Seasonal Dynamic of CDOM in a Shelf Site of the South-Eastern Ligurian Sea (Western Mediterranean)
Abstract
1. Introduction
2. Materials and Methods
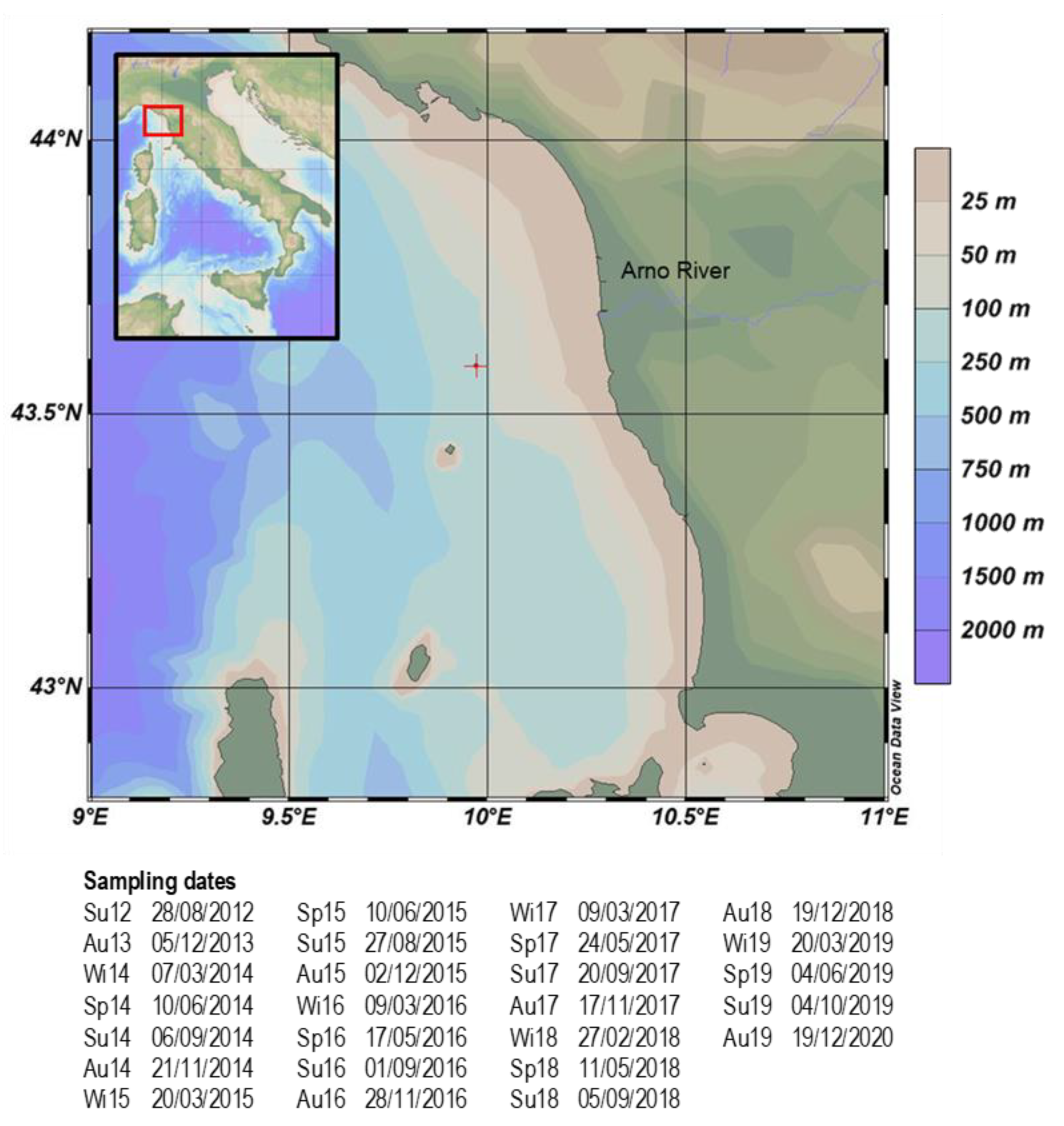
2.1. Study Area
2.2. Sampling
2.3. Phytoplankton Pigments and Taxonomic Composition
+1.27[19′Hexanoyloxyfucoxanthin])/DP
+0.35[19′Butanoylfucoxanthin] + 0.86[Zeaxanthin] + 1.01[Chlb + Divinyl-Chlb]
2.4. CDOM Absorption Measurements
3. Results
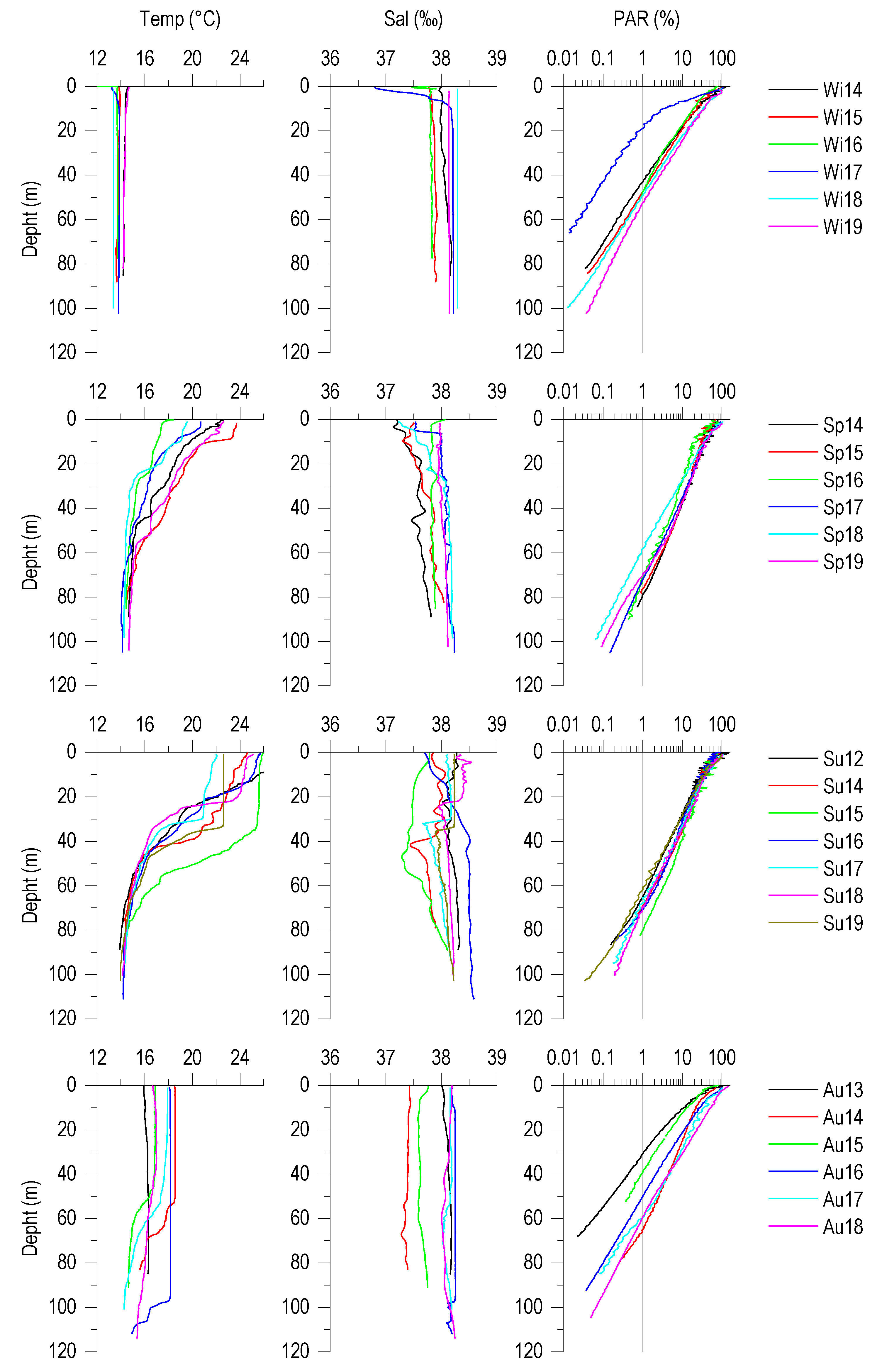
3.1. Vertical Profiles of Temperature, Salinity and PAR (%)
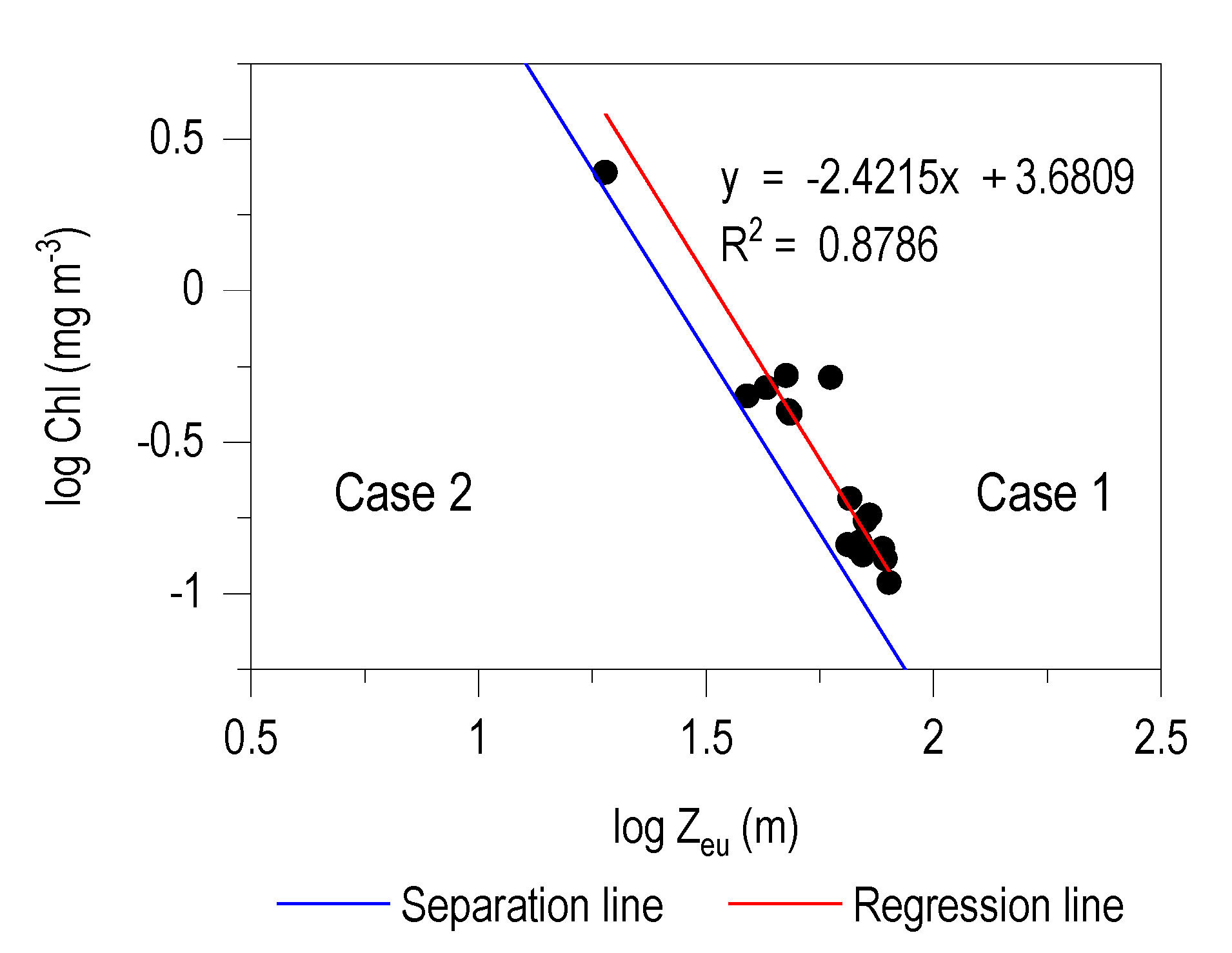
3.2. Zeu/Chl Relationship
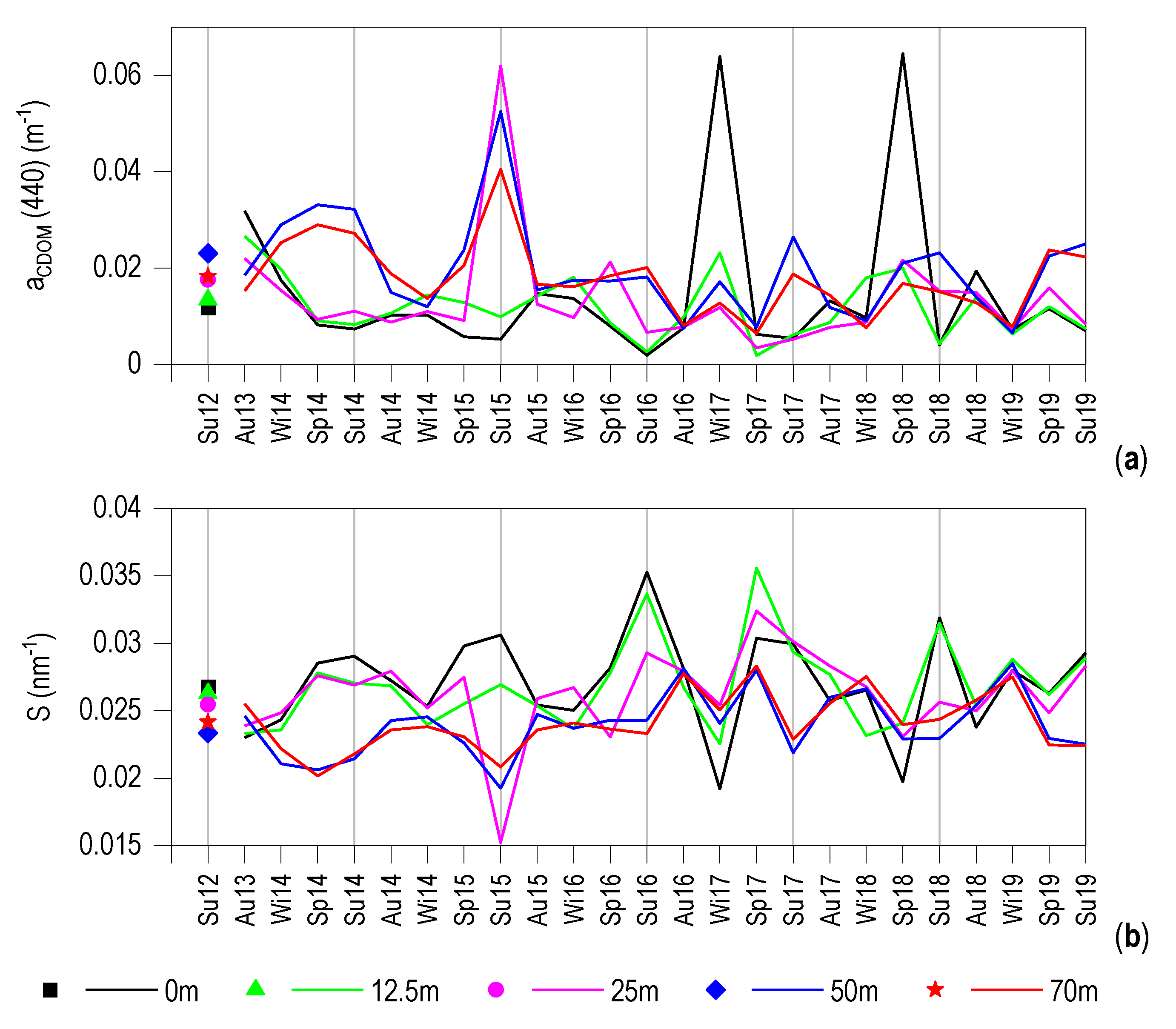
3.3. CDOM Seasonal Evolution
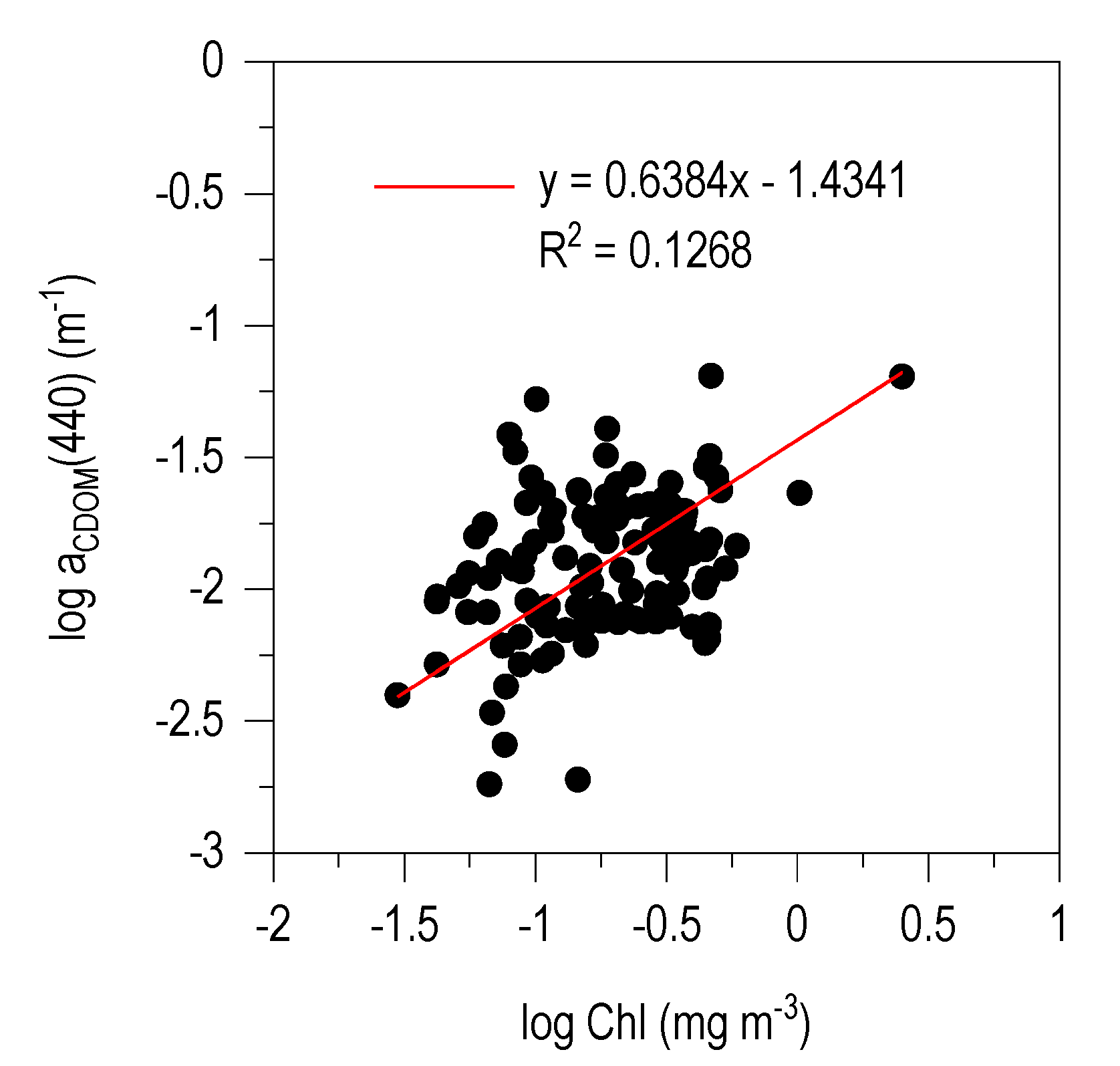
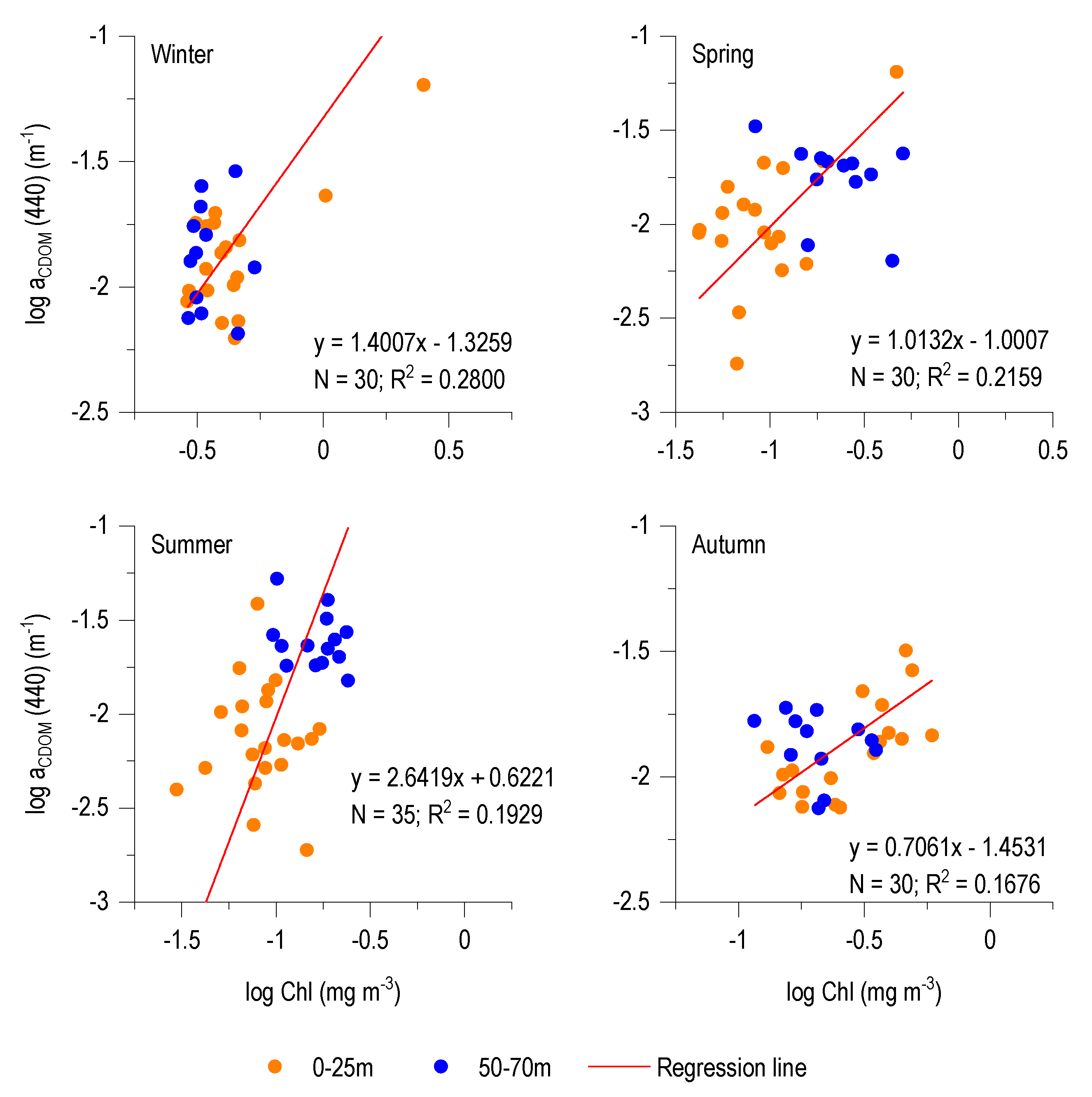
3.4. CDOM-Chl Relationship
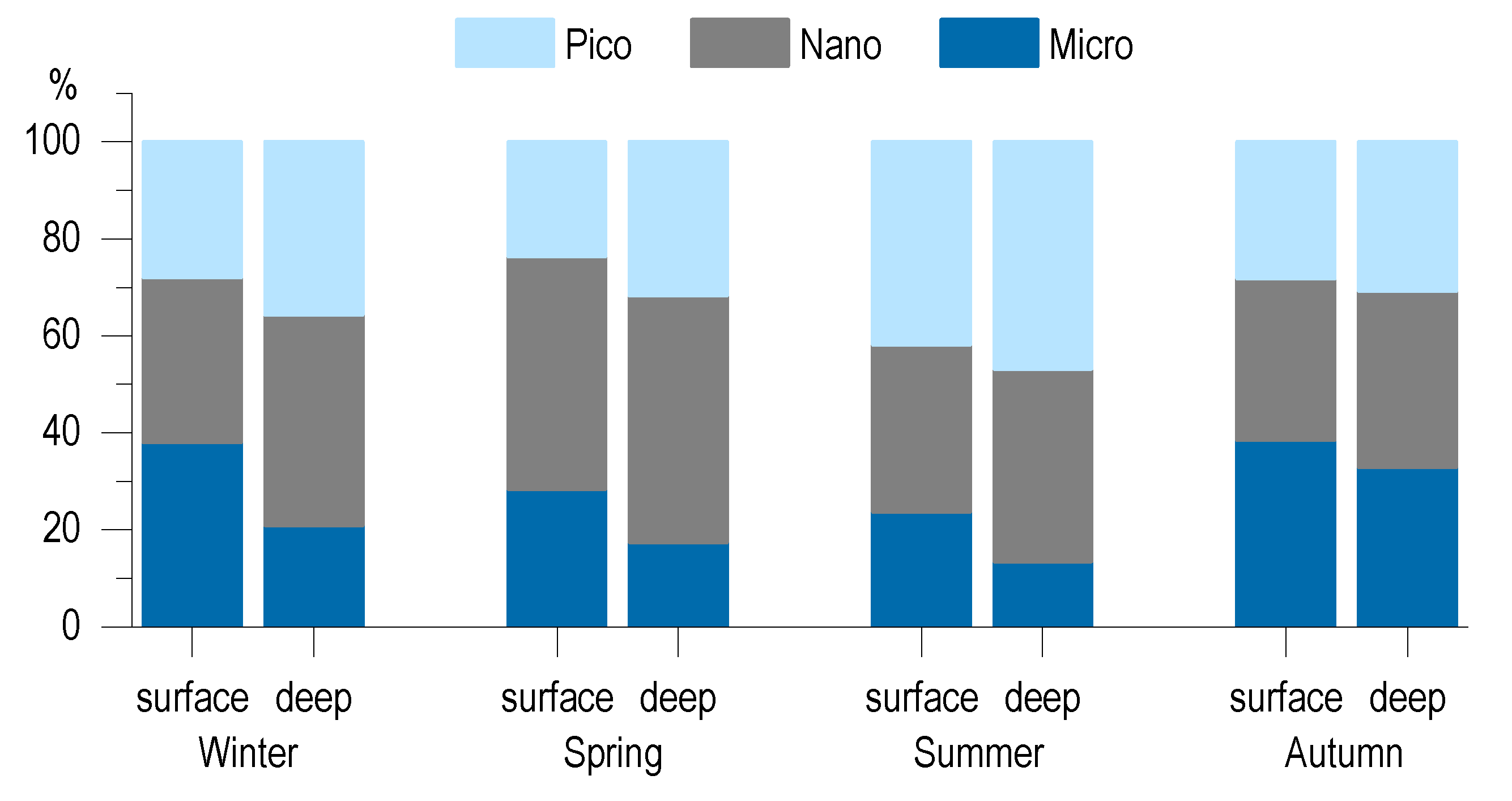
3.5. Phytoplankton Community
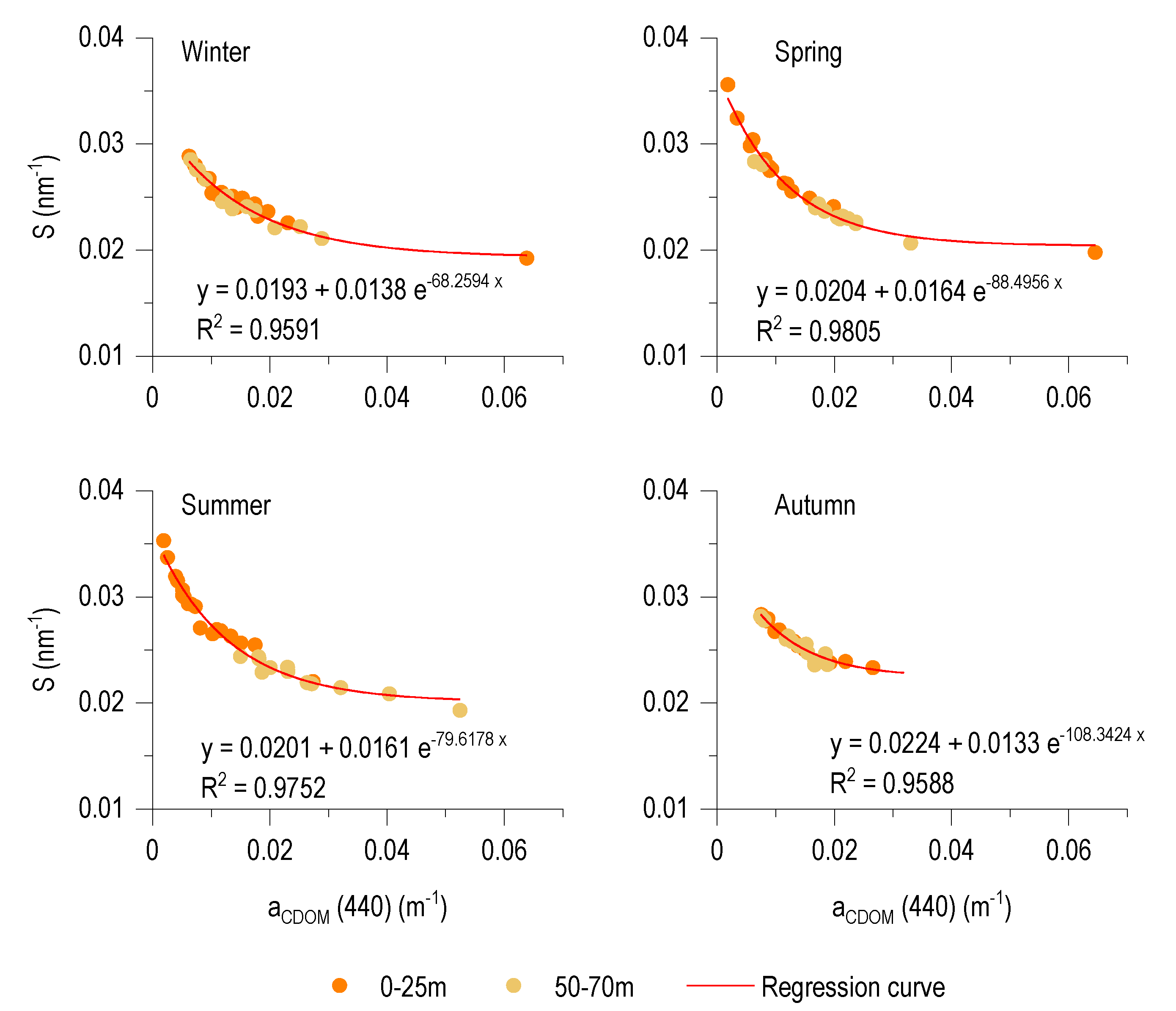
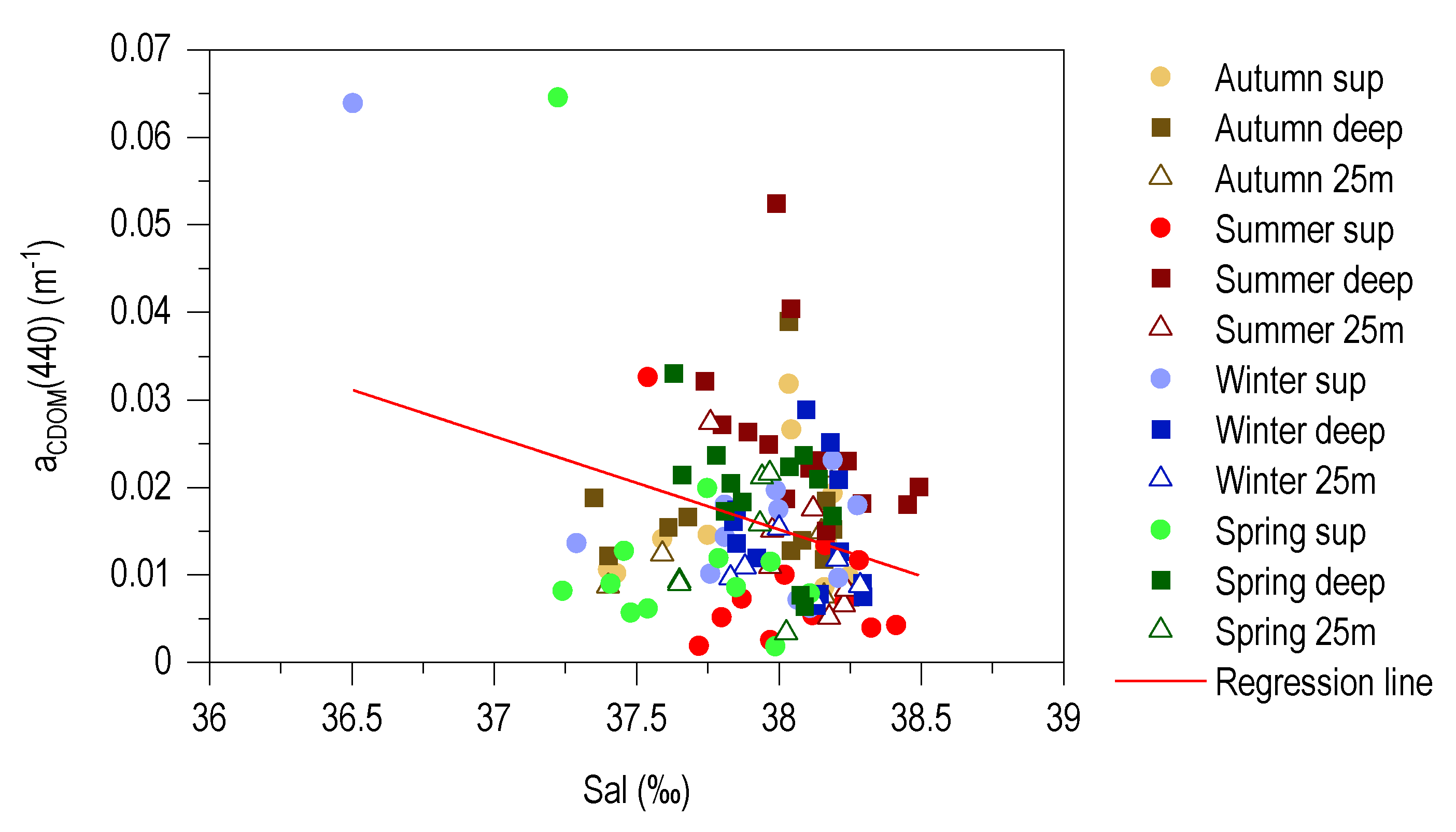
3.6. Relationship between aCDOM (440) and S
4. Discussion
4.1. Seasonal Dynamic of CDOM
4.2. aCDOM (440)–Chl Relationship
4.3. Spectral Characteristics and Origin of CDOM
- (1)
- The surface one linked to terrestrial input events.
- (2)
- The surface summer one, with low absorption and high S.
- (3)
- The winter entire water column one.
- (4)
- The deep summer layer one characterized by high aCDOM (440) and low S.
4.4. Implications for Remote Sensing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blough, N.V.; Del Vecchio, R. Chromophoric DOM in the Coastal Environment. In Biogeochemistry of Marine Dissolved Organic Matter; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 509–546. [Google Scholar] [CrossRef]
- Thurman, E.M. Aquatic Humic Substances. In Organic Geochemistry of Natural Waters. Developments in Biogeochemistry; Springer: Dordrecht, The Netherlands, 1985; Volume 2, pp. 273–361. [Google Scholar] [CrossRef]
- Mopper, K.; Kieber, D.J. Photochemistry and the Cycling of Carbon, Sulfur, Nitrogen and Phosphorus. In Biogeochemistry of Marine Dissolved Organic Matter; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 455–507. [Google Scholar] [CrossRef]
- Bushaw, K.L.; Zepp, R.G.; Tarr, M.A.; Shulz-Jander, D.; Bourbonniere, R.A.; Hodson, R.E.; Miller, W.L.; Bronk, D.A.; Moran, M.A. Photochemical release of biologically available nitrogen from aquatic dissolved organic matter. Nature 1996, 381, 404–407. [Google Scholar] [CrossRef]
- Miller, W.L. Effects of UV Radiation on Aquatic Humus: Photochemical Principles and Experimental Considerations. In Aquatic Humic Substances. Ecological Studies (Analysis and Synthesis); Hessen, D.O., Tranvik, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 133, pp. 125–126. [Google Scholar]
- Wrigley, T.J.; Chambers, J.M.; McComb, A.J. Nutrient and gilvin levels in waters of coastal-plain wetlands in an agricultural area of Western Australia. Mar. Freshwater Res. 1988, 39, 685–694. [Google Scholar] [CrossRef]
