The Effects of Glyphosate and Its Commercial Formulations to Marine Invertebrates: A Review
Abstract
1. Introduction
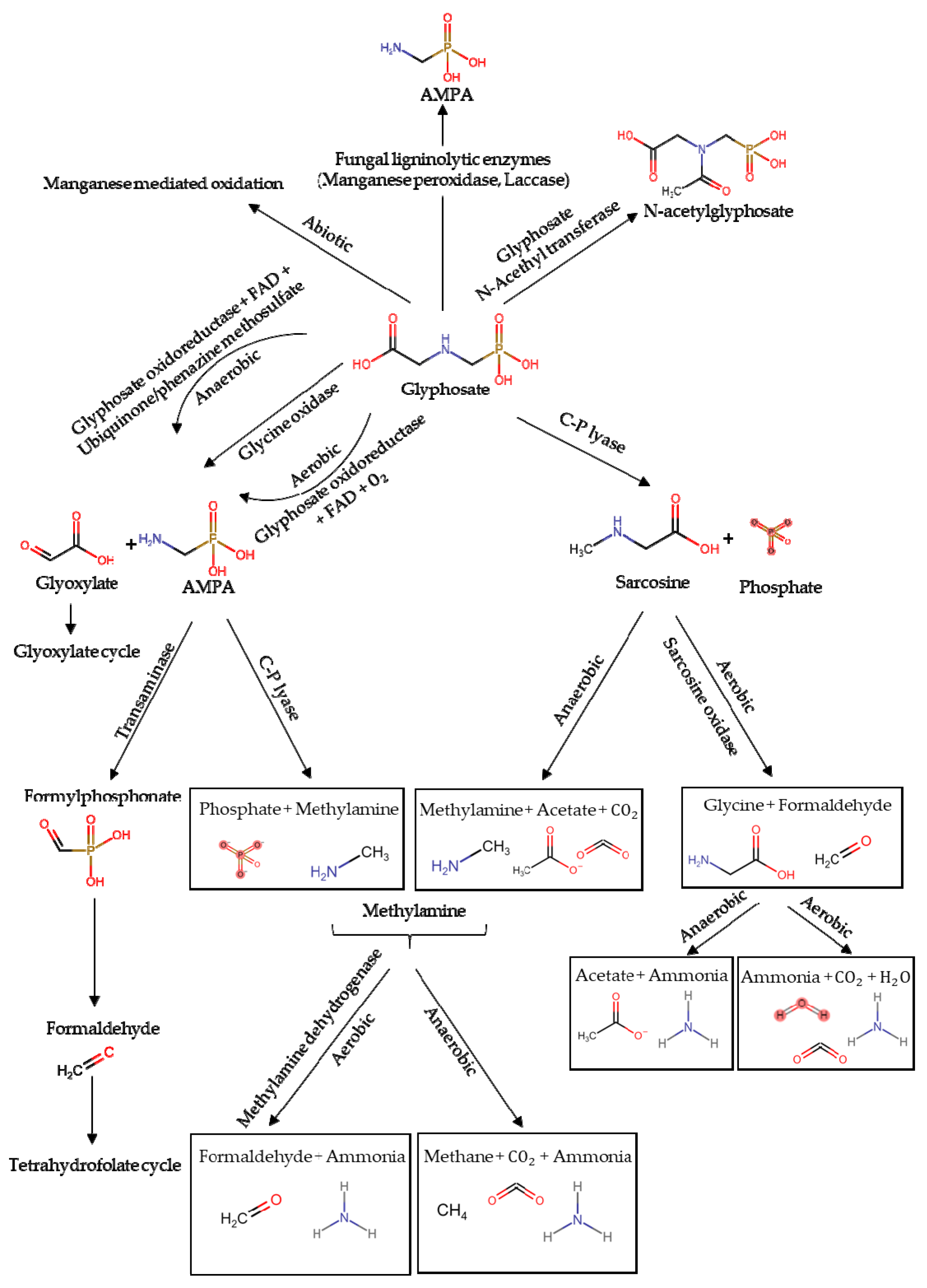
1.1. Environmental Fate of Glyphosate
1.2. Occurrence of Glyphosate in Aquatic Environments
2. The Effects of Glyphosate to Marine Invertebrates
2.1. Mollusks
2.2. Other Marine Invertebrates
3. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaner, L.D. Role of translocation as a mechanism of resistance to glyphosate. Weed Sci. 2009, 57, 118–123. [Google Scholar] [CrossRef]
- Saunders, L.E.; Pezeshki, R. Glyphosate in runoff waters and in the root-zone: A review. Toxics 2015, 3, 462–480. [Google Scholar] [CrossRef]
- Steinrücken, H.; Amrhein, N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvylshikimic acid-3-phosphate synthase. Biochem. Biophys. Res. Commun. 1980, 94, 1207–1212. [Google Scholar] [CrossRef]
- Schönbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.S.; Kabsch, W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. PNAS 2001, 98, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.; Höss, S.; Neumann, G.; Afzal, J.; Reichenbecher, W. Glyphosate, a chelating agent-relevant for ecological risk assessment? Environ. Sci. Pollut. Res. 2018, 25, 5298–5317. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B.; Chaney, R.L.; Hammerschmidt, R. Glyphosate effects on plant mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J. Agric. Food. Chem. 2012, 60, 10375–10397. [Google Scholar] [CrossRef]
- Herrmann, K.M. The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol. 1995, 107, 7–12. [Google Scholar] [CrossRef]
- Richards, T.A.; Dacks, J.B.; Campbell, S.A.; Blanchard, J.L.; Foster, P.G.; McLeod, R.; Roberts, C.W. Evolutionary origins of the eukaryotic shikimate pathway: Gene fusions, horizontal gene transfer, and endosymbiotic replacements. Eukaryot. Cell 2006, 5, 1517–1531. [Google Scholar] [CrossRef]
- Roberts, F.; Roberts, C.W.; Johnson, J.J.; Kyle, D.E.; Krell, T.; Coggins, J.R.; Coombs, G.H.; Milhous, W.K.; Tzipori, S.; Ferguson, D.J.; et al. Evidence for the shikimate pathway in apicomplexan parasites. Nature 1998, 393, 801–805. [Google Scholar] [CrossRef]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. Shikimate and phenylalanine biosynthesis in the green lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.; Tutus, Y.; Ozturk, L. Glyphosate reduced seed and leaf concentrations of calcium, manganese, magnesium, and iron in non-glyphosate resistant soybean. Eur. J. Agron. 2009, 31, 114–119. [Google Scholar] [CrossRef]
- Barrett, K.A.; McBride, M.B. Trace element mobilization in soils by glyphosate. Soil Sci. Soc. Am. J. 2006, 70, 1882–1888. [Google Scholar] [CrossRef]
- Eker, S.; Ozturk, L.; Yazici, A.; Erenoglu, B.; Romheld, V.; Cakmak, I. Foliar-applied glyphosate substantially reduced uptake and transport of iron and manganese in sunflower (Helianthus annuus L.) plant. J. Agric. Food Chem. 2006, 54, 10019–10025. [Google Scholar] [CrossRef] [PubMed]
- Johal, G.S.; Huber, D.M. Glyphosate effects on diseases of plants. Eur. J. Agron. 2009, 31, 144–152. [Google Scholar] [CrossRef]
- EPA, United States Environmental Protection Agency Washington, D.C., 20460. Glyphosate-Response to Comments Usage and Benefits–Final. 2019. Available online: https://www.epa.gov/sites/production/files/2019-04/documents/glyphosate-response-comments-usage-benefits-final.pdf (accessed on 1 May 2020).
