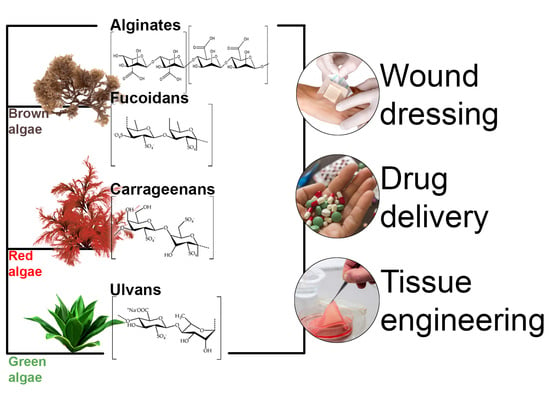
Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering: A Review
Abstract
1. Introduction
2. Wound Healing and Current Trends in the Design of Wound Dressings
3. Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering
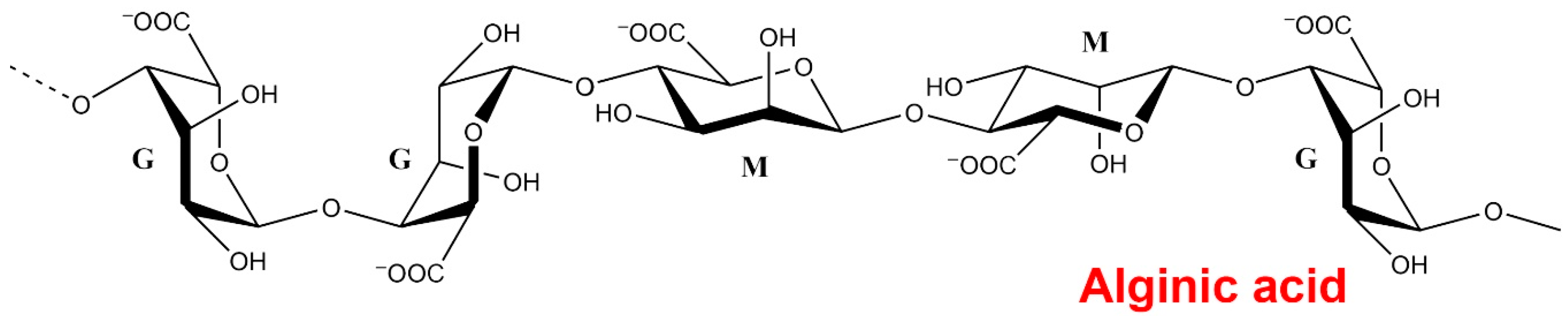
3.1. Alginates
3.2. Fucoidans
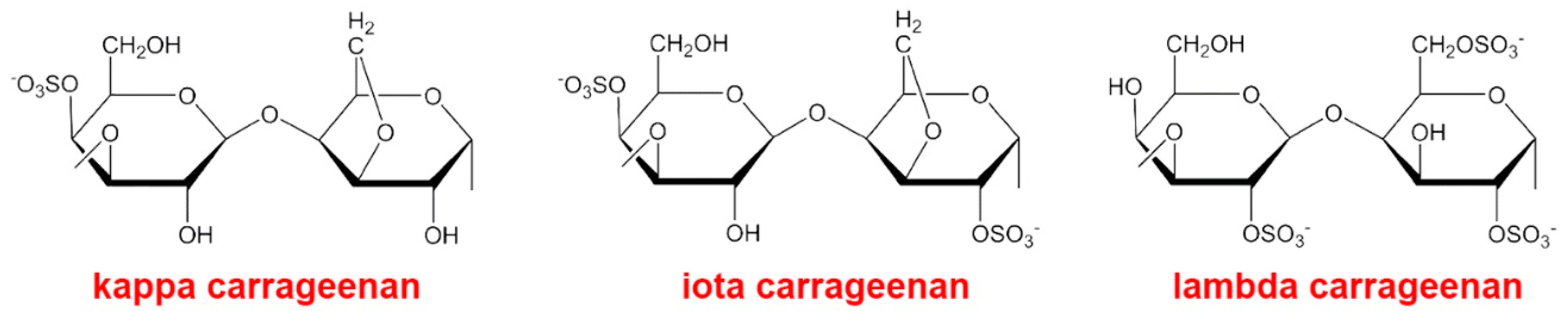
3.3. Carrageenans
3.4. Ulvans
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pereira, L. Biological and therapeutic properties of the seaweed polysaccharides. Int. Boil. Rev. 2018, 2, 2. [Google Scholar] [CrossRef]
- Jovic, T.; Jessop, Z.; Al-Sabah, A.; Whitaker, I.S. The clinical need for 3D printed tissue in reconstructive surgery. 3D Bioprinting for Reconstructive Surgery 2018, 235–244. [Google Scholar] [CrossRef]
- Jovic, T.H.; Kungwengwe, G.; Mills, A.C.; Whitaker, I.S. Plant-Derived Biomaterials: A Review of 3D Bioprinting and Biomedical Applications. Front. Mech. Eng. 2019, 5, 19. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector. Mar. Drugs 2019, 18, 7. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Elango, S. Wound dressings – a review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, А.N.; Obluchinskaya, E.; Vuorela, H. The Pharmacokinetics of Fucoidan after Topical Application to Rats. Mar. Drugs 2019, 17, 687. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Das, S.; Baker, A. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surg. (Oxford) 2017, 35, 489–494. [Google Scholar] [CrossRef]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A Comprehensive Review of Advanced Biopolymeric Wound Healing Systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Bianchera, A.; Catanzano, O.; Boateng, J.; Elviri, L. The Place of Biomaterials in Wound Healing. In Therapeutic Dressings and Wound Healing Applications; Wiley: New York, NY, USA, 2020; pp. 337–366. [Google Scholar]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2013, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Anil, S.-K.; Kim, M.S. Shim Seaweed polysaccharide-based nanoparticles: Preparation and applications for drug delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef]
- Annabi, N.; Rana, D.; Sani, E.S.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef]
- Mishra, S.; Sharma, S.; Javed, M.N.; Pottoo, F.H.; Barkat, M.A.; Alam, S.; Amir, M.; Sarafroz, M. Bioinspired Nanocomposites: Applications in Disease Diagnosis and Treatment. Pharm. Nanotechnol. 2019, 7, 206–219. [Google Scholar] [CrossRef]
- Lokhande, G.; Carrow, J.K.; Thakur, T.; Xavier, J.R.; Parani, M.; Bayless, K.J.; Gaharwar, A.K. Nanoengineered injectable hydrogels for wound healing application. Acta Biomater. 2018, 70, 35–47. [Google Scholar] [CrossRef]
- Lee, E.J.; Huh, B.K.; Na Kim, S.; Lee, J.Y.; Park, C.G.; Mikos, A.G.; Bin Choy, Y. Application of materials as medical devices with localized drug delivery capabilities for enhanced wound repair. Prog. Mater. Sci. 2017, 89, 392–410. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Nooshabadi, V.T.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. Part A 2019, 108, 545–556. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Rupérez, P.; Gómez-Ordóñez, E.; Jiménez-Escrig, A. Biological Activity of Algal Sulfated and Nonsulfated Polysaccharides. Bioactive Compounds from Marine Foods 2013, 11, 219–247. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Boil. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Stößlein, S.; Grunwald, I.; Stelten, J.; Hartwig, A. In-situ determination of time-dependent alginate-hydrogel formation by mechanical texture analysis. Carbohydr. Polym. 2019, 205, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.-I.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Thomas, S. Alginate dressings in surgery and wound management — part 1. J. Wound Care 2000, 9, 56–60. [Google Scholar] [CrossRef]
- Sudarsan, S.; Franklin, D.S.; Guhanathan, S. Imbibed salts and pH-responsive behaviours of sodium-alginate based eco-friendly biopolymeric hydrogels-A solventless approach. MMAIJ. 2015, 11, 24–29. [Google Scholar]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles- a review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2015, 57, 1133–1152. [Google Scholar] [CrossRef]
