2007, 20, 36–45. [Google Scholar] [CrossRef]
- Baumann-Pickering, S.; McDonald, M.A.; Simonis, A.E.; Solsona Berga, A.; Merkens, K.P.B.; Oleson, E.M.; Roch, M.A.; Wiggins, S.M.; Rankin, S.; Yack, T.M.; et al. Species-specific beaked whale echolocation signals. J. Acoust. Soc. Am. 2013, 134, 2293–2301. [Google Scholar] [CrossRef]
- Zimmer, W.M.X.; Johnson, M.P.; Madsen, P.T.; Tyack, P.L. Echolocation clicks of free-ranging Cuvier’s beaked whales (Ziphius cavirostris). J. Acoust. Soc. Am. 2005, 117, 3919–3927. [Google Scholar] [CrossRef] [PubMed]
- Cholewiak, D.; Baumann-Pickering, S.; Van Parijs, S. Description of sounds associated with Sowerby’s beaked whales (Mesoplodon bidens) in the western North Atlantic Ocean. J. Acoust. Soc. Am. 2013, 134, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- O’Cadhla, O.; Mackey, M.; Aguilar de Soto, N.; Rogan, E.; Connolly, N. Cetaceans and Seabirds of Ireland’s Atlantic Margin. Volume II: Cetacean Distribution and Abundance. Technical Report, Irish Infracture Programme (PIP): Rockall Studies Group Projects 98/6 and 00/13, Porcupine Studies Group Project P00/15 and Offshore Support Group Project 99/38. 2004. Available online: https://www.ucc.ie/research/crc/publications/reports/Vol2_Cetaceans_Final.pdf (accessed on 1 October 2021).
- Belkin, I.M.; Cornillon, P. Fronts in the world ocean’s large marine ecosystems. ICES CM 2007, 21, 1–33. [Google Scholar]
- Dransfeld, L.; Maxwell, H.; Moriarty, M.; Nolan, C.; Kelly, E.; Pedreschi, D.; Slattery, N.; Connolly, P. North Western Waters Atlas, 3rd ed.; Marine Institute: Oranmore, Ireland, 2014. [Google Scholar]
- Shoosmith, D.R.; Richardson, P.L.; Bower, A.S.; Rossby, H.T. Discrete eddies in the northern North Atlantic as observed by looping RAFOS floats. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 627–650. [Google Scholar] [CrossRef]
- Clarke, M.R. Oceanic cephalopod distribution and species diversity in the Eastern North Atlantic. Arquipel. Life Mar. Sci. 2006, 23A, 27–46. [Google Scholar]
- Hastie, L.; Pierce, G.; Wang, J.; Bruno, I.; Moreno, A.; Piatkowski, U.; Robin, J. Cephalopods In The North-eastern Atlantic. Oceanogr. Mar. Biol. Annu. Rev. 2009, 47, 111–190. [Google Scholar] [CrossRef]
- Berrow, S.; Meade, R.; Marrinan, M.; Mckeogh, E.; Brien, J.O. First confirmed sighting of Sowerby’s beaked whale (Mesoplodon bidens (Sowerby, 1804)) with calves in the Northeast Atlantic. Mar. Biodivers. Rec. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Berrow, S. Biological diversity of cetaceans (whales, dolphins and porpoises) in Irish waters. Mar. Biodivers. Irel. Adjac. Waters 2001, 26, 115–120. [Google Scholar]
- Hernandez-Milian, G.; Lusher, A.; O’Brian, J.; Fernandez, A.; O’Connor, I.; Berrow, S.; Rogan, E. New information on the diet of True’s beaked whale (Mesoplodon mirus, Gray 1850), with insights into foraging ecology on mesopelagic prey. Mar. Mammal Sci. 2017, 33, 1245–1254. [Google Scholar] [CrossRef]
- Bruton, T.; Cotton, D.; Enright, M. Gulf Stream Beaked Whale Mesoplodon Europaeus (Gervais). Ir. Nat. J. 1989, 23, 156. [Google Scholar]
- Arranz, P.; Borchers, D.L.; De Soto, N.A.; Johnson, M.P.; Cox, M.J. A new method to study inshore whale cue distribution from land-based observations. Mar. Mammal Sci. 2014, 30, 810–818. [Google Scholar] [CrossRef]