- Rolando, C.A.; Baillie, B.R.; Thompson, D.G.; Little, K.M. The risks associated with glyphosate-based herbicide use in planted forests. Forests 2017, 8, 208. [Google Scholar] [CrossRef]
- Torstensson, L.; Börjesson, E.; Stenström, J. Efficacy and fate of glyphosate on Swedish railway embankments. Pest. Manag. Sci. 2005, 61, 881–886. [Google Scholar] [CrossRef]
- Tang, T.; Boënne, W.; Desmet, N.; Seuntjens, P.; Bronders, J.; van Griensven, A. Quantification and characterization of glyphosate use and loss in a residential area. Sci. Total Environ. 2015, 517, 207–214. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest. Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Valavanidis, A. Glyphosate, the Most Widely Used Herbicide. Health and Safety Issues. Why Scientists Differ in Their Evaluation of Its Adverse Health Effects. Scientific Reviews. 2018. Available online: www.chem-tox-ecotox.org/ScientificReviews (accessed on 1 May 2020).
- APVMA. Final Pesticide and Veterinary Medicines Product Sales 2016–2017; Commonwealth of Australia Gazette No APVMA 6; Australian Pesticides and Veterinary Medicines Authority: Canberra, Australia, 2018. Available online: https://apvma.gov.au/node/10756 (accessed on 26 May 2020).
- Travlos, I.; Cheimona, N.; Bilalis, D. Glyphosate Efficacy of Different Salt Formulations and Adjuvant Additives on Various Weeds. Agronomy 2017, 7, 60. [Google Scholar] [CrossRef]
- Hess, F.; Foy, C. Interaction of Surfactants with Plant Cuticles. Weed Technol. 2000, 14, 807–813. [Google Scholar] [CrossRef]
- Defarge, N.; Takács, E.; Lozano, V.L.; Mesnage, R.; Spiroux de Vendômois, J.; Séralini, G.E.; Székács, A. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int. J. Environ. Res. Public Health 2016, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Benbrook, C.; Antoniou, M.N. Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem. Toxicol. 2019, 128, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Baylis, A.D. Why glyphosate is a global herbicide: Strengths, weaknesses and prospects. Pest. Manag. Sci. 2000, 56, 299–308. [Google Scholar] [CrossRef]
- Monsanto. Backgrounder History of Monsanto’s Glyphosate Herbicides. 2002. Available online: http://www.bingeiagung.com/files/back_history.pdf (accessed on 31 May 2020).
- Grube, A.H.; Donaldson, D.; Kiely, T.; Wu, L. Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates; Off. Pestic. Programs, US Environ. Prot. Agency: Washington, DC, USA, 2011. [Google Scholar]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Richmond, M.E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci. 2018, 8, 416–434. [Google Scholar] [CrossRef]
- African Centre for Biosafety. Glyphosate in SA: Risky Pesticide at Large and Unregulated in Our Soil. 2015. Available online: https://www.acbio.org.za/en/glyphosate-sa-risky-pesticide-large-and-unregulated-our-soil-and-water (accessed on 31 May 2020).
- Maggi, F.; Tang, F.H.M.; la Cecilia, D.; McBratney, A. PEST-CHEMGRIDS, global gridded maps of the top 20 crop-specific pesticide application rates from 2015 to 2025. Sci. Data 2019, 6, 170. [Google Scholar] [CrossRef]
- Sørensen, S.R.; Schultz, A.; Jacobsen, O.S.; Aamand, J. Sorption, desorption and mineralisation of the herbicides glyphosate and MCPA in samples from two Danish soil and subsurface profiles. Environ. Pollut. 2006, 141, 184–194. [Google Scholar] [CrossRef]
- von Wirén-Lehr, S.; Komoßa, D.; Gläßgen, W.E.; Sandermann, H.; Scheunert, I., Jr. Mineralization of [14C] glyphosate and its plant-associated residues in arable soils originating from different farming systems. Pestic. Sci. 1997, 51, 436–442. [Google Scholar] [CrossRef]
- Helander, M.; Saloniemi, I.; Saikkonen, K. Glyphosate in northern ecosystems. Trends Plant Sci. 2012, 17, 56–574. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Gimsing, A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: A review. Pest. Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. Rev. Environ. Toxicol. 2000, 167, 35–120. [Google Scholar]
- Singh, B.K.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Glyphosate renewal assessment report of 18 December 2013. Rapporteur Member State (RMS): Germany, Co-RMS: Slovakia. 2013. Available online: http://www.pan-germany.org/download/Glyphosat-Studie_Campact_PAN_korrigiert.pdf (accessed on 31 May 2020).