- Davies-Colley, R.J. Yellow substance in coastal and marine waters around the South Island, New Zealand. N. Z. J. Mar. Fresh. 1992, 26, 311–322. [Google Scholar] [CrossRef][Green Version]
- Carder, K.L.; Hawes, S.K.; Baker, K.A.; Smith, R.C.; Steward, R.G.; Mitchell, B.G. Reflectance model for quantifying chlorophyll a in the presence of productivity degradation products. J. Geophys. Res. 1991, 96, 20599–20611. [Google Scholar] [CrossRef]
- Carder, K.L.; Chen, F.R.; Lee, Z.P.; Hawes, S.K.; Kamykowski, D. Semianalytic Moderate-resolution imaging spectrometer algorithms for chlorophyll A and absorption with bio-optical domains based on nitrate-depletion temperatures. J. Geophys. Res. 1999, 104, 5403–5421. [Google Scholar] [CrossRef]
- Kalle, K. Zum Problem des Meercswasserfarbc. Ann. Hydrol. Mar. Mitt. 1938, 66, 1–13. [Google Scholar]
- Jerlov, N.G. Influence of suspended and dissolved matter on the transparency of sea water. Tellus 1953, 5, 59–65. [Google Scholar] [CrossRef]
- Vodacek, A.; Blough, N.V.; DeGrandpre, M.D.; Peltzer, E.T.; Nelson, R.K. Seasonal variation in CDOM and DOC in the Middle Atlantic Bight: Terrestrial inputs and photo-oxidation. Limnol. Oceanogr. 1997, 42, 674–768. [Google Scholar] [CrossRef]
- Nelson, N.B.; Siegel, D.A.; Michaels, A.F. Seasonal dynamics of colored dissolved material in the Sargasso Sea. Deep Sea Res. Part I 1998, 45, 931–957. [Google Scholar] [CrossRef]
- Nelson, N.B.; Siegel, D.A. Chromophoric DOM in the Open Ocean. In Biogeochemistry of Marine Dissolved Organic Matter; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 547–578. [Google Scholar] [CrossRef]
- Nelson, N.B.; Siegel, D.A. The global distribution and dynamics of chromophoric dissolved organic matter. Annu. Rev. Mar. Sci. 2013, 5, 447–476. [Google Scholar] [CrossRef]
- Coble, P.G. Marine optical biogeochemistry: The chemistry of ocean color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef] [PubMed]
- DeGrandpre, M.D.; Vodacek, A.; Nelson, R.K.; Bruce, E.J.; Blough, N.V. Seasonal seawater optical properties of the U.S. Middle Atlantic Bight. J. Geophys. Res. 1996, 101, 22727–22736. [Google Scholar] [CrossRef]
- Morel, A.; Gentili, B. The dissolved yellow substance and the shades of blue in the Mediterranean Sea. Biogeosciences 2009, 6, 2625–2636. [Google Scholar] [CrossRef]
- Kitidis, V.; Stubbins, A.P.; Uher, G.; Upstill Goddard, R.C.; Lawb, C.S.; Woodward, E.M.S. Variability of chromophoric organic matter in surface waters of the Atlantic Ocean. Deep Sea Res. Part II 2006, 53, 1666–1684. [Google Scholar] [CrossRef]
- Antoine, D.; Morel, A.; André, J.M. Algal pigment distribution and primary production in the Eastern Mediterranean as derived from coastal zone color scanner observations. J. Geophys. Res. 1995, 100, 193–209. [Google Scholar] [CrossRef]