- Cardoso, M.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Saarai, A.; Sedlacek, T.; Kašpárková, V.; Kitano, T.; Saha, P. On the characterization of sodium alginate/gelatine-based hydrogels for wound dressing. J. Appl. Polym. Sci. 2012, 126, E79–E88. [Google Scholar] [CrossRef]
- Singh, R.; Singh, D. Radiation synthesis of PVP/alginate hydrogel containing nanosilver as wound dressing. J. Mater. Sci. Mater. Electron. 2012, 23, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Xing, N.; Tian, F.; Yang, J.; Li, Y.K., II. Characterizations of Alginate-Chitosan Hydrogel for Wound Dressing Application. Adv. Mater. Res. 2012, 490, 3124–3128. [Google Scholar] [CrossRef]
- Straccia, M.C.; d’Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate hydrogels for wound dressing. Mar. Drugs. 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [PubMed]
- Algicell® Ag Antimicrobial Alginate Dressing. Available online: http://www.woundsource.com/product/algicell-ag-antimicrobial-alginate-dressing (accessed on 13 December 2017).
- Algidex, Ag. Available online: https://www.deroyal.com›catalog-item-preview (accessed on 13 December 2017).
- Andreev, D.Y.; Paramonov, B.A.; Mukhtarova, A.M. Modern wound dressing. Vestnik ckirurgii. 2009, 168, 98–102. [Google Scholar]
- AlgiSite M™. Available online: http://www.smith-nephew.com/professional/products/advanced-wound-management/algisite-m/ (accessed on 13 December 2017).
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.; Pile, J.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 infectious diseases society of America Clinical Practice guidelines for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012, 54, 132–173. [Google Scholar] [CrossRef]
- Algivon. Available online: http://www.advancis.co.uk/products/activon-manuka-honey/algivon (accessed on 13 December 2017).
- Amerx. Available online: https://www.woundsource.com/company/amerx-health-care-corp (accessed on 13 December 2017).
- Biatain. Available online: https://www.coloplast.us/biatain-non-adhesive-en-us.aspx. (accessed on 13 December 2017).
- Udovichenko, O.V.; Bublik, E.V. Use of Biatain dressing material in patients with diabetic foot syndrome: Randomized comparative study. Diabetes Mellitus 2009, 1, 18–21. [Google Scholar] [CrossRef][Green Version]
- Pogorelov, A.G.; Gavrilyuk, V.B.; Pogorelova, V.N.; Gavrilyuk, B.K. Scanning electron microscopy of wound dressings made of biosynthetic materials of the Biokol. Bull. Exp. Biol. Med. 2012, 3, 176–180. [Google Scholar] [CrossRef]
- CalciCare. Available online: https://www.woundsource.com/print/product/calcicare-calcium-alginate (accessed on 13 December 2017).
- SMTL Dressings Datacard. Available online: http://www.dressings.org/Dressings/comfeel-plus.html (accessed on 13 December 2017).
- Disa, J.J.; Alizadeh, K.; Smith, J.W.; Hu, Q.-Y.; Cordeiro, P.G. Evaluation of a Combined Calcium Sodium Alginate and Bio-occlusive Membrane Dressing in the Management of Split-Thickness Skin Graft Donor Sites. Ann. Plast. Surg. 2001, 46, 405–408. [Google Scholar] [CrossRef]
- CovaWound. Available online: https://www.covalon.co.uk/ (accessed on 13 December 2017).
- DermaGinate. Available online: http://dermarite.com/product/dermaginate/ (accessed on 13 December 2017).
- ExcelGinate. Available online: https://www.woundsource.com/product/excelginate (accessed on 13 December 2017).
- Fibracol™ Plus Collagen Wound Dressing with Alginate. Available online: http://www.woundsource.com/product/fibracol-plus-collagen-wound-dressing-alginate (accessed on 13 December 2017).
- Guardix-SG. Available online: https://www.cphi-online.com/guardixsg-prod594275.html (accessed on 13 December 2017).
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek-Skibińska, P.; Winnicka, K.; Pucił Owska, A.; Szymań Ska, E. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Hyalogran® Biodegradable Wound Dressing. Available online: http://www.anikatherapeutics.com/products/dermal/hyalogran/ (accessed on 13 December 2017).
- Kaltostat Calcium Sodium Alginate Dressing. Available online: https://fsastore.com/KALTOSTAT-Calcium-Sodium-Alginate-Dressing-3-x-4-34-Box-of-10-P23356.aspx (accessed on 13 December 2017).