- Bergström, L.; Börjesson, E.; Stenström, J. Laboratory and lysimeter studies of glyphosate and aminomethylphosphonic acid in a sand and a clay soil. J. Environ. Qual. 2011, 40, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, P.; Rämö, S.; Nikunen, U.; Jauhiainen, L.; Siimes, K.; Turtola, E. Glyphosate and phosphorus leaching and residues in boreal sandy soil. Plant Soil 2009, 323, 267–283. [Google Scholar] [CrossRef]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef]
- Vera, M.S.; Lagomarsino, L.; Sylvester, M.; Pérez, G.L.; Rodríguez, P.; Mugni, H.; Sinistro, R.; Ferraro, M.; Bonetto, C.; Zagarese, H.; et al. New evidences of Roundup (glyphosate formulation) impact on the periphyton community and the water quality of freshwater ecosystems. Ecotoxicology 2010, 19, 710–721. [Google Scholar] [CrossRef]
- Vera, M.S.; Di Fiori, E.; Lagomarsino, L.; Sinistro, R.; Escaray, R.; Iummato, M.M.; Juárez, A.; Ríos de Molina, M.; Tell, G.; Pizarro, H. Direct and indirect effects of the glyphosate formulation Glifosato Atanor® on freshwater microbial communities. Ecotoxicology 2012, 21, 1805–1816. [Google Scholar] [CrossRef]
- Pérez, G.L.; Torremorell, A.; Mugni, H.; Rodríguez, P.; Solange Vera, M.; do Nascimento, M.; Allende, L.; Bustingorry, J.; Escaray, R.; Ferraro, M.; et al. Effects of the herbicide Roundup on freshwater microbial communities: A mesocosm study. Ecol. Appl. 2007, 17, 2310–2322. [Google Scholar] [CrossRef]
- Goldsborough, L.G.; Beck, A.E. Rapid dissipation of glyphosate in small forest ponds. Arch. Environ. Contam. Toxicol. 1989, 18, 537–544. [Google Scholar] [CrossRef]
- Mallat, E.; Barceló, D. Analysis and degradation study of glyphosate and of aminomethylphosphonic acid in natural waters by means of polymeric and ion-exchange solid-phase extraction columns followed by ion chromatography-post-column derivatization with fluorescence detection. J. Chromatogr. A. 1998, 823, 129–136. [Google Scholar] [CrossRef]
- Pizarro, H.; Vera, M.S.; Vinocur, A.; Perez, G.; Ferraro, M.; Menendez Helman, R.J.; Dos Santos Afonso, M. Glyphosate input modifies microbial community structure in clear and turbid freshwater systems. Environ. Sci. Pollut. Res. 2016, 23, 5143–5153. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, P.; Flores, F.; Mueller, J.F.; Carter, S.; Negri, A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2014, 85, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Kryuchkova, Y.V.; Burygin, G.L.; Gogoleva, N.E.; Gogolev, Y.V.; Chernyshova, M.P.; Makarov, O.E.; Fedorov, E.E.; Turkovskaya, O.V. Isolation and characterization of a glyphosate-degrading rhizosphere strain, Enterobacter cloacae K7. Microbiol. Res. 2014, 169, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.A.; McBride, M.B. Oxidative degradation of glyphosate and aminomethylphosphonate by manganese oxide. Environ. Sci. Technol. 2005, 39, 9223–9228. [Google Scholar] [CrossRef]
- Paudel, P.; Negusse, A.; Jaisi, D.P. Birnessite-catalyzed degradation of glyphosate: A mechanistic study aided by kinetics batch studies and NMR spectroscopy. Soil Sci. Soc. Am. J. 2015, 79, 815–825. [Google Scholar] [CrossRef]
- Pollegioni, L.; Schonbrunn, E.; Siehl, D. Molecular basis of glyphosate resistance-different approaches through protein engineering. FEBS J. 2011, 278, 2753–2766. [Google Scholar] [CrossRef]
- la Cecilia, D.; Maggi, F. Analysis of glyphosate degradation in a soil microcosm. Environ. Pollut. 2018, 233, 201–207. [Google Scholar] [CrossRef]
- Sviridov, A.V.; Shushkova, T.V.; Zelenkova, N.F.; Vinokurova, N.G.; Morgunov, I.G.; Ermakova, I.T.; Leontievsky, A.A. Distribution of glyphosate and methylphosphonate catabolism systems in soil bacteria Ochrobactrum anthropi and Achromobacter sp. Appl. Microbiol. Biotechnol. 2012, 93, 787–796. [Google Scholar] [CrossRef]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Epiktetov, D.O.; Leont’evskii, A.A. Microbial Degradation of Glyphosate Herbicides (Review). Prikl. Biokhim. Mikrobiol. 2015, 51, 183–190. [Google Scholar]
- Hove-Jensen, B.; Zechel, D.L.; Jochimsen, B. Utilization of glyphosate as phosphate source: Biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol. Mol. Biol. R. 2014, 78, 176–197. [Google Scholar] [CrossRef] [PubMed]
- Shushkova, T.V.; Vinokurova, N.G.; Baskunov, B.P.; Zelenkova, N.F.; Sviridov, A.V.; Ermakova, I.T.; Leontievsky, A.A. Glyphosate acetylation as a specific trait of Achromobacter sp. Kg 16 physiology. Appl. Microbiol. Biotechnol. 2016, 100, 847–855. [Google Scholar] [CrossRef]
- Pizzul, L.; Castillo, M.D.P.; Stenström, J. Degradation of glyphosate and other pesticides by ligninolytic enzymes. Biodegradation 2009, 20, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Sprankle, P.; Meggitt, W.F.; Penner, D. Adsorption, mobility, and microbial degradation of glyphosate in the soil. Weed Sci. 1975, 23, 229–234. [Google Scholar] [CrossRef]
- Vereecken, H. Mobility and leaching of glyphosate: A review. Pest. Manag. Sci. 2005, 61, 1139–1151. [Google Scholar] [CrossRef]
- Yang, X.; Wang, F.; Bento, C.; Xue, S.; Gai, L.; van Dam, R.; Mol, H.; Ritsema, C.J.; Geissen, V. Short-term transport of glyphosate with erosion in Chinese loess soil–a flume experiment. Sci. Total Environ. 2015, 512–513, 406–414. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate: Peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 4302. [Google Scholar]
- Botta, F.; Lavison, G.; Couturier, G.; Alliot, F.; Moreau-Guigon, E.; Fauchon, N.; Guery, B.; Chevreuil, M.; Blanchoud, H. Transfer of glyphosate and its degradate AMPA to surface waters through urban sewerage systems. Chemosphere 2009, 77, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Peruzzo, P.J.; Porta, A.A.; Ronco, A.E. Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environ. Pollut. 2008, 156, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Mahler, B.J.; Van Metre, P.C.; Burley, T.E.; Loftin, K.A.; Meyer, M.T.; Nowell, L.H. Similarities and differences in occurrence and temporal fluctuations in glyphosate and atrazine in small Midwestern streams (USA) during the 2013 growing season. Sci. Total Environ. 2017, 579, 149–158. [Google Scholar] [CrossRef]
- Okada, E.; Allinson, M.; Barral, M.P.; Clarke, B.; Allinson, G. Glyphosate and aminomethylphosphonic acid (AMPA) are commonly found in urban streams and wetlands of Melbourne, Australia. Water Res. 2020, 168, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in U.S.; soils, surface water, groundwater, and precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Okada, E.; Pérez, D.; De Gerónimo, E.; Aparicio, V.; Massone, H.; Costa, J.L. Non-point source pollution of glyphosate and AMPA in a rural basin from the southeast Pampas, Argentina. Environ. Sci. Pollut. Res. 2018, 25, 15120–15132. [Google Scholar] [CrossRef] [PubMed]