- Coomber, F.; Moulins, A.; Tepsich, P.; Rosso, M. Sexing free-ranging adult Cuvier’s beaked whales (Ziphius cavirostris) using natural marking thresholds and pigmentation patterns. J. Mammal. 2016, 97, 879–890. [Google Scholar] [CrossRef]
- Falcone, E.A.; Schorr, G.S.; Watwood, S.L.; DeRuiter, S.L.; Zerbini, A.N.; Andrews, R.D.; Morrissey, R.P.; Moretti, D.J. Diving behaviour of cuvier’s beaked whales exposed to two types of military sonar. R. Soc. Open Sci. 2017, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Whitehead, H. Population analysis of Endangered northern bottlenose whales on the Scotian Shelf seven years after the establishment of a Marine Protected Area. Endanger. Species Res. 2013, 21, 273–284. [Google Scholar] [CrossRef]
- Fedutin, I.D.; Filatova, O.A.; Mamaev, E.G.; Burdin, A.M.; Hoyt, E. Occurrence and social structure of Baird’s beaked whales, Berardius bairdii, in the Commander Islands, Russia. Mar. Mammal Sci. 2015, 31, 853–865. [Google Scholar] [CrossRef]
- MacLeod, C.; Mitchell, G. Key areas for beaked whales worldwide. J. Cetacean Res Manag. 2006, 7, 309–322. [Google Scholar]
- Baird, R. Behavior and ecology of social odontocetes: Cuvier’s and Blainville’s beaked whales. In Ethology and Behavioral Ecology of Toothed Whales and Dolphins, The Odontocetes; Wursig, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- European Commission. Guidance Document on the Strict Protection of Animal Species of Community Interest under the Habitats Directive 92/43/EEC. 2007. Available online: https://ec.europa.eu/environment/nature/conservation/species/guidance/pdf/guidance_en.pdf (accessed on 1 October 2021).
- Bernaldo de Quirós, Y.; Fernandez, A.; Baird, R.W.; Brownell, R.L.; Aguilar de Soto, N.; Allen, D.; Arbelo, M.; Arregui, M.; Costidis, A.; Fahlman, A.; et al. Advances in research on the impacts of anti-submarine sonar on beaked whales. Proc. R. Soc. Biol. Sci. 2019, 286, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Simonis, A.; Brownell, R.; Thayre, B.; Trickey, J.; Oleson, E.; Huntington, R.; Baumann-Pickering, S. Co-occurrence of beaked whale strandings and naval sonar in the Mariana Islands, Western Pacific. Proc. Biol. Sci. 2020, 287, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.M.; Ragen, T.J.; Read, A.J.; Vos, E.; Baird, R.W.; Balcomb, K.; Barlow, J.; Caldwell, J.; Cranford, T.; Crum, L.; et al. Understanding the impacts of anthropogenic sound on beaked whales. J. Cetacean Res. Manag. 2006, 7, 177–187. [Google Scholar]
- D’Amico, A.; Gisiner, R.C.; Ketten, D.R.; Hammock, J.A.; Johnson, C.; Tyack, P.L.; Mead, J. Beaked whale strandings and naval exercises. Aquat. Mamm. 2009, 35, 452–472. [Google Scholar] [CrossRef]
- Filadelfo, R.; Mintz, J.; Michlovich, E.; D’Amico, A.; Tyack, P.L.; Ketten, D.R. Correlating military sonar use with beaked whale mass strandings: What do the historical data show? Aquat. Mamm. 2009, 35, 435–444. [Google Scholar] [CrossRef]
- Joyce, T.; Durban, J.; Claridge, D.; Dunn, C.; Hickmott, L.; Fearnbach, H.; Dolan, K.; Moretti, D. Behavioral responses of satellite tracked Blainville’s beaked whales (Mesoplodon densirostris) to mid-frequency active sonar. Mar. Mammal Sci. 2020, 36, 29–46. [Google Scholar] [CrossRef]