- Bricaud, A.; Bosc, E.; Antoine, D. Algal biomass and sea surface temperature in the Mediterranean Basin: Intercomparison of data from various satellite sensors, and implications for primary production estimates. Remote Sens. Environ. 2002, 81, 163–178. [Google Scholar] [CrossRef]
- Volpe, G.; Santoleri, R.; Vellucci, V.; Ribera d’Alcalà, M.; Marullo, S.; D’Ortenzio, F. The colour of the Mediterranean Sea: Global versus regional bio-optical algorithms evaluation and implication for satellite chlorophyll estimates. Remote Sens. Environ. 2007, 107, 625–638. [Google Scholar] [CrossRef]
- Antoine, D.; D’Ortenzio, F.; Hooker, S.B.; Bécu, G.; Gentili, B.; Tailliez, D.; Scott, A.J. Assessment of uncertainty in the ocean reflectance determined by three satellite ocean color sensors (MERIS, SeaWiFS and MODIS-A) at an offshore site in the Mediterranean Sea (BOUSSOLE project). J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef]
- Lapucci, C.; Rella, M.A.; Brandini, C.; Ganzin, N.; Gozzini, B.; Maselli, F.; Massi, L.; Nuccio, C.; Ortolani, A.; Trees, C. Evaluation of empirical and semi-analytical chlorophyll algorithms in the Ligurian and North Tyrrhenian Seas. J. Appl. Remote Sens. 2012, 6. [Google Scholar] [CrossRef]
- Xing, X.; Morel, A.; Claustre, H.; D’Ortenzio, F.; Poteau, A. Combined processing and mutual interpretation of radiometry and fluorometry from autonomous profiling Bio-Argo floats: 2. Colored dissolved organic matter absorption retrieval. J. Geophys. Res. 2012, 117, C04022. [Google Scholar] [CrossRef]
- Lee, Z.; Hu, C. Global distribution of Case-1 waters: An analysis from SeaWiFS measurements. Remote Sens. Environ. 2006, 101, 270–276. [Google Scholar] [CrossRef]
- Babin, M.; Stramski, D.; Ferrari, G.M.; Claustre, H.; Bricaud, A.; Obolensky, G.; Hoepffner, N. Variations in the light absorption coefficients of phytoplankton, nonalgal particles, and dissolved organic matter in coastal waters around Europe. J. Geophys. Res. 2003, 108, 3211. [Google Scholar] [CrossRef]
- Bracchini, L.; Tognazzi, A.; Dattilo, A.M.; Decembrini, F.; Rossi, C.; Loiselle, S.A. Sensitivity analysis of CDOM spectral slope in artificial and natural samples: An application in the central eastern Mediterranean Basin. Aquat. Sci. 2010, 72, 485–498. [Google Scholar] [CrossRef]
- Para, J.; Coble, P.G.; Charrière, B.; Tedetti, M.; Fontana, C.; Sempéré, R. Fluorescence and absorption properties of chromophoric dissolved organic matter (CDOM) in coastal surface waters of the Northwestern Mediterranean Sea (Bay of Marseilles, France). Biogeosciences 2010, 7, 4083–4103. [Google Scholar] [CrossRef]
- Massi, L.; Santini, C.; Pieri, M.; Nuccio, C.; Maselli, F. Use of MODIS imagery for the optical characterization of Western Mediterranean water. Ital. J. Remote Sens. 2011, 43, 19–37. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Álvarez-Salgado, X.A.; Galí, M.; Gasol, J.M.; Marrasé, C. Combined effect of light exposure and microbial activity on distinct dissolved organic matter pools. A seasonal field study in an oligotrophic coastal system (Blanes Bay, NW Mediterranean). Mar. Chem. 2013, 148, 44–51. [Google Scholar] [CrossRef]