- Kendall. Available online: https://www.cardinalhealth.com.br/alginate-dressings/kendall-calcium-alginate-dressings.html (accessed on 13 December 2017).
- Luofucon Extra Silver Alginate Dressing. Available online: https://foryoumedical.en.made-in-china.com/ (accessed on 13 December 2017).
- Maxorb® ES. Available online: https://www.woundsource.com/product/maxorb-es (accessed on 13 December 2017).
- Melgisorb. Available online: https://www.molnlycke.com/products-solutions/melgisorb-plus/ (accessed on 13 December 2017).
- Nu-derm. Available online: https://www.woundsource.com›product›nu-derm-hydrocolloid-dressing (accessed on 13 December 2017).
- Restore. Available online: https://www.hollister.com/en/products/wound-care-products/wound-dressings/restore-calcium-alginate-dressing (accessed on 13 December 2017).
- SeaSorb, Ag. Available online: https://www.medbis.nl/framework/modules/Catalog/media/products/SeaSorb_Ag-datablad.pdf (accessed on 13 December 2017).
- Sorbalgon. Available online: https://www.hartmann.info/en-us/our-products/wound-management/hydroactive-wound-dressings/alginates/sorbalgon%C2%AE#products (accessed on 13 December 2017).
- Sorbsan Flat. Available online: http://www.aspenmedicaleurope.com/specialist_wound_car/sorbsan-flat/ (accessed on 13 December 2017).
- Suprasorb. Available online: https://www.lohmann-rauscher.com/us-en/products/wound-care/modern-wound-care/suprasorb-f/ (accessed on 13 December 2017).
- Moura, L.; Dias, A.M.A.; Carvalho, E.; De Sousa, H.C.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef]
- O’Meara, S.; James, M.M. Alginate dressings for venous leg ulcers. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Tromboguard®. Available online: http://matopat.ro/wp-content/uploads/sites/2/2013/12/tromboguard-leaflet.pdf (accessed on 13 December 2017).
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and Aloe vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Liakos, I.L.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef]
- Fu, R.; Li, C.; Yu, C.; Xie, H.; Shi, S.; Li, Z.; Wang, Q.; Lu, L. A novel electrospun membrane based on moxifloxacin hydrochloride/poly (vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Deliv. 2016, 23, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Türe, H. Characterization of hydroxyapatite-containing alginate–gelatin composite films as a potential wound dressing. Int. J. Boil. Macromol. 2019, 123, 878–888. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Hegge, A.B.; Andersen, T.; Melvik, J.; Bruzell, E.; Kristensen, S.; Tønnesen, H. Formulation and Bacterial Phototoxicity of Curcumin Loaded Alginate Foams for Wound Treatment Applications: Studies on Curcumin and Curcuminoides XLII. J. Pharm. Sci. 2011, 100, 174–185. [Google Scholar] [CrossRef]
- Valerón Bergh, V.J.; Johannessen, E.; Andersen, T.; Hjorth Hanne Tønnesen, H.H. Evaluation of porphyrin loaded dry alginate foams containing poloxamer 407 and β-cyclodextrin-derivatives intended for wound treatment. Pharm. Dev. Technol. 2018, 23, 1–36. [Google Scholar]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun Nanofibers as Dressings for Chronic Wound Care: Advances, Challenges, and Future Prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Gargallo, V.A.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart Dressings Based on Nanostructured Fibers Containing Natural Origin Antimicrobial, Anti-Inflammatory, and Regenerative Compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- Hu, C.; Gong, R.H.; Zhou, F.-L. Electrospun Sodium Alginate/Polyethylene Oxide Fibers and Nanocoated Yarns. Int. J. Polym. Sci. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate-lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef]
- Matthews, K.; Stevens, H.; Auffret, A.; Humphrey, M.; Eccleston, G. Lyophilised wafers as a drug delivery system for wound healing containing methylcellulose as a viscosity modifier. Int. J. Pharm. 2005, 289, 51–62. [Google Scholar] [CrossRef]
- Boateng, J.S.; Burgos-Amador, R.; Okeke, O.; Pawar, H. Composite alginate and gelatin based bio-polymeric wafers containing silver sulfadiazine for wound healing. Int. J. Boil. Macromol. 2015, 79, 63–71. [Google Scholar] [CrossRef]
- Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.; et al. Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics 2020, 12, 120. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Jahangi, M.A.; Saleem, M.A.; Kazmi, I.; Bhavani, P.D.; Muheem, A. Formulation and Evaluation of Fucidin Topical Gel Containing Wound Healing Modifiers. Am. J. Pharm.Tech. Res. 2015, 5, 232–242. [Google Scholar]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently polysaccharide-based alginate/chitosan hydrogel embedded alginate microspheres for BSA encapsulation and soft tissue engineering. Int. J. Boil. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-S.; Park, J.-H.; Shin, U.S.; Kim, H.-W. Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering. Acta Biomater. 2011, 7, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Kalvadia, P.; Patel, H.; Shah, R.; Gupta, A.; Shah, D. Mucoadhesive microcapsules of amoxicillin trihydrate for effective treatment of H. pylori. Der Pharmacia Sinica. 2014, 5, 45–55. [Google Scholar]
- Adebisi, A.O.; Conway, B.R. Preparation and characterisation of gastroretentive alginate beads for targeting H. pylori. J. Microencapsul. 2013, 31, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gowda, D.V.; Fredric, S.; Srivastava, A.; Osmani, R.A. Design and development of antimicrobial wafers for chronic wound healing. Pharm. Lett. 2016, 8, 70–79. [Google Scholar]