- Wensveen, P.J.; Isojunno, S.; Hansen, R.R.; Von Benda-Beckmann, A.M.; Kleivane, L.; Van Ijsselmuide, S.; Lam, F.P.A.; Kvadsheim, P.H.; Deruiter, S.L.; Curé, C.; et al. Northern bottlenose whales in a pristine environment respond strongly to close and distant navy sonar signals. Proc. R. Soc. B 2019, 286, 1–10. [Google Scholar] [CrossRef]
- Aguilar Soto, N.; Johnson, M.; Madsen, P.T.; Tyack, P.L.; Bocconcelli, A.; Fabrizio Borsani, J. Does intense ship noise disrupt foraging in deep-diving cuvier’s beaked whales (Ziphius cavirostris)? Mar. Mammal Sci. 2006, 22, 690–699. [Google Scholar] [CrossRef]
- Cholewiak, D.; DeAngelis, A.I.; Palka, D.; Corkeron, P.J.; Van Parijs, S.M. Beaked whales demonstrate a marked acoustic response to the use of shipboard echosounders. R. Soc. Open Sci. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Brownlow, A.; Davsion, N.; Ten Doeschate, M.; Berrow, S.; Dagleish, M.; Deaville, R.; van Geel, N.; Hantke, G.; Jepson, P.; Onoufriou, A.; et al. Deep trouble: Investigation into an unprecedented number of beaked whale strandings, eastern Atlantic, July–October 2018. In Proceedings of the World Marine Mammal Science Conference, Barcelona, Spain, 8–12 December 2019. [Google Scholar]
- Dolman, S.J.; Pinn, E.; Reid, R.J.; Barley, J.P.; Deaville, R.; Jepson, P.D.; O’Connell, M.; Berrow, S.; Penrose, R.S.; Stevick, P.T.; et al. A note on the unprecedented strandings of 56 deep-diving whales along the UK and Irish coast. Mar. Biodivers. Rec. 2010, 3, 1–8. [Google Scholar] [CrossRef]
- Beck, S.; O’Connor, I.; Berrow, S.; O’Brien, J. Report Series No. 120. Assessment and Monitoring of Ocean Noise in Irish Waters; Environmental Protection Agency: Dublin, Ireland, 2013; Number 101, p. 120. Available online: https://www.epa.ie/publications/research/water/STRIVE-120---Assessment-and-Monitoring-of-Ocean-Noise-in-Irish-Waters.pdf (accessed on 1 October 2021).
- Redfern, J.V.; Ferguson, M.C.; Becker, E.A.; Hyrenbach, K.D.; Good, C.; Barlow, J.; Kaschner, K.; Baumgartner, M.F.; Forney, K.A.; Ballance, L.T.; et al. Techniques for cetacean—Habitat modeling. Mar. Ecol. Prog. Ser. 2006, 310, 271–295. [Google Scholar] [CrossRef]
- Eguiguren, A.; Pirotta, E.; Cantor, M.; Rendell, L.; Whitehead, H. Habitat use of culturally distinct Galápagos sperm whale Physeter macrocephalus clans. Mar. Ecol. Prog. Ser. 2019, 609, 257–270. [Google Scholar] [CrossRef]
- Pirotta, E.; Matthiopoulos, J.; MacKenzie, M.; Scott-Hayward, L.; Rendell, L. Modelling sperm whale habitat preference: A novel approach combining transect and follow data. Mar. Ecol. Prog. Ser. 2011, 436, 257–272. [Google Scholar] [CrossRef]
- Kowarski, K.; Delarue, J.; Martin, B.; O’Brien, J.; Meade, R.; Cadhla, O.; Berrow, S. Signals from the deep: Spatial and temporal acoustic occurrence of beaked whales off western Ireland. PLoS ONE 2018, 13, e0199431. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J. On a simple algorithm to calculate the ’energy’ of a signal. In Proceedings of the International Conference on Acoustics, Speech, and Signal Processing, Albuquerque, NM, USA, 3–6 April 1990; pp. 381–384. [Google Scholar] [CrossRef]
- Mahalanobis, P.C. On the Generalized Distance in Statistics. Proc. Natl. Inst. Sci. India 1936, 2, 49–55. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Mendelssohn, R. Package ’rerddapXtracto’. R Package Version 1.0.2. 2020. Available online: https://cran.r-project.org/web/packages/rerddapXtracto/rerddapXtracto.pdf (accessed on 2 February 2021).