- Hanke, I.; Singer, H.; Hollender, J. Ultratrace-level determination of glyphosate, aminomethylphosphonic acid and glufosinate in natural waters by solid-phase extraction followed by liquid chromatography-tandem mass spectrometry: Performance tuning of derivatization, enrichment and detection. Anal. Bioanal. Chem. 2008, 391, 2265–2276. [Google Scholar] [CrossRef]
- Scribner, E.A.; Battaglin, W.A.; Gilliom, R.J.; Meyer, M.T. Concentrations of Glyphosate, Its Degradation Product, Aminomethylphosphonic Acid, and Glufosinate in Ground- and Surface-Water, Rainfall, and Soil Samples Collected in the United States, 2001–2006; U.S. Geological Survey Scientific Investigations Report 2007–5122; U.S. Geological Survey: Reston, VA, USA, 2007; 111p. [Google Scholar]
- Kemp, W.M.; Twilley, R.R.; Stevenson, J.C.; Boynton, W.R.; Means, J.C. The decline of submerged vascular plants in upper Chesapeake Bay: Summary and results concerning possible causes. Mar. Technol. Soc. J. 1983, 2, 78–89. [Google Scholar]
- Burgeot, T.; Gagnaire, B.; Renault, T.; Haure, J.; Moraga, D.; David, E.; Boutet, I.; Sauriau, P.G.; Malet, N.; Bouchet, V.; et al. Oyster summer mortality risks associated with environmental stress. In Summer Mortality of Pacific Oyster Crassostrea Gigas; Samain, J.F., McCombie, H., Eds.; Editions Quæ: Versailles, France, 2007; pp. 107–151. [Google Scholar]
- Wang, S.; Liu, B.; Yuan, D.; Ma, J. A simple method for the determination of glyphosate and aminomethylphosphonic acid in seawater matrix with high performance liquid chromatography and fluorescence detection. Talanta 2016, 161, 700–706. [Google Scholar] [CrossRef]
- Skeff, W.; Neumann, C.; Schulz-Bull, D.E. Glyphosate and AMPA in the estuaries of the Baltic Sea method optimization and field study. Mar. Pollut. Bull. 2015, 100, 577–585. [Google Scholar] [CrossRef]
- Kaiser, K. Preliminary study of pesticide drift into the Maya Mountain protected areas of Belize. Bull. Environ. Contam. Toxicol. 2011, 86, 56–59. [Google Scholar] [CrossRef]
- Chang, F.C.; Simcik, M.F.; Capel, P.D. Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ. Toxicol. Chem. 2011, 30, 548–555. [Google Scholar] [CrossRef]
- Solomon, K.R.; Thompson, D.G. Ecological risk assessment for aquatic organisms from over-water uses of glyphosate. J. Toxicol. Env. Heal. B 2003, 6, 289–324. [Google Scholar] [CrossRef]
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest. Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Avigliano, E.; Schenone, N.F. Human health risk assessment and environmental distribution of trace elements, glyphosate, fecal coliform and total coliform in Atlantic Rainforest mountain rivers (South America). Microchem. J. 2015, 122, 149–158. [Google Scholar] [CrossRef]
- Castro Berman, M.; Marino, D.; Quiroga, M.V.; Zagarese, H. Occurrence and levels of glyphosate and AMPA in shallow lakes from the Pampean and Patagonian regions of Argentina. Chemosphere 2018, 200, 513–522. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Castro, R.; Rivero-Pérez, N.; Bello-Mendoza, R.; Sánchez, D. Occurrence of glyphosate in water bodies derived from intensive agriculture in a tropical region of southern Mexico. Bull. Environ. Contam. Toxicol. 2014, 93, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Masiol, M.; Giannì, B.; Prete, M. Herbicides in river water across the northeastern Italy: Occurrence and spatial patterns of glyphosate, aminomethylphosphonic acid, and glufosinate ammonium. Environ. Sci. Pollut. Res. 2018, 25, 24368–24378. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.J.; Okada, E.; De Gerónimo, E.; Menone, M.L.; Aparicio, V.C.; Costa, J.L. Spatial and temporal trends and flow dynamics of glyphosate and other pesticides within an agricultural watershed in Argentina. Environ. Toxicol. Chem. 2017, 36, 3206–3216. [Google Scholar] [CrossRef] [PubMed]
- Daouk, S.; Copin, P.J.; Rossi, L.; Chèvre, N.; Pfeifer, H.R. Dynamics and environmental risk assessment of the herbicide glyphosate and its metabolite AMPA in a small vineyard river of the Lake Geneva catchment. Environ. Toxicol. Chem. 2013, 32, 2035–2044. [Google Scholar] [CrossRef]
- Huntscha, S.; Stravs, M.A.; Bühlmann, A.; Ahrens, C.H.; Frey, J.E.; Pomati, F.; Hollender, J.; Buerge, I.J.; Balmer, M.E.; Poiger, T. Seasonal dynamics of glyphosate and AMPA in Lake Greifensee: Rapid microbial degradation in the epilimnion during summer. Environ. Sci. Technol. 2018, 52, 4641–4649. [Google Scholar] [CrossRef]
- Rzymski, P.; Klimaszyk, P.; Kubacki, T.; Poniedziałek, B. The effect of glyphosate-based herbicide on aquatic organisms—A case study. Limnol. Rev. 2013, 4, 215–220. [Google Scholar] [CrossRef]
- John, J.; Liu, H. Glyphosate monitoring in water, foods, and urine reveals an association between urinary glyphosate and tea drinking: A pilot study. Int. J. Env. Health Eng. 2018, 7, 2. [Google Scholar]
- Poiger, T.; Buerge, I.J.; Bächli, A.; Müller, M.D.; Balmer, M.E. Occurrence of the herbicide glyphosate and its metabolite AMPA in surface waters in Switzerland determined with on-line solid phase extraction LC-MS/MS. Environ. Sci. Pollut. Res. 2017, 24, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Tzaskos, D.F.; Marcovicz, C.; Dias, N.M.P.; Rosso, N.D. Development of sampling for quantification of glyphosate in natural waters. Cienc. Agrotec. 2012, 36, 399–405. [Google Scholar] [CrossRef]
- Popp, M.; Hann, S.; Mentler, A.; Fuerhacker, M.; Stingeder, G.; Koellensperger, G. Determination of glyphosate and AMPA in surface and waste water using high-performance ion chromatography coupled to inductively coupled plasma dynamic reaction cell mass spectrometry (HPIC–ICP–DRC–MS). Anal. Bioanal. Chem. 2008, 391, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Montiel-León, J.M.; Munoz, G.; Vo Duy, S.; Do, D.T.; Vaudreuil, M.A.; Goeury, K.; Guillemette, F.; Amyot, M.; Sauvé, S. Widespread occurrence and spatial distribution of glyphosate, atrazine, and neonicotinoids pesticides in the St. Lawrence and tributary rivers. Environ. Pollut. 2019, 250, 29–39. [Google Scholar]
- Zhang, Q.; Zhou, H.; Li, Z.; Zhu, J.; Zhou, C.; Zhao, M. Effects of glyphosate at environmentally relevant concentrations on the growth of and microcystin production by Microcystis aeruginosa. Environ. Monit. Assess. 2016, 188, 632. [Google Scholar] [CrossRef]
- Aparicio, V.C.; De Gerónimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef]
- Giroux, I. Présence de pesticides dans l’eau au Québec: Portrait et tendances dans les zones de maïs et de soya - 2011 à 2014, Québec, ministère du Développement durable, de l’Environnement et de la Lutte contre les changements climatiques, Direction du suivi de l’état de l’environnement. ISBN 978-2-550-73603-5. Available online: http://www.mddelcc.gouv.qc.ca/eau/flrivlac/pesticides.htm (accessed on 24 May 2020).