- Pawar, H.V.; Boateng, J.S.; Ayensu, I.; Tetteh, J. Multifunctional Medicated Lyophilised Wafer Dressing for Effective Chronic Wound Healing. J. Pharm. Sci. 2014, 103, 1720–1733. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, Y.-Y.; Sun, T.-W.; Qi, C.; Zhao, H.; Chen, F.; Shi, Z.; Zhu, Y.-J.; Chen, D.; He, Y. Design of a novel wound dressing consisting of alginate hydrogel and simvastatin-incorporated mesoporous hydroxyapatite microspheres for cutaneous wound healing. RSC Adv. 2016, 6, 104375–104387. [Google Scholar] [CrossRef]
- Mohandas, A.; Raja, B.; Lakshmanan, V.K.; Jayakumar, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10, 53–66. [Google Scholar] [CrossRef]
- Yu, C.-C.; Chang, J.-J.; Lee, Y.-H.; Lin, Y.-C.; Wu, M.-H.; Yang, M.-C.; Chien, C.-T. Electrospun scaffolds composing of alginate, chitosan, collagen and hydroxyapatite for applying in bone tissue engineering. Mater. Lett. 2013, 93, 133–136. [Google Scholar] [CrossRef]
- Nakaoka, R.; Hirano, Y.; Mooney, D.J.; Tsuchiya, T.; Matsuoka, A. Study on the potential of RGD- and PHSRN-modified alginates as artificial extracellular matrices for engineering bone. J. Artif. Organs 2013, 16, 284–293. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.-K. Chitosan-Alginate Biocomposite Containing Fucoidan for Bone Tissue Engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, E.V.; Fedotov, A.; E Mamonov, V.; Komlev, V.; Panteleyev, A.A. Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomed. Mater. 2018, 13, 025007. [Google Scholar] [CrossRef]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process. Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Boil. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyagintseva, T.N.; Maluarenko, O.S.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from brown alga Fucus evanescens: Structure and biological activit. Front. Mar. Sci. 2017, 10, 3389. [Google Scholar] [CrossRef]
- Prokofjeva, M.M.; Imbs, T.I.; Shevchenko, N.M.; Spirin, P.V.; Horn, S.; Fehse, B.; Zvyagintseva, T.N.; Prassolov, V.S. Fucoidans as Potential Inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Jeong, M.-R.; Choi, S.-M.; Na, S.-S.; Cha, J.-D. Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Arch. Oral Boil. 2013, 58, 482–492. [Google Scholar] [CrossRef]
- Purnama, A.; Aid-Launais, R.; Haddad, O.; Maire, M.; Mantovani, D.; Letourneur, D.; Hlawaty, H.; Le Visage, C.; Maire, M. Fucoidan in a 3D scaffold interacts with vascular endothelial growth factor and promotes neovascularization in mice. Drug Deliv. Transl. Res. 2013, 5, 187–197. [Google Scholar] [CrossRef]
- Obluchinsksya, E.D.; Makarova, M.N.; Pozharitskaya, O.N.; Shikov, А.N. 0Effects of Ultrasound Treatment on the Chemical Composition and Anticoagulant Properties of Dry Fucus Extract. Pharm. Chem. J. 2015, 49, 183–186. [Google Scholar] [CrossRef]
- Pomin, V.H. Marine Non-Glycosaminoglycan Sulfated Glycans as Potential Pharmaceuticals. Pharmaceuticals 2015, 8, 848–864. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Marinval, N.; Saboural, P.; Haddad, O.; Maire, M.; Bassand, K.; Geinguenaud, F.; Djaker, N.; Ben Akrout, K.; De La Chapelle, M.L.; Robert, R.; et al. Identification of a Pro-Angiogenic Potential and Cellular Uptake Mechanism of a LMW Highly Sulfated Fraction of Fucoidan from Ascophyllum nodosum. Mar. Drugs 2016, 14, 185. [Google Scholar] [CrossRef]
- Park, J.-H.; Choi, S.-H.; Park, S.-J.; Lee, Y.J.; Park, J.H.; Song, P.H.; Cho, C.-M.; Ku, S.K.; Song, C.-H. Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model. Mar. Drugs 2017, 15, 112. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective Effect of Sulfated Polysaccharides from Celluclast-Assisted Extract of Hizikia fusiforme Against Ultraviolet B-Induced Skin Damage by Regulating NF-κB, AP-1, and MAPKs Signaling Pathways In Vitro in Human Dermal Fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Ben Slima, S.; Trabelsi, I.; Ktari, N.; Bardaa, S.; Elkaroui, K.; Bouaziz, M.; Abdeslam, A.; Ben Salah, R. Novel Sorghum bicolor (L.) seed polysaccharide structure, hemolytic and antioxidant activities, and laser burn wound healing effect. Int. J. Boil. Macromol. 2019, 132, 87–96. [Google Scholar] [CrossRef]
- Matou, S.; Helley, D.; Chabut, D.; Bros, A.; Fischer, A.-M. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb. Res. 2002, 106, 213–221. [Google Scholar] [CrossRef]
- O’Leary, R.; Rerek, M.; Wood, E.J. Fucoidan modulates the effect of transforming growth factor (TGF)-beta1 on fibroblast proliferation and wound repopulation in in vitro models of dermal wound repair. Boil. Pharm. Bull. 2004, 27, 266–270. [Google Scholar] [CrossRef]
- Song, Y.S.; Li, H.; Balcos, M.C.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S.; Choi, H.-R.; Park, K.-C.; Kim, D.-S. Fucoidan Promotes the Reconstruction of Skin Equivalents. Korean J. Physiol. Pharmacol. 2014, 18, 327–331. [Google Scholar] [CrossRef]
- Changotade, S.I.T.; Korb, G.; Bassil, J.; Barroukh, B.; Willig, C.; Colliec-Jouault, S.; Durand, P.; Godeau, G.; Senni, K. Potential effects of a low-molecular-weight fucoidan extracted from brown algae on bone biomaterial osteoconductive properties. J. Biomed. Mater. Res. Part A 2008, 87, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kang, H.-J.; Park, J.-Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, e128. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-A.; Hung, Y.-L.; Phan, N.N.; Hieu, B.-T.-N.; Chang, P.-M.; Li, K.-L.; Lin, Y.-C. The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology 2015, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, L.; Li, X.; Jia, J.; Zhang, Y.; Sun, X.; Ma, J.; Liu, Z.; Ma, X. Low-molecular weight fucoidan inhibits the differentiation of osteoclasts and reduces osteoporosis in ovariectomized rats. Mol. Med. Rep. 2016, 15, 890–898. [Google Scholar] [CrossRef]