- Organelli, E.; Bricaud, A.; Antoine, D.; Matsuoka, A. Seasonal dynamics of light absorption by chromophoric dissolved organic matter (CDOM) in the NW Mediterranean Sea (BOUSSOLE site). Deep Sea Res. Part I 2014, 91, 72–85. [Google Scholar] [CrossRef]
- Santinelli, C. DOC in the Mediterranean Sea. In Biogeochemistry of Marine Dissolved Organic Matter, 2nd ed.; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 579–608. [Google Scholar] [CrossRef]
- Galletti, Y.; Gonnelli, M.; Retelletti Brogi, S.; Vestri, S.; Santinelli, C. DOM dynamics in open waters of the Mediterranean Sea: New insights from optical properties. Deep. Sea Res. I. 2019, 144, 95–114. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Bustilloz-Guzmàn, J.; Cailliau, C.; Marty, J.C. Rapid HPLC method for determination of phytoplankton chemotaxinomic pigments: Separation of chlorophyll a from divinyl-chlorophyll a and zeaxanthin from lutein. J. Plankton Res. 1996, 18, 2377–2382. [Google Scholar] [CrossRef]
- Barlow, R.G.; Cummings, D.G.; Gibb, S.W. Improved resolution of mono- and divinyl chlorophylls a and b and zeaxanthin and lutein in phytoplankton extracts using reverse phase C-8 HPLC. Mar. Ecol. Prog. Ser. 1997, 161, 303–307. [Google Scholar] [CrossRef]
- Bricaud, A.; Claustre, H.; Ras, J.; Oubelkheir, K. Natural variability of phytoplanktonic absorption in oceanic waters: Influence of the size structure of algal populations. J. Geophys. Res. 2004, 109, 1–12. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Manca, B.; Luchetta, A.; Marty, J. Phytoplankton pigment distribution in relation to upper thermocline circulation in the Eastern Mediterranean Sea during winter. J. Geophys. Res. 2001, 106, 19939–19956. [Google Scholar] [CrossRef]
- Zingone, A.; Totti, C.; Sarno, D.; Caroppo, C.; Giacobbe, M.G.; Lugliè, A.; Nuccio, C.; Socal, G. Fitoplancton: Metodiche di analisi quali-quantitativa. In Metodologie di Campionamento e di Studio del Plancton Marino; Socal, G., Buttino, I., Cabrini, M., Mangoni, O., Penna, A., Totti, C., Eds.; Manuali e Linee Guida ISPRA-SIBM: Rome, Italy, 2010; Volume 56, pp. 213–237. [Google Scholar]
- Nayar, S.; Chou, L.M. Relative efficiencies of different filters in retaining phytoplankton for pigment and productivity studies. Estuar. Coast. Shelf. Sci. 2000, 58, 241–248. [Google Scholar] [CrossRef]
- Ferrari, G. The relationship between chromophoric dissolved organic matter and dissolved organic carbon in the European Atlantic coastal area and in the West Mediterranean Sea (Gulf of Lions). Mar. Chem. 2000, 70, 339–357. [Google Scholar] [CrossRef]
- Bricaud, A.; Morel, A.; Prieur, L. Absorption by dissolved organic matter of the sea (yellow substance) in the UV and visible domains. Limnol. Oceanogr. 1981, 26, 43–53. [Google Scholar] [CrossRef]