- Jaquet, N. How spatial and temporal scales influence understanding of Sperm Whale distribution: A review. Mammal Rev. 1996, 26, 51–65. [Google Scholar] [CrossRef]
- Silva, T.L.; Mooney, T.A.; Sayigh, L.S.; Baumgartner, M.F. Temporal and spatial distributions of delphinid species in Massachusetts Bay (USA) using passive acoustics from ocean gliders. Mar. Ecol. Prog. Ser. 2019, 631, 1–17. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized additive models. Monogr. Stat. Appl. Probab. 1990, 43, 205–208. [Google Scholar]
- Zuur, A.F. A Beginner’s Guide to Generalized Additive Models with R; Highland Statistics Limited: Newburgh, NY, USA, 2012. [Google Scholar]
- Liang, K.Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Stanistreet, J.E.; Nowacek, D.P.; Bell, J.T.; Cholewiak, D.M.; Hildebrand, J.A.; Hodge, L.E.; Van Parijs, S.M.; Read, A.J. Spatial and seasonal patterns in acoustic detections of sperm whales Physeter macrocephalus along the continental slope in the western North Atlantic Ocean. Endanger. Species Res. 2018, 35, 1–13. [Google Scholar] [CrossRef]
- Højsgaard, S.; Halekoh, U.; Yan, J.; Ekstrøm, C. Geepack: Generalized Estimating Equations Package. R Package, Version 1.2-1. 2016. Available online: https://CRAN.r-project.org/package=geepack (accessed on 2 February 2021).
- Pan, W. Akaike’s information criterion in generalized estimating equations. Biometrics 2001, 57, 120–125. [Google Scholar] [CrossRef]
- Fielding, A.; Bell, J. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Sing, T.; Sander, O.; Beerenwinkel, N.; Lengauer, T. ROCR: Visualizing Classifier Performance in R. R Package, Version 1.0-11. Bioinformatics 2005, 21, 7881. [Google Scholar] [CrossRef]
- Boyce, M.; Vernier, P.; Nielsen, S.; Schmiegelow, F. Evaluating resource selection functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 23 February 2021).
- Frouin-Mouy, H.S.; Hipsey, S.; Denes Burns, R. Soundscape Characterisation and Cetacean Presence in the Porcupine Basin: June to November 2016; Document 01320, Version 1.0. Technical Report; JASCO Applied Sciences for Woodside Energy (Ireland) Pty Ltd.: Dublin, Ireland, 2017. [Google Scholar]
- Berrow, S.; O’Brien, J.; Meade, R.; Delarue, J.; Kowarski, K.; Martin, B.; Moloney, J.; Wall, D.; Gillepsie, D.; Leaper, R.; et al. Acoustic Surveys of Cetaceans in the Irish Atlantic Margin in 2015–2016: Occurrence, Distribution and Abundance. Technical Report; 2018. Available online: https://secure.dccae.gov.ie/downloads/SDCU_DOWNLOAD/ObSERVE_Acoustic_Report.pdf (accessed on 16 October 2019).