- Ronco, A.E.; Marino, D.J.; Abelando, M.; Almada, P.; Apartin, C.D. Water quality of the main tributaries of the Paraná Basin: Glyphosate and AMPA in surface water and bottom sediments. Environ. Monit. Assess. 2016, 188, 458. [Google Scholar] [CrossRef]
- Byer, J.D.; Struger, J.; Klawunn, P.; Todd, A.; Sverko, E. Low cost monitoring of glyphosate in surface waters using the ELISA method: An evaluation. Environ. Sci. Technol. 2008, 42, 6052–6057. [Google Scholar] [CrossRef]
- ISPRA. Rapporto Nazionale Pesticidi Nelle Acque, Dati 2015–2016. 2018. Available online: http://www.confagricolturave.it/ambiente-archivio/694-rapporto-nazionale-pesticidi-nelle-acque-dati-2015-e-2016 (accessed on 31 May 2020).
- Glozier, N.E.; Struger, J.; Cessna, A.J.; Gledhill, M.; Rondeau, M.; Ernst, W.R.; Sekela, M.A.; Cagampan, S.J.; Sverko, E.; Murphy, C.; et al. Occurrence of glyphosate and acidic herbicides in select urban rivers and streams in Canada, 2007. Environ. Sci. Pollut. Res. 2012, 19, 821–834. [Google Scholar] [CrossRef]
- Freire, R.; Schneider, R.M.; de Freitas, F.H.; Bonifácio, C.M.; Tavares, C.R.G. Monitoring of toxic chemical in the basin of Maringá stream. Acta Sci. Technol. 2012, 34, 295–302. [Google Scholar] [CrossRef]
- Medalie, L.; Baker, N.T.; Shoda, M.E.; Stone, W.W.; Meyer, M.T.; Stets, E.G.; Wilson, M. Influence of land use and region on glyphosate and aminomethylphosphonic acid in streams in the USA. Sci. Total Environ. 2020, 707, 136008. [Google Scholar] [CrossRef] [PubMed]
- Mörtl, M.; Németh, G.; Juracsek, J.; Darvas, B.; Kamp, L.; Rubio, F.; Székács, A. Determination of glyphosate residues in Hungarian water samples by immunoassay. Microchem. J. 2013, 107, 143–151. [Google Scholar] [CrossRef]
- Struger, J.; Thompson, D.; Staznik, B.; Martin, P.; McDaniel, T.; Marvin, C. Occurrence of glyphosate in surface waters of Southern Ontario. Bull. Environ. Contam. Toxicol. 2008, 80, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Bonansea, R.I.; Filippi, I.; Wunderlin, D.A.; Marino, D.; Amé, M.V. The fate of glyphosate and AMPA in a freshwater endorheic basin: An ecotoxicological risk assessment. Toxics 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Tierney, K.B.; Sekela, M.A.; Cobbler, C.E.; Xhabija, B.; Gledhill, M.; Ananvoranich, S.; Zielinski, B.S. Evidence for behavioral preference toward environmental concentrations of urban-use herbicides in a model adult fish. Environ. Toxicol. Chem. 2011, 30, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Thurman, E.M.; Lee, E.A.; Meyer, M.T.; Furlong, E.T.; Glassmeyer, S.T. Urban contributions of glyphosate and its degradate AMPA to streams in the United States. Sci. Total Environ. 2006, 354, 191–197. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Kolpin, D.W.; Scribner, E.A.; Kuivila, K.M.; Sandstrom, M.W. Glyphosate, other herbicides, and transformation products in midwestern streams, 2002. J. Am. Water. Resour. As. 2005, 41, 323–332. [Google Scholar] [CrossRef]
- Brüsch, W.; Rosenbom, A.E.; Badawi, N.; Gudmondsson, L.; von Platten-Hallermund, F.; Nielsen, C.B.; Plauborg, F.; Laier, T.; Olsen, P. The Danish Pesticide Leaching Assessment Programme; GEUS: København, Denmark, 2015; ISBN 978-87-7871-388-9. [Google Scholar]
- Ramirez, C.E.; Bellmund, S.; Gardinali, P.R. A simple method for routine monitoring of glyphosate and its main metabolite in surface waters using lyophilization and LC-FLD+MS/MS. Case study: Canals with influence on Biscayne National Park. Sci. Total Environ. 2014, 496, 389–401. [Google Scholar] [CrossRef]
- Trégouët, B.; Nirascou, F. Les pesticides dans les eaux, données 2003 et 2004. Les dossiers ifen 2006, 5, 1–40. [Google Scholar]
- Horth, H.; Blackmore, K. Survey of Glyphosate and AMPA in Groundwaters and Surface Waters in Europe; WRC Report No. UC8073.02; WRc plc: Swindon, UK, 2009. [Google Scholar]
- Sanchís, J.; Kantiani, L.; Llorca, M.; Rubio, F.; Ginebreda, A.; Fraile, J.; Garrido, T.; Farré, M. Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 2335–2345. [Google Scholar] [CrossRef]
- Van Stempvoort, D.R.; Roy, J.W.; Brown, S.J.; Bickerton, G. Residues of the herbicide glyphosate in riparian groundwater in urban catchments. Chemosphere 2014, 95, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rendon-von Osten, J.; Dzul-Caamal, R. Glyphosate Residues in Groundwater, Drinking Water and Urine of Subsistence Farmers from Intensive Agriculture Localities: A Survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef] [PubMed]
- Van Stempvoort, D.R.; Spoelstra, J.; Senger, N.D.; Brown, S.J.; Post, R.; Struger, J. Glyphosate residues in rural groundwater, Nottawasaga River Watershed, Ontario, Canada. Pest. Manag. Sci. 2016, 72, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Akcha, F.; Spagnol, C.; Rouxel, J. Genotoxicity of diuron and glyphosate in oyster spermatozoa and embryos. Aquat. Toxicol. 2012, 106–107, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Mottier, A.; Séguin, A.; Devos, A.; Le Pabic, C.; Voiseux, C.; Lebel, J.M.; Serpentini, A.; Fievet, B.; Costil, K. Effects of subchronic exposure to glyphosate in juvenile oysters (Crassostrea gigas): From molecular to individual levels. Mar. Pollut. Bull. 2015, 95, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Favret, K.P.; Lynn, J.W. Flow-cytometric analyses of viability biomarkers in pesticide-exposed sperm of three aquatic invertebrates. Arch. Environ. Contam. Toxicol. 2010, 58, 973–984. [Google Scholar] [CrossRef]