- Kowalczuk, P.; Cooper, W.J.; Whitehead, R.F.; Durako, M.J.; Sheldon, W. Characterization of CDOM in an organic-rich river and surrounding coastal ocean in the South Atlantic Bight. Aquat. Sci. 2003, 65, 384–401. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. The optics of chromophoric dissolved organic matter (CDOM) in the Greenland Sea: An algorithm for differentiation between marine and terrestrially derived organic matter. Limnol. Oceanogr. 2001, 46, 2087–2093. [Google Scholar] [CrossRef]
- Twardowski, M.S.; Boss, E.; Sullivan, J.M.; Donaghay, P.L. Modeling the spectral shape of absorption by chromophoric dissolved organic matter. Mar. Chem. 2004, 89, 69–88. [Google Scholar] [CrossRef]
- Morel, A. Optical modeling of the upper ocean in relation to its biogenous matter content (case 1 water). J. Geophys. Res. 1988, 93, 10749–10768. [Google Scholar] [CrossRef]
- Bricaud, A.; Babin, M.; Claustre, H.; Ras, J.; Tièche, F. Light absorption properties and absorption budget of Southeast Pacific waters. J. Geophys. Res. 2010, 115, C08009. [Google Scholar] [CrossRef]
- Röttgers, R.; Koch, B. Spectroscopic detection of a ubiquitous dissolved pigment degradation product in subsurface waters of the global ocean. Biogeosciences 2012, 9. [Google Scholar] [CrossRef]
- D’Ortenzio, F.; Iudicone, D.; de Boyer Montégut, C.; Testor, P.; Antoine, D.; Marullo, S.; Santoleri, R.; Madec, G. Seasonal variability of the mixed layer depth in the Mediterranean Sea as derived from in situ profiles. Geophys. Res. Lett. 2005, 32, L12605. [Google Scholar] [CrossRef]
- D’Ortenzio, F.; Prieur, L. The Upper Mixed Layer. In Life in the Mediterranean Sea: A Look at Habitat Changes; Stambler, N., Ed.; Nova Science Publisher: Hauppage, NY, USA, 2012; pp. 127–156. [Google Scholar]
- Bricaud, A.; Ciotti, A.M.; Gentili, B. Spatial-temporal variations in phytoplankton size and colored detrital matter absorption at global and regional scales, as derived from twelve years of SeaWiFS data (1998–2009). Glob. Biogeochem. Cycles 2012, 26, GB1010. [Google Scholar] [CrossRef]
- Xing, X.; Claustre, H.; Wang, H.; Poteau, A.; D’Ortenzio, F. Seasonal dynamics in colored dissolved organic matter in the Mediterranean Sea: Patterns and drivers. Deep Sea Res. Part I 2014, 83, 93–101. [Google Scholar] [CrossRef]
- Organelli, E.; Barbieux, M.; Claustre, H.; Schmechtig, C.; Poteau, A.; Bricaud, A.; Boss, E.; Briggs, N.; Dall’Olmo, G.; D’Ortenzio, F.; et al. Two databases derived from BGC-Argo float measurements for marine biogeochemical and bio-optical applications. Earth Syst. Sci. Data 2017, 9, 861–880. [Google Scholar] [CrossRef]
- De Souza Sierra, M.M.; Donard, O.F.X.; Lamotte, M. Spectral identification and behaviour of dissolved organic fluorescent material during estuarine mixing processes. Mar. Chem. 1997, 58, 51–58. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Behaviour of the optical properties of coloured dissolved organic matter under conservative mixing. Estuar. Coast. Shelf Sci. 2003, 57, 973–979. [Google Scholar] [CrossRef]