- Xu, W.; Miller, P.I.; Quartly, G.D.; Pingree, R.D. Seasonality and interannual variability of the European Slope Current from 20 years of altimeter data compared with in situ measurements. Remote. Sens. Environ. 2015, 162, 196–207. [Google Scholar] [CrossRef]
- Dinter, W. Biogeography of the OSPAR maritime area. Bonn Ger. Fed. Agency Nat. Conserv. 2001, 167, 1–130. [Google Scholar]
- Huang, W.G.; Cracknell, A.P.; Vaughan, R.A.; Davies, P.A. A satellite and field view of the Irish Shelf front. Cont. Shelf Res. 1991, 11, 543–562. [Google Scholar] [CrossRef]
- Boelens, R.; Maloney, D.; Parsons, A.; Walsh, A. Ireland’s Marine and Coastal Areas and Adjacent Seas: An Environmental Assessment; Technical Report; Marine Institute: Dublin, Ireland, 1999. [Google Scholar]
- Forney, K.A.; Becker, E.A.; Foley, D.G.; Barlow, J.; Oleson, E.M. Habitat-based models of cetacean density and distribution in the central North Pacific. Endanger. Species Res. 2015, 27, 1–20. [Google Scholar] [CrossRef]
- Hátún, H.; Payne, M.R.; Beaugrand, G.; Reid, P.C.; Sandø, A.B.; Drange, H.; Hansen, B.; Jacobsen, J.A.; Bloch, D. Large bio-geographical shifts in the north-eastern Atlantic Ocean: From the subpolar gyre, via plankton, to blue whiting and pilot whales. Prog. Oceanogr. 2009, 80, 149–162. [Google Scholar] [CrossRef]
- Hazen, E.L.; Nowacek, D.P.; Laurent St, L.; Halpin, P.N.; Moretti, D.J. The relationship among oceanography, prey fields, and beaked whale foraging habitat in the tongue of the ocean. PLoS ONE 2011, 6, e19269. [Google Scholar] [CrossRef]
- Diogou, N.; Palacios, D.M.; Nystuen, J.A.; Papathanassiou, E.; Katsanevakis, S.; Klinck, H. Sperm whale (Physeter macrocephalus) acoustic ecology at Ocean Station PAPA in the Gulf of Alaska—Part 2: Oceanographic drivers of interannual variability. Deep Sea Res. Part I Oceanogr. Res. Pap. 2019, 150, 103044. [Google Scholar] [CrossRef]
- Houghton, L.; Ramirez-Martinez, N.; Mikkelsen, B.; Víkingsson, G.; Gunnlaugsson, T.; Øien, N.; Hammond, P. Oceanic Drivers of Sei Whale Distribution in the North Atlantic. Nammco Sci. Publ. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Correia, A.M.; Tepsich, P.; Rosso, M.; Caldeira, R.; Sousa-Pinto, I. Cetacean occurrence and spatial distribution: Habitat modelling for offshore waters in the Portuguese EEZ (NE Atlantic). J. Mar. Syst. 2015, 143, 73–85. [Google Scholar] [CrossRef]
- Ferguson, M.C.; Barlow, J.; Reilly, S.B.; Gerrodette, T. Predicting Cuvier’s (Ziphius cavirostris) and Mesoplodon beaked whale population density from habitat characteristics in the eastern tropical Pacific Ocean. J. Cetacean Res. Manag. 2006, 7, 287–299. [Google Scholar]