- Boutet, I.; Tanguy, A.; Moraga, D. Characterisation and expression of four mRNA sequences encoding glutathione S-transferases pi, mu, omega and sigma classes in the Pacific oyster Crassostrea gigas exposed to hydrocarbons and pesticides. Mar. Biol. 2004, 146, 53–64. [Google Scholar] [CrossRef]
- Tanguy, A.; Boutet, I.; Laroche, J.; Moraga, D. Molecular identification and expression study of differentially regulated genes in the Pacific oyster Crassostrea gigas in response to pesticide exposure. FEBS J. 2005, 272, 390–403. [Google Scholar] [CrossRef]
- Milan, M.; Dalla Rovere, G.; Smits, M.; Ferraresso, S.; Pastore, P.; Marin, M.G.; Bogialli, S.; Patarnello, T.; Bargelloni, L.; Matozzo, V. Ecotoxicological effects of the herbicide glyphosate in non-target aquatic species: Transcriptional responses in the mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 237, 442–451. [Google Scholar] [CrossRef]
- Matozzo, V.; Fabrello, J.; Masiero, L.; Ferraccioli, F.; Finos, L.; Pastore, P.; Di Gangi, I.M.; Bogialli, S. Ecotoxicological risk assessment for the herbicide glyphosate to non-target aquatic species: A case study with the mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 233, 623–632. [Google Scholar] [CrossRef]
- Matozzo, V.; Zampieri, C.; Munari, M.; Marin, M.G. Glyphosate affects haemocyte parameters in the clam Ruditapes philippinarum. Mar. Environ. Res. 2019, 146, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, J.Z.; Rola, R.C.; Lopes, F.M.; Buffon, H.F.; Freitas, M.M.; Martins, C.d.M.G.; da Rosa, C.E. Effects of glyphosate on cholinesterase activity of the mussel Perna perna and the fish Danio rerio and Jenynsia multidentata: In vitro studies. Aquat. Toxicol. 2013, 130–131, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Séguin, A.; Mottier, A.; Perron, C.; Lebel, J.M.; Serpentini, A.; Costil, K. Sub-lethal effects of a glyphosate-based commercial formulation and adjuvants on juvenile oysters (Crassostrea gigas) exposed for 35 days. Mar. Pollut. Bull. 2017, 117, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Mottier, A.; Serpentini, A.; Dallas, L.; James, A.; Lebel, J.M.; Costil, K. In vitro effects of glyphosate-based herbicides and related adjuvants on primary culture of hemocytes from Haliotis tuberculata. Fish Shellfish Immunol. 2020, 100, 1–8. [Google Scholar] [CrossRef]
- Hanana, H.; Simon, G.; Kervarec, N.; Mohammadou, B.A.; Cérantola, S. HRMAS NMR as a tool to study metabolic responses in heart clam Ruditapes decussatus exposed to Roundup®. Talanta 2012, 97, 425–431. [Google Scholar] [CrossRef]
- Fabrello, J.; Grapputo, A.; Munari, M.; Marin, M.G.; Masiero, L.; Pacchioni, B.; Millino, C.; Matozzo, V. Molecular and biochemical responses of vitellogenin in the mussel Mytilus galloprovincialis exposed to the glyphosate-based herbicide Roundup® Power 2.0. Environ. Sci. Pollut. Res. 2020, in press. [Google Scholar] [CrossRef]
- de Brito Rodrigues, L.; de Oliveira, R.; Abe, F.R.; Brito, L.B.; Moura, D.S.; Valadares, M.C.; Grisolia, C.K.; de Oliveira, D.P.; de Oliveira, G.A.R. Ecotoxicological assessment of glyphosate-based herbicides: Effects on different organisms. Environ. Toxicol. Chem. 2017, 36, 1755–1763. [Google Scholar] [CrossRef]
- Tsui, M.T.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Osterberg, J.S.; Darnell, K.M.; Blickley, T.M.; Romano, J.A.; Rittschof, D. Acute toxicity and sub-lethal effects of common pesticides in post-larval and juvenile blue crabs, Callinectes sapidus. J. Exp. Mar. Bio. Ecol. 2012, 424–425, 5–14. [Google Scholar] [CrossRef]
- Avigliano, L.; Canosa, I.S.; Medesani, D.A.; Rodríguez, E. Effects of glyphosate on somatic and ovarian growth in the estuarine crab Neohelice granulata, during the pre-reproductive period. Water Air Soil Pollut. 2018, 229, 44. [Google Scholar] [CrossRef]
- Canosa, I.S.; Silveyra, G.R.; Avigliano, L.; Medesani, D.A.; Rodríguez, E.M. Ovarian growth impairment after chronic exposure to Roundup Ultramax® in the estuarine crab Neohelice granulata. Environ. Sci. Pollut. Res. 2018, 25, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Canosa, I.S.; Zanitti, M.; Lonné, N.; Medesani, D.A.; López Greco, L.S.; Rodríguez, E.M. Imbalances in the male reproductive function of the estuarine crab Neohelice granulata, caused by glyphosate. Ecotoxicol. Environ. Saf. 2019, 182, 109405. [Google Scholar] [CrossRef] [PubMed]
- His, E.; Heyvang, I.; Geffard, O.; De Montaudouin, X. A comparison between oyster (Crassostrea gigas) and sea urchin (Paracentrotus lividus) larval bioassays for toxicological studies. Water Res. 1999, 33, 1706–1718. [Google Scholar] [CrossRef]
- Amid, C.; Olstedt, M.; Gunnarsson, J.S.; Le Lan, H.; Tran Thi Minh, H.; Van den Brink, P.J.; Hellström, M.; Tedengren, M. Additive effects of the herbicide glyphosate and elevated temperature on the branched coral Acropora formosa in Nha Trang, Vietnam. Environ. Sci. Pollut. Res. 2018, 25, 13360–13372. [Google Scholar] [CrossRef] [PubMed]
- de Melo Tarouco, F.; de Godoi, F.G.A.; Velasques, R.R.; da Silveira Guerreiro, A.; Geihs, M.A.; da Rosa, C.E. Effects of the herbicide Roundup on the polychaeta Laeonereis acuta: Cholinesterases and oxidative stress. Ecotoxicol. Environ. Saf. 2017, 135, 259–266. [Google Scholar] [CrossRef] [PubMed]
| IUPAC Name | N-(phosphonomethyl) Glycine |
|---|---|
| CAS Number | 1071-83-6 |
| Molecular Formula | C3H8NO5P |
| Chemical Structure |  |
| Molecular Weight | 169.07 g/mol |
| Physical state and Color | White crystalline powder |
| Melting Point | 189.5–230 °C |
| Density at 20 °C | 1.705 |
| Water Solubility at 25 °C | 1.2 g/100 mL |