- Carson, M.A.; Clarke, S.A. Bioactive Compounds from Marine Organisms: Potential for Bone Growth and Healing. Mar. Drugs 2018, 16, 340. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, R.Y.; Chen, J.Y.; Chen, J.K. Biphasic release of gentamycin from chitosan/fucoidan nanoparticles for pulmonary delivery. Carbohydr. Polym. 2016, 138, 114–122. [Google Scholar]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Hatıpoğlu, F.; Oğurtan, Z.; Bas, A.L.; Akbuğa, J.; Hatipoglu, F. Preparation of Fucoidan-Chitosan Hydrogel and Its Application as Burn Healing Accelerator on Rabbits. Boil. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef]
- Murakami, K.; Ishihara, M.; Aoki, H.; Nakamura, S.; Nakamura, S.-I.; Yanagibayashi, S.; Takikawa, M.; Kishimoto, S.; Yokoe, H.; Kiyosawa, T.; et al. Enhanced healing of mitomycin C-treated healing-impaired wounds in rats with hydrosheets composed of chitin/chitosan, fucoidan, and alginate as wound dressings. Wound Repair Regen. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Yanagibayashi, S.; Kishimoto, S.; Ishihara, M.; Murakami, K.; Aoki, H.; Takikawa, M.; Fujita, M.; Sekido, M.; Kiyosawa, T. Novel hydrocolloid-sheet as wound dressing to stimulate healing-impaired wound healing in diabetic db/db mice. Bio-Medical Mater. Eng. 2012, 22, 301–310. [Google Scholar] [CrossRef]
- Pielesz, A. Temperature-dependent FTIR spectra of collagen and protective effect of partially hydrolysed fucoidan. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2014, 118, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Feki, A.; Bardaa, S.; Hajji, S.; Ktari, N.; Hamdi, M.; Chabchoub, N.; Kallel, R.; Boudawara, T.; Nasri, M.; Ben Amara, I. Falkenbergia rufolanosa polysaccharide - Poly(vinyl alcohol) composite films: A promising wound healing agent against dermal laser burns in rats. Int. J. Boil. Macromol. 2019, 144, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nambu, M.; Ishizuka, T.; Hattori, H.; Kanatani, Y.; Takase, B.; Kishimoto, S.; Amano, Y.; Aoki, H.; Kiyosawa, T.; et al. Effect of controlled release of fibroblast growth factor-2 from chitosan/fucoidan micro complex-hydrogel on in vitro and in vivo vascularization. J. Biomed. Mater. Res. A. 2008, 85, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lowe, B.; Venkatesan, J.; Anil, S.; Shim, M.S.; Kim, S.-K. Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Boil. Macromol. 2016, 93, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Sangha, J.S.; Ravichandran, S.; Prithiviraj, K.; Critchley, A.T.; Prithiviraj, B. Sulfated macroalgal polysaccharides λ-carrageenan and ι-carrageenan differentially alter Arabidopsis thaliana resistance to Sclerotinia sclerotiorum. Physiol. Mol. Plant Pathol. 2010, 75, 38–45. [Google Scholar] [CrossRef]
- Shen, Y.-R.; Kuo, M.-I. Effects of different carrageenan types on the rheological and water-holding properties of tofu. LWT 2017, 78, 122–128. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Torres, M.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. Environ. Boil. Fishes 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Jaballi, I.; Sellem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Ben Amara, I. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Boil. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef]
- Wurm, F.; Pham, T.; Bechtold, T. Modelling of phase separation of alginate-carrageenan gels based on rheology. Food Hydrocoll. 2019, 89, 765–772. [Google Scholar] [CrossRef]
- Prasad, K.; Kaneko, Y.; Kadokawa, J.I. Novel gelling systems of κ-, ι-and λ-carrageenans and their composite gels with cellulose using ionic liquid. Macromol. Biosci. 2009, 9, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.S.; Chellappa, N.; Fathima, N.N. Gelatin–carrageenan hydrogels: Role of pore size distribution on drug delivery process. Colloids Surfaces B: Biointerfaces 2014, 113, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, B.; Qian, Y.; Wang, Q.; Han, R.; Hao, T.; Shu, Y.; Zhang, Y.; Yao, F.; Wang, C. Iota-carrageenan/chitosan/gelatin scaffold for the osteogenic differentiation of adipose-derived MSCsin vitro. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2014, 103, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, C.; Liu, Z.; Li, Q.; Yan, X.; Liu, Y.; Lu, W. Enhancement in bioavailability of ketorolac tromethamine via intranasal in situ hydrogel based on poloxamer 407 and carrageenan. Int. J. Pharm. 2014, 474, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hezaveh, H.; Muhamad, I.I. Impact of metal oxide nanoparticles on oral release properties of pH-sensitive hydrogel nanocomposites. Int. J. Boil. Macromol. 2012, 50, 1334–1340. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Balding, P.; Iii, R.G.; Spinks, G.M.; Panhuis, M.I.H. Printed ionic-covalent entanglement hydrogels from carrageenan and an epoxy amine. RSC Adv. 2014, 4, 38088–38092. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Makino, K.; Idenuma, R.; Murakami, T.; Ohshima, H. Design of a rate- and time-programming drug release device using a hydrogel: Pulsatile drug release from j-carrageenan hydrogel device by surface erosion of the hydrogel. Colloids Surf B: Biointerfaces 2001, 12, 355–359. [Google Scholar] [CrossRef]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell Delivery Systems Using Alginate–Carrageenan Hydrogel Beads and Fibers for Regenerative Medicine Applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef]
- Boateng, J.S.; Pawar, H.V.; Tetteh, J. Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. Int. J. Pharm. 2013, 441, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Hezaveh, H.; Muhamad, .I.I. Modification and swelling kinetic study of kappa-carrageenan-based hydrogel for controlled release study. J. Chin. Inst. Chem. Eng. 2013, 44, 182–191. [Google Scholar] [CrossRef]
- Guan, J.; Li, L.; Mao, S. Applications of Carrageenan in Advanced Drug Delivery. In Seaweed Polysaccharides; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 283–303. [Google Scholar]