- Del Vecchio, R.; Subramaniam, A. Influence of the Amazon River on the surface optical properties of the western tropical North Atlantic Ocean. J. Geophys. Res. 2004, 109, C11001. [Google Scholar] [CrossRef]
- Del Castillo, C.E.; Gilbes, F.; Coble, P.G.; Müller-Karger, F.E. On the dispersal of riverine colored dissolved organic matter over the West Florida Shelf. Limnol. Oceanogr. 2000, 45, 1425–1432. [Google Scholar] [CrossRef]
- Matsuoka, A.; Bricaud, A.; Benner, R.; Para, J.; Sempere, R.; Prieur, L.; Belanger, S.; Babin, M. Tracing the transport of colored dissolved organic matter in water masses of the Southern Beaufort Sea: Relationship with hydrographic characteristics. Biogeosciences 2012, 9, 925–940. [Google Scholar] [CrossRef]
- Seritti, A.; Russo, D.; Nannicini, L.; Del Vecchio, R. DOC, absorption and fluorescence properties of estuarine and coastal waters of the Northern Tyrrhenian Sea. Chem. Spec. Bioavailab. 1998, 10, 95–106. [Google Scholar] [CrossRef]
- Vignudelli, S.; Santinelli, C.; Murru, E.; Nannicini, L.; Seritti, A. Distributions of dissolved organic carbon (DOC) and chromophoric dissolved organic matter (CDOM) in coastal waters of the northern Tyrrhenian Sea (Italy). Estuar. Coast. Shelf Sci. 2004, 60, 133–149. [Google Scholar] [CrossRef]
- Swan, C.M.; Siegel, D.A.; Nelson, N.B.; Carlson, C.A.; Nasir, E. Biogeochemical and hydrographic controls on chromophoric dissolved organic matter distribution in the Pacific Ocean. Deep Sea Res. Part I 2009, 56, 2175–2192. [Google Scholar] [CrossRef]
- Del Vecchio, R.; Blough, N. Photobleaching of chromophoric dissolved organic matter in natural waters: Kinetics and modeling. Mar. Chem. 2002, 78, 231–253. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Swan, C.M.; Nelson, N.B.; Siegel, D.A.; Kostadinov, T.S. The effect of surface irradiance on the absorption spectrum of chromophoric dissolved organic matter in the global ocean. Deep Sea Res. Part I 2012, 63, 52–64. [Google Scholar] [CrossRef]
- Kouassi, A.M.; Zika, R.G. Light-induced destruction of the absorbance property of dissolved organic matter in seawater. Toxicol. Environ. Chem. 1992, 35, 195–211. [Google Scholar] [CrossRef]
- Siegel, D.A.; Michaels, A.F. Quantification of non-algal light attenuation in the Sargasso Sea: Implications for biogeochemistry and remote sensing. Deep Sea Res. Part II 1996, 43, 321–345. [Google Scholar] [CrossRef]
- Del Vecchio, R.; Blough, N.V. On the origin of the optical properties of humic substances. Environ. Sci. Technol. 2004, 38, 3885–3891. [Google Scholar] [CrossRef]