| Octanol-water Coeff. (log Kow) | −3.40 to −1.0 |
| Vapor Pressure at 25 °C | 1.31 × 10-2 mPa |
| Glyphosate Concentrations | Water Environment, Country | Ref. |
|---|---|---|
| 0.10 to 0.70 mg/L | Surface waters, Argentina | [66] |
| <LOD-427 µg/L | Surface waters, USA | [69] |
| <LOD-430 μg/L | Surface waters, USA | [80] |
| <LOD-1600 μg/L | Surface waters, Argentina | [81] |
| <LOD-4.52 μg/L (2.11 * μg/L) | Surface waters, Argentina | [82] |
| <LOD-36.71 μg/L (3.02 * μg/L) | Surface waters, Mexico | [83] |
| <LOD-27.8 μg/L (1.68 ** μg/L) | Surface waters, USA | [67] |
| <0.1–427 μg/L | Surface waters, USA | [72] |
| <LOD-2.1 μg/L | Surface waters, Italy | [84] |
| <LOD-8.2 μg/L (0.4 * μg/L) | Surface waters, Argentina | [70] |
| LOD-2.9 μg/L (0.78 * μg/L) | Surface waters, Argentina | [85] |
| <LOD-4970 ng/L | Surface waters, Switzerland | [86] |
| <LOD-145 ng/L | Surface waters, Switzerland | [87] |
| 15–390 ng/L | Surface waters, Switzerland | [71] |
| <LOD-90 μg/L | Surface waters, Poland | [88] |
| <LOD-0.08 μg/L | Surface waters, USA | [89] |
| <LOD-2.1 μg/L (0.11 ** μg/L) | Surface waters, Switzerland | [90] |
| 1.258–1.550 mg/L | Surface waters, Brazil | [91] |
| < LOD-1.93 μg/L | Surface waters, Austria | [92] |
| <2–3000 ng/L (109 * ng/L; 26.9 ** ng/L) | Surface waters, Canada | [93] |
| 10 mg/L | Surface waters, China | [94] |
| <LOD-7.6 μg/L | Surface water, Argentina | [95] |
| <LOD-18 μg/L | Surface waters, Canada | [96] |
| <LOD-0.7 μg/L (0.6 * μg/L) | Surface water, Argentina | [97] |
| <LOD-12 μg/L | Surface waters, Canada | [98] |
| <LOD-0.08 μg/L | Surface waters, Italy | [99] |
| <LOD-11.8 μg/L (158.6 * ng/L; 19.8 ** ng/L) | Surface waters, Canada | [100] |
| <LOD-0.041 mg/L | Surface waters, Brazil | [101] |
| <LOD-8.1 μg/L (0.05 ** μg/L) | Surface waters, USA | [102] |
| <LOD-0.68 ng/mL | Surface water, Hungary | [103] |
| <LOD-40.8 μg/L | Surface water, Canada | [104] |
| <LOD-125 μg/L | Surface waters, Argentina | [105] |
| <LOD-455 ng/L | Surface water, Canada | [106] |
| <LOD-14.2 μg/L | Surface waters, Australia | [68] |
| <LOD-2.2 μg/L | Surface waters, USA | [107] |
| <LOD-8.7 μg/L | Surface waters, USA | [108] |
| <LOD-90 μg/L | Surface waters, France | [65] |
| <LOD-31 μg/L | Surface waters, Denmark | [109] |
| <LOD-59.9 μg/L | Surface waters, USA | [110] |
| <LOD-165 μg/L | Surface waters, France | [111] |
| <LOD-1.3 μg/L | Surface waters, Belgium | [112] |
| <LOD-0.46 μg/L | Surface waters, Finland | [112] |
| <LOD-50 μg/L | Surface waters, France | [112] |
| <LOD-4.7 μg/L | Surface waters, Germany | [112] |
| <LOD-1.8 μg/L | Surface waters, Ireland | [112] |
| <LOD-11 μg/L | Surface waters, Italy | [112] |
| <LOD-0.93 μg/L | Surface waters, Norway | [112] |
| <LOD-3.6 μg/L | Surface waters, Slovakia | [112] |
| <LOD-15.3 μg/L | Surface waters, Spain | [112] |
| <LOD-13 μg/L | Surface waters, Sweden | [112] |
| <LOD-8.8 μg/L | Surface waters, UK | [112] |
| <LOD-3.6 μg/L | Surface waters, Austria | [112] |
| <LOD-8.7 μg/L | Groundwaters, Denmark | [112] |
| <LOD-24 μg/L | Groundwaters, France | [112] |
| <LOD-1.2 μg/L | Groundwaters, Italy | [112] |
| <LOD-1.7 μg/L | Groundwaters, Sweden | [112] |
| <LOD-0.21 μg/L | Groundwaters, Switzerland | [112] |
| <LOD-4.7 μg/L | Groundwaters, Netherlands | [112] |
| <LOD-0.47 μg/L | Groundwaters, UK | [112] |
| <LOD-6.8 μg/L | Groundwaters, France | [111] |
| <LOD-0.67 μg/L | Groundwaters, Denmark | [109] |
| <LOD-0.98 ng/mL | Groundwaters, Hungary | [103] |
| <LOD-0.011 μg/L | Groundwaters, Italy | [99] |
| <LOD-2.56 μg/L (202 * ng/L) | Groundwaters, Spain | [113] |
| <LOD-0.025 μg/L | Groundwaters, Switzerland | [90] |
| <LOD-42 ng/L | Groundwater, Canada | [114] |
| <LOD-8.5 μg/L (0.4 * μg/L) | Groundwaters, Argentina | [70] |
| 0.44–1.41 μg/L | Groundwaters, Mexico | [115] |
| <0.1–4.7 μg/L | Groundwaters, USA | [72] |
| <LOD-663 ng/L | Groundwaters, Canada | [116] |
| <LOD-1690 ng/L (up to 665 * ng/L) | Sea water, Baltic Sea estuaries | [76] |
| 13–1377 μg/L | Sea waters, Western Pacific | [75] |
| <LOD-1.2 μg/L | Sea waters, French Atlantic coast | [74] |
| <0.1–2.5 μg/L (0.1–0.2 ** μg/L) | Precipitations, USA | [78] |
| 0.3–1.1 μg/L | Precipitations, USA | [72] |
| <LOD-135 ng/L | Precipitation, Canada Ontario | [116] |
| 0.2210–5 μg/L | Phytotelmic water, Belize | [77] |
| Compound Tested | Concentrations (Exposure Type) | Species | Effects | Ref. |
|---|---|---|---|---|
| Glyphosate (active ingredient) Roundup (commercial formulation) | 0.5, 1, 1.5, 2.5, 5 µg/L (in vitro exposure) | Crassostrea gigas (gametes and embryos) | increases in the percentage of abnormal D-larvae no genotoxic effects on oyster spermatozoa | [117] |
| Glyphosate (acid, 97% purity) | 0.1, 1, 100 µg/L (in vivo exposure) | Crassostrea gigas (juveniles) | no mortality no growth no histological alterations moderate alterations of enzyme activities moderate alterations in gene expression | [118] |
| Roundup Ready-To-Use Plus® | 0.25, 1, 4, 16 mg/L (in vitro exposure) | Crassostrea virginica (sperm) | no significant alterations in mitochondrial membrane potential in the sperm | [119] |
| Glyphosate (active ingredient) | 2 µg/L (in vivo exposure) | Crassostrea gigas (adults) | gills: decreases in expression of GSTs digestive gland: increases in expression of GSTs | [120] |
| Glyphosate (active ingredient) | 2 µg/L (in vivo exposure) | Crassostrea gigas (adults) | differentially regulated gene expression in gills and digestive gland | [121] |