- Gannier, A.; Praca, E. SST fronts and the summer sperm whale distribution in the north-west Mediterranean Sea. J. Mar. Biol. Assoc. 2007, 87, 187–193. [Google Scholar] [CrossRef]
- Mizroch, S.A.; Rice, D.W. Ocean nomads: Distribution and movements of sperm whales in the North Pacific shown by whaling data and Discovery marks. Mar. Mammal Sci. 2013, 29, 136–165. [Google Scholar] [CrossRef]
- Spitz, J.; Cherel, Y.; Bertin, S.; Kiszka, J.; Dewez, A.; Ridoux, V. Prey preferences among the community of deep-diving odontocetes from the Bay of Biscay, Northeast Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 273–282. [Google Scholar] [CrossRef]
- Häkkinen, S.; Rhines, P.; Worthen, D. Northern North Atlantic sea surface height and ocean heat content variability. J. Geophys. Res. Ocean. 2013, 118, 3670–3678. [Google Scholar] [CrossRef]
- Baird, R.W.; Schorr, G.S.; Webster, D.L.; Mahaffy, S.D.; McSweeney, D.J.; Hanson, M.B.; Andrews, R.D. Open-ocean movements of a satellite-tagged Blainville’s beaked whale (Mesoplodon densirostris): Evidence for an offshore population in Hawai’i? Aquat. Mamm. 2011, 37, 506–511. [Google Scholar] [CrossRef]
- Cribb, N.; Miller, C.; Seuront, L.; Cribb, N. Towards a Standardized Approach of Cetacean Habitat: Past Achievements and Future Directions. Open J. Mar. Sci. 2015, 5, 335–357. [Google Scholar] [CrossRef]
- Scales, K.L.; Hazen, E.L.; Jacox, M.G.; Edwards, C.A.; Boustany, A.M.; Oliver, M.J.; Bograd, S.J. Scale of inference: On the sensitivity of habitat models for wide-ranging marine predators to the resolution of environmental data. Ecography 2017, 40, 210–220. [Google Scholar] [CrossRef]
- Pirotta, E.; Thompson, P.M.; Miller, P.I.; Brookes, K.L.; Cheney, B.; Barton, T.R.; Graham, I.M.; Lusseau, D. Scale-dependent foraging ecology of a marine top predator modelled using passive acoustic data. Funct. Ecol. 2014, 28, 206–217. [Google Scholar] [CrossRef]
- Pirotta, E.; Brotons, J.M.; Cerdà, M.; Bakkers, S.; Rendell, L.E. Multi-scale analysis reveals changing distribution patterns and the influence of social structure on the habitat use of an endangered marine predator, the sperm whale Physeter macrocephalus in the Western Mediterranean Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 155, 103169. [Google Scholar] [CrossRef]