- Siegel, D.A.; Maritorena, S.; Nelson, N.B.; Behrenfeld, M.J.; McClain, C.R. Colored dissolved organic matter and its influence on the satellite-based characterization of the ocean biosphere. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nosaka, Y.; Suzuki, K.; Ogawa, H.; Takahashi, K.; Saito, H. Photobleaching as a factor controlling spectral characteristics of chromophoric dissolved organic matter in open ocean. Biogeosciences 2013, 10, 7207–7217. [Google Scholar] [CrossRef]
- Nelson, N.B.; Carlson, C.A.; Steinberg, D.K. Production of chromophoric dissolved organic matter by Sargasso Sea microbes. Mar. Chem. 2004, 89, 273–287. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; p. 535. [Google Scholar]
- Platt, T.; Sathyendranath, S. Ecological indicators for the pelagic zone of the ocean from remote sensing. Remote Sens. Environ. 2008, 112, 3426–3436. [Google Scholar] [CrossRef]
- Edwards, K.F.; Litchman, E.; Klausmeier, C.A. Functional traits explain phytoplankton responses to environmental gradients across lakes of the United States. Ecology 2013, 94, 1626–1635. [Google Scholar] [CrossRef]
- Brun, P.; Vogt, M.; Payne, M.R.; Gruber, N.; O’Brien, C.J.; Buitenhuis, E.T.; Le Quéré, C.; Leblanc, K.; Luo, Y.-W. Ecological niches of open ocean phytoplankton taxa. Limnol. Oceanogr. 2015, 60, 1020–1038. [Google Scholar] [CrossRef]
- D’Alelio, D.; Libralato, S.; Wyatt, T.; Ribera d’Alcalà, M. Ecological-network models link diversity, structure and function in the plankton food-web. Sci. Rep. 2016, 6, 21806. [Google Scholar] [CrossRef]
- Lochte, K.; Ducklow, H.W.; Fasham, M.J.R.; Stienen, C. Plankton succession and carbon cycling at 47° N 20° W during the JGOFS North Atlantic Bloom Experiment. Deep Sea Res. Part II 1993, 40, 91–114. [Google Scholar] [CrossRef]
- Lee, Y.C.; Park, M.O.; Jung, J.; Yang, E.J.; Lee, S.H. Taxonomic variability of phytoplankton and relationship with production of CDOM in the polynya of the Amundsen Sea, Antarctica. Deep Sea Res. Part II 2016, 123, 30–41. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Malinsky-Rushansky, N.Z.; Legrand, C. Excretion of dissolved organic carbon by phytoplankton of different sizes and subsequent bacterial uptake. Mar. Ecol. Prog. Ser. 1996, 132, 249–255. [Google Scholar] [CrossRef]
- Lagaria, A.; Psarra, S.; Gogou, A.; Tuğrul, S.; Christaki, U. Particulate and dissolved primary production along a pronounced hydrographic and trophic gradient (Turkish Straits System–NE Aegean Sea). J. Mar. Syst. 2013, 119–120, 1–10. [Google Scholar] [CrossRef]
- Livanou, E.; Lagaria, A.; Psarra, S.; Lika, K. Dissolved organic matter release by phytoplankton in the context of the Dynamic Energy Budget theory. Biogeosci. Dis. 2017. [Google Scholar] [CrossRef]
- Organelli, E.; Bricaud, A.; Gentili, B.; Antoine, D.; Vellucci, V. Evaluation of bio-optical inversion models for the retrieval of Colored Dissolved Organic Matter (CDOM) and Colored Detrital Matter (CDM) light absorption coefficients in the Mediterranean Sea using field and satellite ocean color radiometry. Remote Sens. Environ. 2016, 186, 297–310. [Google Scholar] [CrossRef]