| Glyphosate (active ingredient) | 10, 100, 1000 µg/L (in vivo exposure) | Mytilus galloprovincialis (adults) | effects on the transcriptional regulation of genes involved in important cell functions | [122] |
| Glyphosate (active ingredient) | 10, 100, 1000 µg/L (in vivo exposure) | Mytilus galloprovincialis (adults) | alterations in hemocyte parameters no marked alteration in antioxidant enzyme activity in gills and digestive gland alterations in gill AChE activity | [123] |
| Glyphosate (active ingredient) | 10, 100, 1000 µg/L (in vivo exposure) | Ruditapes philippinarum (adults) | reductions in THC increases in hemocyte volume and diameter increases in hemocyte proliferation increase in HL acid phosphatase activity | [124] |
| Glyphosate (pure) | from 0.075 to 15 mM (in vitro exposure) | Perna perna (tissue from juveniles) | significant inhibition of cholinesterase activity in gills and muscle | [125] |
| Roundup Express® (REX) | 0.1, 1, 100 μg/L (in vivo exposure) | Crassostrea gigas (juveniles) | no mortality no effects on condition index delay in gametogenesis decreases in shell length slight reduction in whole weight moderate alterations of digestive gland enzyme activities | [126] |
| Glyphosate (active ingredient) REX (commercial formulation) | from 0.1 to 100,000 μg/L (in vitro exposure) | Haliotis tuberculata (hemocytes) | cell viability: significant reduction due to REX, but not to glyphosate phagocytosis: significant reduction due to REX, but not to glyphosate lysosomal stability: significant effects of low concentrations of glyphosate and high levels of REX | [127] |
| Roundup® 3plus | 0.2, 1 g/L (in vivo exposure) | Ruditapes decussatus (adults) | alteration of energy metabolism and metabolic biomarkers | [128] |
| Roundup® Power 2.0 | 100, 1000 μg/L (in vivo exposure) | Mytilus galloprovincialis (adults) | no significant alterations in vtg gene expression in female gonads reduction in ALP levels if female gonads at 21 days reduction in ALP levels if male gonads at 7 days and increases at 21 days | [129] |
| Compound Tested | Concentrations (Exposure Type) | Species Crustacea | Effects | Ref. |
|---|---|---|---|---|
| Roundup® Glyphosate AKB 480 | 5, 10, 25, 50, 100 mg/L, corresponding to 1.8, 3.6, 9, 18 and 36 mg/L of glyphosate (in vivo exposure) | Artemia salina (nauplii) | increased mortality rates, even if Roundup was more toxic than AKB | [130] |
| Glyphosate acid Isopropylamine salt of glyphosate Roundup® | 1 to 100 mg acid equivalent/L (in vivo exposure) | Acartia tonsa (adults) | LC50 determination | [131] |
| Roundup® Pro | 103 to 107 μg/L (in vivo exposure) | Callinectes sapidus (megalopae and juvenile stages) | LC50 determination | [132] |
| Glyphosate (active ingredient) | 0.02, 0.2, 1 mg/L (in vivo exposure) | Neohelice granulata (adult females) | decrease in body weight no effects on gonadosomatic index no effects on vitellogenic protein content of the ovary increased percentage of reabsorbed vitellogenic oocytes | [133] |
| Roundup Ultramax® | 0.01 and 0.2 mg/L asacid equivalent (in vivo exposure) | Neohelice granulata (adult females) | increases in glycemia no effects on muscle glycogen content no effects on gonadosomatic index increased percentage of reabsorbed vitellogenic oocytes decrease in Vtg levels in the ovary | [134] |
| Glyphosate (as-active ingredient) Roundup Ultramax® | 1 mg/L (in vivo exposure) | Neohelice granulata (adult males) | reductions in weight reductions in total protein levels in muscle increase in glycogen content in muscle increased percentage of abnormal spermatophores | [135] |
| Echinoderms | ||||
| Roundup Ready-To-Use Plus® | 0.25, 1, 4, 16 mg/L (in vitro exposure) | Lytechinus variegatus (sperm) | no effects on mitochondrial membrane potential of sperm | [119] |
| Glyphosate | 10, 25, 50, 100, 150, 200 μg/L (in vivo exposure) | Paracentrotus lividus (larvae) | no abnormal development of larvae | [136] |
| Corals | ||||
| Glyphosate + Increased temperature | 0.12, 1.2, 6, 12 mg/L (in vivo exposure) | Acropora formosa (adults) | significant effects of temperature*glyphosate interaction on coral pigmentation and chlorophyll a | [137] |
| Polychaetes | ||||
| Roundup | from 0.065 to 65 mg/L (in vivo exposure) | Laeonereis acuta (adults) | LC50 determination no significant change in oxygen consumption significant decrease in AChE activity significant decrease in ROS production after 24 h significant decrease in antioxidant capacity against peroxyl radicals after 24 h no significant alterations in antioxidant enzyme activities, except for SOD significant alterations in lipid peroxidation | [138] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matozzo, V.; Fabrello, J.; Marin, M.G. The Effects of Glyphosate and Its Commercial Formulations to Marine Invertebrates: A Review. J. Mar. Sci. Eng. 2020, 8, 399. https://doi.org/10.3390/jmse8060399
Matozzo V, Fabrello J, Marin MG. The Effects of Glyphosate and Its Commercial Formulations to Marine Invertebrates: A Review. Journal of Marine Science and Engineering. 2020; 8(6):399. https://doi.org/10.3390/jmse8060399
Chicago/Turabian StyleMatozzo, Valerio, Jacopo Fabrello, and Maria Gabriella Marin. 2020. "The Effects of Glyphosate and Its Commercial Formulations to Marine Invertebrates: A Review" Journal of Marine Science and Engineering 8, no. 6: 399. https://doi.org/10.3390/jmse8060399
APA StyleMatozzo, V., Fabrello, J., & Marin, M. G. (2020). The Effects of Glyphosate and Its Commercial Formulations to Marine Invertebrates: A Review. Journal of Marine Science and Engineering, 8(6), 399. https://doi.org/10.3390/jmse8060399