- Dunlop, K.M.; Jarvis, T.; Benoit-Bird, K.J.; Waluk, C.M.; Caress, D.W.; Thomas, H.; Smith, K.L. Detection and characterisation of deep-sea benthopelagic animals from an autonomous underwater vehicle with a multibeam echosounder: A proof of concept and description of data-processing methods. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 134, 64–79. [Google Scholar] [CrossRef]
- Breen, P.; Pirotta, E.; Allcock, L.; Bennison, A.; Boisseau, O.; Bouch, P.; Hearty, A.; Jessopp, M.; Kavanagh, A.; Taite, M.; et al. Insights into the habitat of deep diving odontocetes around a canyon system in the northeast Atlantic ocean from a short multidisciplinary survey. Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 159, 103236. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Stelzenmüller, V.; South, A.; Sørensen, T.K.; Jones, P.J.; Kerr, S.; Badalamenti, F.; Anagnostou, C.; Breen, P.; Chust, G.; et al. Ecosystem-based marine spatial management: Review of concepts, policies, tools, and critical issues. Ocean. Coast. Manag. 2011, 54, 807–820. [Google Scholar] [CrossRef]
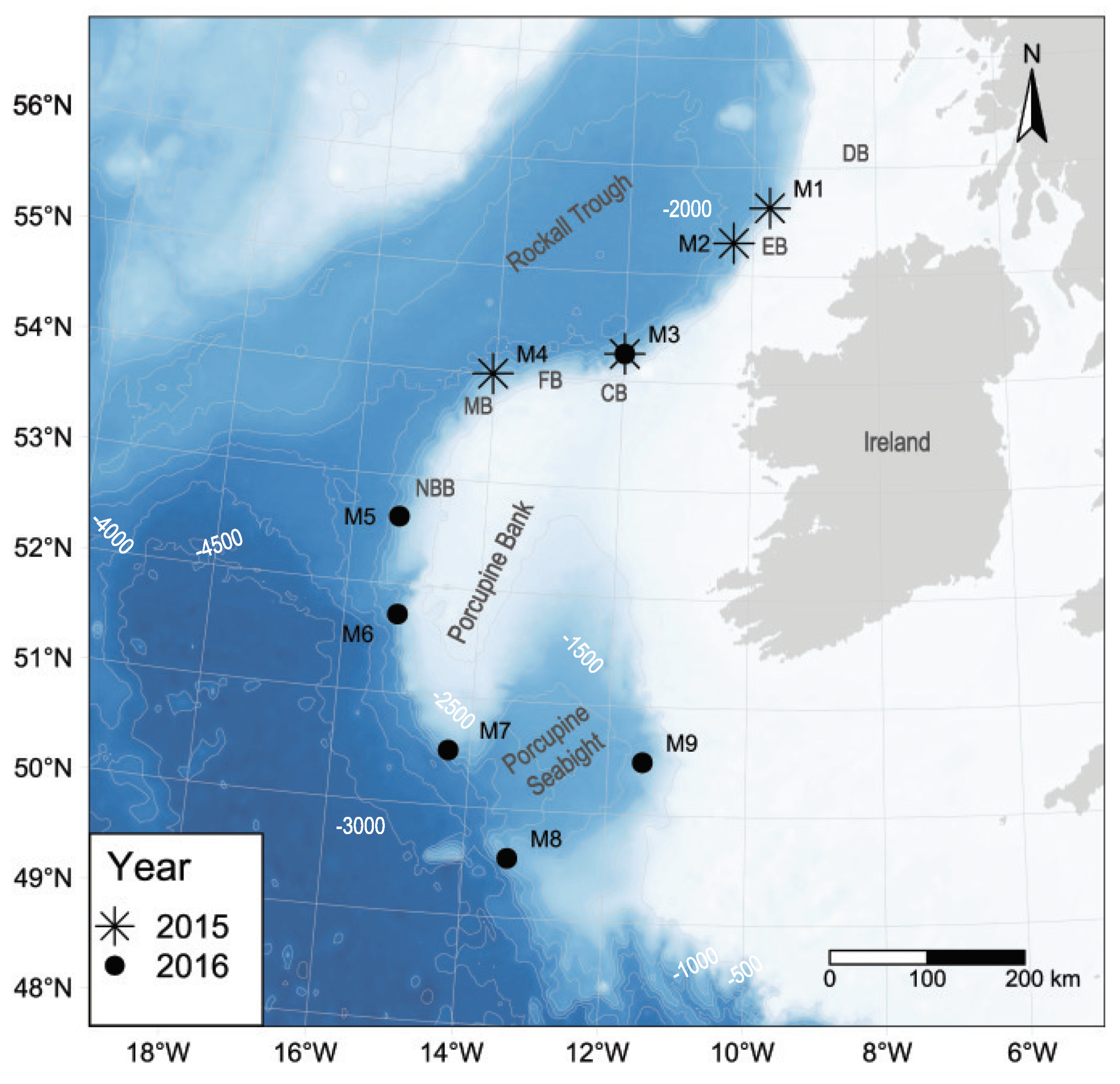



| Mooring | Latitude (°N) | Longitude (°W) | Depth (m) | Start | End | Recording Duration Per Day (Hours) | Recording Period (Cumulative Days) | Total Recording Time (Hours) |
|---|---|---|---|---|---|---|---|---|
| M1 | 55.6302 | −9.7302 | 1600 | 8 May 2015 | 22 August 2015 | 6.5 | 214 | 1391 |
| 55.6323 | −9.7252 | 1620 | 29 August 2015 | 13 December 2015 | 6.5 | |||
| M2 | 55.3018 | −10.3084 | 1995 | 8 May 2015 | 22 August 2015 | 6.5 | 214 | 1391 |
| 55.3011 | −10.3037 | 1971 | 29 August 2015 | 13 December 2015 | 6.5 | |||
| M3 | 54.2513 | −11.9940 | 1850 | 30 August 2015 | 14 December 2015 | 6.5 | 107 | 696 |
| 54.2502 | −11.9926 | 1770 | 24 March 2016 | 29 June 2016 | 2.6 | 98 | 255 | |
| M4 | 54.0014 | −14.0424 | 1920 | 7 May 2015 | 20 August 2015 | 6.5 | 212 | 1378 |
| 54.0015 | −14.0429 | 1944 | 30 August 2015 | 13 December 2015 | 6.5 | |||
| M5 | 52.6225 | −15.3045 | 1752 | 21 March 2016 | 10 July 2016 | 2.6 | 227 | 590 |
| 52.6221 | −15.3046 | 1750 | 10 July 2016 | 2 November 2016 | 2.6 | |||
| M6 | 51.7226 | −15.2077 | 1765 | 20 March 2016 | 11 July 2016 | 2.6 | 210 | 546 |
| 51.7245 | −15.2342 | 1745 | 11 July 2016 | 15 October 2016 | 2.6 | |||
| M7 | 50.5096 | −14.3124 | 1750 | 19 March 2016 | 11 July 2016 | 2.6 | 230 | 598 |
| 50.5085 | −14.3150 | 1750 | 11 July 2016 | 3 November 2016 | 2.6 | |||
| M8 | 49.5477 | −13.3730 | 1760 | 19 March 2016 | 9 August 2016 | 2.6 | 122 | 598 |
| 49.5478 | −13.3723 | 1760 | 9 August 2016 | 3 November 2016 | 2.6 | |||
| M9 | 50.4867 | −11.4963 | 1530 | 2 June 2016 | 4 November 2016 | 3.2 | 156 | 499 |