| Full Data Set | N | aCDOM (440) | S | Chl |
|---|---|---|---|---|
| mean | 120 | 0.0154 | 0.0257 | 0.2565 |
| standard error | - | 0.001 | 0.0003 | 0.0242 |
| min | - | 0.0018 | 0.0192 | 0.0298 |
| max | - | 0.0645 | 0.0356 | 2.5134 |
| (0.5–70 m) | ||||
| winter | 30 | 0.0154 | 0.0249 | 0.4660 |
| spring | 30 | 0.016 | 0.0258 | 0.1652 |
| summer | 30 | 0.016 | 0.0263 | 0.1231 |
| autumn | 30 | 0.014 | 0.0258 | 0.2696 |
| (0.5–25 m) | ||||
| winter | 18 | 0.0184 | 0.0245 | 0.5379 |
| spring | 18 | 0.0138 | 0.0271 | 0.1136 |
| summer | 18 | 0.0067 | 0.0297 | 0.0897 |
| autumn | 18 | 0.0141 | 0.026 | 0.3035 |
| (50–70 m) | ||||
| winter | 12 | 0.0148 | 0.0237 | 0.3483 |
| spring | 12 | 0.0194 | 0.0238 | 0.2530 |
| summer | 12 | 0.0259 | 0.0225 | 0.1732 |
| autumn | 12 | 0.014 | 0.0255 | 0.2187 |
| This Study | [31] | [28] | [29] | [32] | [34] | [27] | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| aCDOM (λ) | 440 | 254 | 300 | 350 | 254 | 300 | 350 | 440 | 254 | 443 |
| mean | 0.0154 | 1.53 | 0.48 | 0.137 | 1.59 | 0.103 | ||||
| Standard Error | 0.001 | 0.02 | 0.01 | 0.005 | 0.02 | 0.003 | ||||
| Min | 0.0018 | 1.13 | 0.26 | 0.045 | 0.08 | 0.060 | 0.015 | 0.69 | ||
| Max | 0.0645 | 2.54 | 1.02 | 0.382 | 0.58 | 0.130 | 0.045 | 1.54 | ||
| S | S (250–600) | S (350–400) | S (270–400) | S (350–500) | S (350–500) | S (275–295) | S (350–500) | |||
| Mean | 0.0257 | 0.0165 | 0.0212 | 0.019 | 0.017 | |||||
| Standard Error | 0.0003 | 0.0004 | 0.0004 | 0.001 | 0.0038 | |||||
| Min | 0.0192 | 0.014 | 0.015 | 0.02 | 0.0114 | |||||
| Max | 0.0356 | 0.026 | 0.021 | 0.04 | 0.0251 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massi, L.; Frittitta, L.; Melillo, C.; Polonelli, F.; Bianchi, V.; De Biasi, A.M.; Nuccio, C. Seasonal Dynamic of CDOM in a Shelf Site of the South-Eastern Ligurian Sea (Western Mediterranean). J. Mar. Sci. Eng. 2020, 8, 703. https://doi.org/10.3390/jmse8090703
Massi L, Frittitta L, Melillo C, Polonelli F, Bianchi V, De Biasi AM, Nuccio C. Seasonal Dynamic of CDOM in a Shelf Site of the South-Eastern Ligurian Sea (Western Mediterranean). Journal of Marine Science and Engineering. 2020; 8(9):703. https://doi.org/10.3390/jmse8090703
Chicago/Turabian StyleMassi, Luca, Laura Frittitta, Chiara Melillo, Francesca Polonelli, Veronica Bianchi, Anna Maria De Biasi, and Caterina Nuccio. 2020. "Seasonal Dynamic of CDOM in a Shelf Site of the South-Eastern Ligurian Sea (Western Mediterranean)" Journal of Marine Science and Engineering 8, no. 9: 703. https://doi.org/10.3390/jmse8090703
APA StyleMassi, L., Frittitta, L., Melillo, C., Polonelli, F., Bianchi, V., De Biasi, A. M., & Nuccio, C. (2020). Seasonal Dynamic of CDOM in a Shelf Site of the South-Eastern Ligurian Sea (Western Mediterranean). Journal of Marine Science and Engineering, 8(9), 703. https://doi.org/10.3390/jmse8090703