- Akiyode, O.; Boateng, J.S. Composite Biopolymer-Based Wafer Dressings Loaded with Microbial Biosurfactants for Potential Application in Chronic Wounds. Polymers 2018, 10, 918. [Google Scholar] [CrossRef]
- Chudinova, Y.V.; Kurek, D.; Varlamov, V.P. Molecular Architecture of Natural Polysaccharide Based Thin Films. Solid State Phenom. 2016, 258, 358–361. [Google Scholar] [CrossRef]
- Rode, M.P.; Angulski, A.B.B.; Gomes, F.A.; Da Silva, M.M.; Jeremias, T.D.S.; De Carvalho, R.G.; Vieira, D.G.I.; Oliveira, L.F.C.; Maia, L.; Trentin, A.G.; et al. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Zamorano, C.; González-Ávila, M.; Díaz-Blas, G.; Smith, C.T.; González-Correa, C.; Garcia-Cancino, A. Increased anti-Helicobacter pylori effect of the probiotic Lactobacillus fermentum UCO-979C strain encapsulated in carrageenan evaluated in gastric simulations under fasting conditions. Food Res. Int. 2018, 121, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, I.I.; Fen, L.S.; Hui, N.H.; Mustapha, N.A. Genipin-cross-linked kappa-carrageenan/carboxymethyl cellulose beads and effects on beta-carotene release. Carbohydr. Polym. 2011, 83, 1207–1212. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. Part A 2009, 9999, 1265–1272. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Liu, H.; Zhang, Z.; Jam, M.; Dudeja, P.K.; Michel, G.; Linhardt, R.J.; Tobacman, J.K. Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds. J. Nutr. Biochem. 2009, 21, 906–913. [Google Scholar] [CrossRef]
- Popa, E.G.; Caridade, S.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Chondrogenic potential of injectable κ -carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J. Tissue Eng. Regen. Med. 2013, 9, 550–563. [Google Scholar] [CrossRef]
- Popa, E.G.; Reis, R.L.; Gomes, M.E. Seaweed polysaccharide-based hydrogels used for the regeneration of articular cartilage. Crit. Rev. Biotechnol. 2014, 35, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, X.; Xu, Q.; Lu, Y.; Zhang, Y.; Xia, H.; Lu, A.; Zhang, L. Rubbery Chitosan/Carrageenan Hydrogels Constructed through an Electroneutrality System and Their Potential Application as Cartilage Scaffolds. Biomacromolecules 2018, 19, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Khanbabaee, B.; Steuber, M.; Bhaw-Luximon, A.; Jonas, U.; Pietsch, U.; Jhurry, D.; Schönherr, H. κ-Carrageenan Enhances the Biomineralization and Osteogenic Differentiation of Electrospun Polyhydroxybutyrate and Polyhydroxybutyrate Valerate Fibers. Biomacromolecules 2017, 18, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, Y.W.; Jung, W.-K.; Oh, J.; Nam, S.Y. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef]
- Johari, N.S.C.; Aizad, S.; Zubairi, S.I. Efficacy Study of Carrageenan as an Alternative Infused Material (Filler) in Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Porous 3D Scaffold. Int. J. Polym. Sci. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Peña-Rodríguez, A.; Mawhinney, T.P.; Ricque-Marie, D.; Cruz-Suárez, L.E. Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chem. 2011, 129, 491–498. [Google Scholar] [CrossRef]
- Kopel, M.; Helbert, W.; Belnik, Y.; Buravenkov, V.; Herman, A.; Banin, E. New Family of Ulvan Lyases Identified in Three Isolates from the Alteromonadales Order*. J. Boil. Chem. 2016, 291, 5871–5878. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Du, C.; Mou, H.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef]
- Konasani, V.R.; Jin, C.; Karlsson, N.G.; Albers, E. Ulvan lyase from Formosa agariphila and its applicability in depolymerisation of ulvan extracted from three different Ulva species. Algal Res. 2018, 36, 106–114. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulate. Nat Prod Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Hou, L.; Qin, L.; He, M.; Li, W.; Mao, W. A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: Characteristics of its structure and antiviral activity. Carbohydr. Polym. 2020, 227, 115280. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Li, H.; Wang, P.; Du, C.; Ye, H.; Zuo, S.; Guan, H.; Wang, P. Structural characterization of ulvan extracted from Ulva clathrata assisted by an ulvan lyase. Carbohydr. Polym. 2019, 229, 115497. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Choi, D.J.; Pohl, R.; Na, Y.S.; Capek, P.; Lattová, E.; Taubner, T.; Choi, J.-W.; Lee, C.-W.; Park, J.K.; et al. Structural Features and Anti-coagulant Activity of the Sulphated Polysaccharide SPS-CF from a Green Alga Capsosiphon fulvescens. Mar. Biotechnol. 2015, 17, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Briseño, J.A.; Cruz-Suárez, L.E.; Sassi, J.-F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. Sulphated Polysaccharides from Ulva clathrata and Cladosiphon okamuranus Seaweeds both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453, 1–9. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Li, W.; Jiang, N.; Li, B.; Wan, M.; Chang, X.; Liu, H.; Zhang, L.; Yin, S.; Qi, H.; Liu, S. Antioxidant activity of purified ulvan in hyperlipidemic mice. Int. J. Boil. Macromol. 2018, 113, 971–975. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.; Dabaghian, E.H.; Surayot, U. Water-soluble polysaccharides from Ulva intestinalis: Molecular properties, structural elucidation and immunomodulatory activities. J. Food Drug Anal. 2017, 26, 599–608. [Google Scholar] [CrossRef]
- Morelli, A.; Chiellini, F. Ulvan as a New Type of Biomaterial from Renewable Resources: Functionalization and Hydrogel Preparation. Macromol. Chem. Phys. 2010, 211, 821–832. [Google Scholar] [CrossRef]
- Toskas, G.; Hund, R.-D.; Laourine, E.; Cherif, C.; Smyrniotopoulos, V.; Roussis, V. Nanofibers based on polysaccharides from the green seaweed Ulva Rigida. Carbohydr. Polym. 2011, 84, 1093–1102. [Google Scholar] [CrossRef]