| Species | Mooring ID | P | R | Classification Threshold | Poptimised | Roptimised | Foptimised |
|---|---|---|---|---|---|---|---|
| Cuvier’s | M1–M8 | 0.87 | 0.79 | 1 | 0.87 | 0.79 | 0.86 |
| M9 | 0.48 | 0.80 | 7 | 1.0 | 0.30 | 0.68 | |
| Sowerby’s | M1–M8 | 0.96 | 0.93 | 1 | 0.96 | 0.93 | 0.95 |
| M9 | 0.96 | 0.57 | 1 | 0.96 | 0.57 | 0.85 |
| Species | Covariate | Scale Selected | Covariate Median (Range) |
|---|---|---|---|
| Cuvier’s | chla | 0.50 × 0.50°-90 days | 0.66 (0.18 to 1.3) |
| sdSST | 0.20 × 0.20°-Monthly | 0.19 (0.00 to 0.46) | |
| relSST | 0.30 × 0.30°-Monthly | 0.22 (−2.9 to 2.7) | |
| SSH | 0.50 × 0.50°-Weekly | −0.51 (−0.65 to −0.35) | |
| Sowerby’s | chla | 0.30 × 0.30°-90 days | 0.62 (0.18 to 1.3) |
| sdSST | 0.20 × 0.20°-Monthly | 0.19 (0.00 to 0.46) | |
| relSST | 0.50 × 0.50°-Monthly | 0.17 (−2.9 to 2.8) | |
| SSH | 0.25 × 0.25°-Monthly | −0.51 (−0.61 to −0.41) |
| Species | AUC | Covariate | p-Value | |
|---|---|---|---|---|
| Cuvier’s | 0.77 | Mooring ID | 1255.7 | <0.001 |
| chla | 57.89 | <0.001 | ||
| SSH | 28.93 | <0.001 | ||
| sdSST | 18.46 | <0.01 | ||
| relSST | 12.37 | <0.05 | ||
| Sowerby’s | 0.70 | Mooring ID | 420.24 | <0.001 |
| chla | 99.23 | <0.001 | ||
| SSH | 26.98 | <0.001 | ||
| relSST | 23.1 | <0.001 | ||
| sdSST | 13.13 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barile, C.; Berrow, S.; O’Brien, J. Oceanographic Drivers of Cuvier’s (Ziphius cavirostris) and Sowerby’s (Mesoplodon bidens) Beaked Whales Acoustic Occurrence along the Irish Shelf Edge. J. Mar. Sci. Eng. 2021, 9, 1081. https://doi.org/10.3390/jmse9101081
Barile C, Berrow S, O’Brien J. Oceanographic Drivers of Cuvier’s (Ziphius cavirostris) and Sowerby’s (Mesoplodon bidens) Beaked Whales Acoustic Occurrence along the Irish Shelf Edge. Journal of Marine Science and Engineering. 2021; 9(10):1081. https://doi.org/10.3390/jmse9101081
Chicago/Turabian StyleBarile, Cynthia, Simon Berrow, and Joanne O’Brien. 2021. "Oceanographic Drivers of Cuvier’s (Ziphius cavirostris) and Sowerby’s (Mesoplodon bidens) Beaked Whales Acoustic Occurrence along the Irish Shelf Edge" Journal of Marine Science and Engineering 9, no. 10: 1081. https://doi.org/10.3390/jmse9101081
APA StyleBarile, C., Berrow, S., & O’Brien, J. (2021). Oceanographic Drivers of Cuvier’s (Ziphius cavirostris) and Sowerby’s (Mesoplodon bidens) Beaked Whales Acoustic Occurrence along the Irish Shelf Edge. Journal of Marine Science and Engineering, 9(10), 1081. https://doi.org/10.3390/jmse9101081