- Alves, A.; Pinho, E.D.; Neves, N.M.; Sousa, R.; Reis, R.L. Processing ulvan into 2D structures: Cross-linked ulvan membranes as new biomaterials for drug delivery applications. Int. J. Pharm. 2012, 426, 76–81. [Google Scholar] [CrossRef]
- Kanno, K.; Akiyoshi, K.; Nakatsuka, T.; Watabe, Y.; Yukimura, S.; Ishihara, H.; Shin, N.; Kawasaki, Y.; Yano, D. Biocompatible Hydrogel from a Green Tide-Forming Chlorophyta. J. Sustain. Dev. 2012, 5, 38–45. [Google Scholar] [CrossRef]
- Yoshimura, T.; Hirao, N.; Fujioka, R. Preparation and Characterization of Biodegradable Hydrogels Based on Ulvan, a Polysaccharide from Green Seaweeds. Polym. Renew. Resour. 2016, 7, 33–41. [Google Scholar] [CrossRef]
- Vlachou, M.; Tragou, K.; Siamidi, A.; Kikionis, S.; Chatzianagnostou, A.-L.; Mitsopoulos, A.; Ioannou, E.; Roussis, V.; Tsotinis, A. Modified in vitro release of the chronobiotic hormone melatonin from matrix tablets based on the marine sulfated polysaccharide ulvan. J. Drug Deliv. Sci. Technol. 2018, 44, 41–48. [Google Scholar] [CrossRef]
- Kikionis, S.; Ioannou, E.; Toskas, G.; Roussis, V. Electrospun biocomposite nanofibers of ulvan/PCL and ulvan/PEO. J. Appl. Polym. Sci. 2015, 132, 42153. [Google Scholar] [CrossRef]
- Alves, A.; Duarte, A.R.C.; Mano, J.F.; Sousa, R.; Reis, R.L. PDLLA enriched with ulvan particles as a novel 3D porous scaffold targeted for bone engineering. J. Supercrit. Fluids 2012, 65, 32–38. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.; Reis, R.L. Processing of degradable ulvan 3D porous structures for biomedical applications. J. Biomed. Mater. Res. Part A 2012, 101, 998–1006. [Google Scholar] [CrossRef]
- Barros, A.; Alves, A.; Nunes, C.; Coimbra, M.A.; Pires, R.A.; Reis, R.L. Carboxymethylation of ulvan and chitosan and their use as polymeric components of bone cements. Acta Biomater. 2013, 9, 9086–9097. [Google Scholar] [CrossRef]
- Dash, M.; Samal, S.K.; Morelli, A.; Bartoli, C.; Declercq, H.A.; Douglas, T.E.L.; Dubruel, P.; Chiellini, F. Ulvan-chitosan polyelectrolyte complexes as matrices for enzyme induced biomimetic mineralization. Carbohydr. Polym. 2018, 182, 254–264. [Google Scholar] [CrossRef] [PubMed]


| Commercially Available Alginate-Based Wound Dressings | Composition | Applications | Links |
|---|---|---|---|
| Algicell™ Algicell® (Integra LifeSciences Corp.) | Sodium alginate, 1.4% silver dressings | To heavily exudative partial or full-thickness wounds. Diabetic foot ulcer, leg ulcers, pressure ulcers, donor sites, and traumatic and surgical wounds. | [37] |
| Algidex Ag® Gel (DeRoyal) Algidex Ag® Foam (DeRoyal) | A combination of ionic silver, alginate and maltodextrin dressings | Venous ulcers, pressure ulcers (stages 1–4), dermal lesions (or secreting skin injuries), second-degree burns or donor sites. Provides immediate and sustained antimicrobial activity. | [38] |
| Algimaf (Palma Co., Ltd., Russia) Algipor (Palma Co., Ltd., Russia) | Porous sheets of lyophilized gel based on sodium alginate, calcium gluconate, mafenide acetate and phenosanoic acid Porous sheets of lyophilized gel based on sodium alginate, calcium gluconate, mafenide acetate and phenosanoic acid | Superficial burns of the II–III degree, long healing ulcers and wounds in the I phase of wound healing, trophic ulcers during the formation of foci of necrosis. Superficial burns of the II–IIIA degree, sluggish wounds, trophic ulcers, bedsores, long healing wounds, deep caries and pulpitis. | [39] |
| AlgiSite M™ (Smith and Nephew, Inc.) | Fast-gelling calcium alginate dressing | Leg ulcers, pressure ulcers, diabetic foot ulcers, surgical wounds. | [40,41] |
| Algivon® (Advancis Medical UK) | Calcium alginate dressing impregnated with 100% Manuka honey dressings | Necrotic wounds, wounds with odors, dorsal and plantar superficial ulcers. | [42] |
| Amerx® (Amerx Health Care Corp.) | Calcium alginate dressings | Heavy exudating chronic and acute wounds. | [43] |
| Biatain™ Biatain® (Coloplast Corp.) | Fast-gelling alginate and sodium carboxymethyl cellulose dressings | Wounds with severe exudation, including lower limb ulcers, pressure sores, diabetic ulcers and second-degree burns. Biatain alginate dressing in the form of a tape is used to treat deep wounds. | [44,45] |
| Biokol (Co., Ltd. Biokol, Russia) | Hydrocolloid monolayer composite film consists of 2 polymers: natural (a mixture of carrageenan, sodium or calcium alginate and methyl cellulose) and a synthetic fluorine-containing polymer (copolymer of vinyldenfluoride with hexafluoropropylene) | Residual wounds after extensive burns, skin graft protection, protection of donor sites for burns and plastic surgeries, protection of excised wound surfaces, trophic ulcers. | [46] |
| CalciCare™ (Hollister Incorporated) | Calcium alginate dressings (high guluronic acid calcium/sodium alginate), strengthening nylon web | To heavily exudating wounds, infected wounds, venous leg ulcers. | [47] |
| Comfeel Plus™ Comfeel Sea-Sorb (Coloplast) | Sodium carboxymethylcellulose and calcium alginate dressings | Ulcers such as venous leg ulcers, pressure ulcers, burns, donor sites, postoperative wounds and necrotic wounds. | [48,49] |
| CovaWound™ (Covalon Technologies, Ltd.) | Alginate dressings (calcium salt of alginic acid riched by mannuronic acid) | Heavily exuding wounds like partial-thickness burns, donor sites, leg, pressure, arterial, diabetic and venous stasis ulcers, cavity wounds, post-surgical incisions, trauma wounds, and most other granulating wounds. | [41,50] |
| DermaGinate™ (DermaRite Industries, LLC) (Dynarex) | Calcium alginate dressings | Acute or chronic partial- to full-thickness wounds with moderate to heavy exudate such as: pressure ulcers, diabetic ulcers, post-operative wounds, trauma wounds, leg ulcers, grafts and donor site. Can absorb up to 17 times its own weight. | [51] |
| ExcelGinate™ (MPM Medical, Inc.) | Calcium alginate dressings | Partial- to full-thickness wounds with moderate to heavy drainage. | [52] |
| Fibracol™Plus (Systagenix) | Calcium alginate (10%) and collagen (90%) dressings | Diabetic ulcers, pressure ulcers, venous ulcers, full-thickness and partial-thickness wounds, second-degree burns, abrasions, ulcers caused by mixed vascular etiologies, donor sites and other bleeding surface wounds, traumatic wound healing by secondary intention, dehisced surgical incisions. | [53] |
| Guardix-SG® (Hanmi Pharm. Co. Ltd.) | Sodium alginate and poloxamer dressings | To prevent post-operative adhesions in thyroid and spine surgeries. | [54,55] |
| Hyalogran® (Haemo Pharma) | An ester of hyaluronic acid and sodium alginate, microgranulate material | Ulcers, diabetic wounds, pressure sores, ischemic, necrotic wounds. | [56] |
| Kaltostat™ Kaltostat® (ConvaTec) | Sodium alginate dressings | Pressure ulcers, venous ulcers, diabetic ulcers, donor sites, and traumatic wounds exuding cavity wounds such as pressure injuries, venous stasis ulcers, arterial ulcers, diabetic ulcers, lacerations, post-surgical wounds and other external wounds inflicted by trauma | [27,57] |
| Kendall™ (Cardinal Health) | Calcium alginate dressings with added benefit of zinc | Arterial, diabetic, pressure and venous insufficiency ulcers, donor sites, abrasions, lacerations and skin tears, partial-thickness (second-degree) burns, deep and tunneling wounds. | [58] |
| Luofucon® Extra Silver (Huizhou Foryou Medical Devices Co., Ltd.) | Antibacterial silver alginate dressings | Heavily exuding wound, venous/arterial leg ulcer, diabetic ulcer, pressure ulcer, donor sites, abrasions, lacerations and post-surgical wound. | [59] |
| Maxorb® ES (Medline Industries, Inc.) Maxorb® Extra (Medline Industries, Inc.) Maxorb® II (Medline Industries, Inc.) | Reinforced CMC/calcium alginate ribbon dressings Calcium alginate and sodium carboxymethyl- cellulose fibers An ultra-absorptive alginate dressing | Pressure injuries, partial- and full-thickness wounds, diabetic ulcers, leg ulcers of various etiologies, surgical wounds, donor sites and first- and second-degree burns. | [41,60] |
| Melgisorb® Plus (Mölnlycke Health Care US, LLC) | Calcium alginate dressings | Heavily exuding partial- to full-thickness wounds, pressure, venous, arterial and diabetic ulcers, donor sites, post-operative wounds, dermal lesions and traumatic wounds. | [41,61] |
| Nu-derm™ (KCI-An Acelity Company) | High guluronic acid alginate and carboxymethylcellulose (CMC) fiber | Heavily exuding chronic wounds, pressure ulcers, leg ulcers, venous stasis ulcers, diabetic ulcers and arterial ulcers, and to control minor bleeding in superficial acute wounds (such as abrasions, lacerations, donor sites and postoperative wounds). | [41,62] |
| Restore® (Hollister Incorporated) | Calcium alginate dressings | Arterial, venous, diabetic and pressure (stage 1–4) ulcers; post-surgical incisions; donor sites; dermal lesions, trauma injuries, incisions or other trauma wounds; superficial (first-degree) and partial-thickness (second-degree) burns. Can also be used under compression bandages, and may also assist in supporting the control of minor bleeding in superficial wounds. | [63] |
| SeaSorb® (Coloplast) | Calcium alginate dressings | High exuding wounds, ulcers such as diabetic and leg pressure ulcers. | [55,64] |
| Sorbalgon® Sorbalgon T (Hartmann USA, Inc.) | Sodium and calcium alginate dressings | Arterial ulcers, diabetic foot ulcers, heavily draining wounds, pressure ulcers/injuries, surgical site infections, bariatric patients, superficial burns (I degree), deep partial-thickness (deep II degree) burns, full-thickness (III and IV degree), chronic wounds. Invasive for plugging into the wound (deep cavities and pockets). | [49,65] |
| Sorbsan™ (Unomedical) | Calcium alginate dressings | Arterial, venous, and diabetic leg ulcers, pressure ulcers, post-operative wounds, donor and graft sites and traumatic wounds. | [49,66] |
| Suprasorb® L&R USA, Inc. (Lohmann & Rauscher) | Calcium alginate dressings | Highly exuding acute and chronic wounds including pressure ulcers, venous ulcers, skin donor sites and post-operative wounds. | [67] |
| 3M™ Tegaderm™ (3M Corporate) | High gelling alginate dressings | Maintain a moist wound healing environment, provides a viral and bacterial barrier. | [68,69] |
| Tegagen™ (3M Corporate) | Sodium alginate dressings | Diabetic and infected wounds. | [70] |
| Tegagel™ (3M Corporate) | Alginate dressings | Skin graft donor sites, diabetic leg ulcers, pressure ulcers, post-operative wounds. | [49] |
| Tromboguard® (Tricomed en) | Sodium alginate, calcium alginate, chitosan, polyurethane and silver cations dressings | Postoperative wounds, traumatic wounds, gun shots, skin graft donor sites, bleeding from accidents. | [71] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N.; Zaporozhets, T.S.; Kalinin, A.V. Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering: A Review. J. Mar. Sci. Eng. 2020, 8, 481. https://doi.org/10.3390/jmse8070481
Kuznetsova TA, Andryukov BG, Besednova NN, Zaporozhets TS, Kalinin AV. Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering: A Review. Journal of Marine Science and Engineering. 2020; 8(7):481. https://doi.org/10.3390/jmse8070481
Chicago/Turabian StyleKuznetsova, Tatyana A., Boris G. Andryukov, Natalia N. Besednova, Tatyana S. Zaporozhets, and Andrey V. Kalinin. 2020. "Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering: A Review" Journal of Marine Science and Engineering 8, no. 7: 481. https://doi.org/10.3390/jmse8070481
APA StyleKuznetsova, T. A., Andryukov, B. G., Besednova, N. N., Zaporozhets, T. S., & Kalinin, A. V. (2020). Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering: A Review. Journal of Marine Science and Engineering, 8(7), 481. https://doi.org/10.3390/jmse8070481