1. Introduction
European Union Atlantic Area countries share an attractive coastline when it comes to the potential of their genetic resources (GR). Marine genetic resources (MGR) have several interesting applications in distinct industries, such as pharmaceutical, cosmetics, and/or other applications [
1]. Among MGR, algae have a significant role in the blue bioeconomy because of their importance for the conservation of marine ecosystems and their value for commercial applications [
2]. In the EU, the algae biomass sector currently employs 14,000 people and has a value of EUR 1.69 billion, which includes research and development, equipment production, and employments [
3]. High added value compounds from microalgae have been identified as a business niche with high growth potential, particularly in the EU Atlantic Area, which is currently heightening interest in the research, innovation, and development of microalgae-based products, especially in the food, feed, cosmetics, and biostimulants sectors [
4].
In this region, international networks comprised by research experts, biotech companies, and technological platforms are able to produce sustainable and high-quality microalgae [
5]. Twenty-four percent of the industrial and scientific members of the European Algae Biomass Association (EABA), mainly represented by SMEs and public institutions, respectively, belong to the Atlantic Area [
6]. Furthermore, according to the dataset on algae production published on the EMODnet Human Activities website, approximately 40% of the companies of microalgae production located in Europe are placed in EU Atlantic Area countries, namely, France, Portugal, Spain, and the UK [
7]. Although this hot hub has a diversity of experts capable of leading the microalgae exploitation, legislative constrains still remain. An example of interest on microalgae in this region is the INTERREG Atlantic Area EnhanceMicroAlgae project EAPA_338/2016 (2017–2020), which is focused on contributing to the competitiveness of the microalgae-based industry in the Atlantic Area through the transfer of technological and economic expertise to the commercial sector [
5].
With the commencement of the Nagoya Protocol (NP) on Access and Benefit-Sharing (ABS) in October 2014, working with GR, such as microalgae, became a subject of great attention and interest in the scientific and industrial communities. The current regulatory requirements to access and utilize GR under the NP constitute a legislative burden that may hinder the development of the microalgae sector. With this in mind, we set out to explore the current legislation under the NP in the EU Atlantic Area countries, namely, France, Ireland, Portugal, Spain, and the United Kingdom (UK) and Northern Ireland (NI). The origins and content of the NP (
Section 2 and
Section 3) and its implication on the EU Atlantic Area countries’ legislation (
Section 4) are reviewed. In addition, a decision framework for microalgae GR users (
Section 4.3) is proposed in order to provide clarity for the research and development sectors.
2. The Origins of the Nagoya Protocol
On 5 June 1992, at the United Nations Conference on Environment and Development, the Convention on Biological Diversity (CBD) was opened for signatures [
8]. The CBD is a unique international instrument that comprehensively addresses the biological diversity and is composed of three objectives: (1) the conservation of biological diversity, (2) the sustainable use of its components, and (3) the fair and equitable sharing of benefits arising from the utilization of genetic resources (see Article 1 of the CBD). CBD Article 3 includes the principle that “States have, in accordance with the Charter of the United Nations and the principles of the international law, the sovereign right to exploit their own resources pursuant to their own environmental policies, and the responsibility to ensure that activities within their jurisdiction or control do not cause damage to the environment of other States or of areas beyond the limits of national jurisdiction”. Hence, as stated in Article 15 of the CBD: (1) “the authority to determine access to genetic resources rests with the national governments and is subject to national legislation”; (2) “Access, where granted, shall be on mutually agreed terms” (MAT) and “subject to prior informed consent of the Contracting Party providing such resources, unless otherwise determined by that Party”; and (3) “Each Contracting Party shall take legislative, administrative or policy measures, as appropriate… with the aim of sharing in a fair and equitable way, the results of research and development and the benefits arising from the commercial and other utilization of genetic resources with the Contracting Party providing such resources. Such sharing shall be upon mutually agreed terms”.
After the CBD established the ABS principles, a challenge for many countries remained: there was no international system that allowed the provider country to guarantee the benefit-sharing after the GR have left its boundaries. For that reason, the “Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the Convention on Biological Diversity” was adopted at the tenth meeting of the Conference of the Parties on 29 October 2010 in Nagoya, Japan [
9]. The NP entered into force on 12 October 2014 and, at the time of writing, 122 countries were Parties to the NP, while 92 had only signed it [
10,
11].
3. The Nagoya Protocol
The NP arises from the need to further develop the implementation of the third objective of the CBD and to effectively implement Articles 15 (Access to Genetic Resources) and 8(j) (Traditional Knowledge). That is why it is applicable to GR and associated traditional knowledge (aTK) within the scope of the CBD and to the benefits arising from the utilization of those resources (see Article 3 of the NP).
The objective of the NP is the fair and equitable sharing of the benefits arising from the utilization of GR and/or aTK in order to contribute to the conservation of biological diversity and sustainable use of its components. Therefore, it is important to comprehend that in the aim of the NP, and according to its Article 2:
- (1)
“Utilization of genetic resources” means to conduct research and development on the genetic and/or biochemical composition of GR, including through the application of biotechnology;
- (2)
“Biotechnology” is defined as any technological application that utilizes biological systems, living organisms, or derivatives thereof to make or modify products or process for specific use and;
- (3)
“Derivative” is a natural biochemical compound resulting from the genetic expression or metabolism of biological GR, even if it does not contain functional units of heredity.
The NP clarifies (Articles 5 and 6) that each State Party is sovereign in the right to grant free access to its GR or to grant access only subjected to prior informed consent (PIC) (Article 6) and mutually agreed terms (MAT) (Article 5) that legalizes the benefit-sharing in a fair and equitable way. To implement this, each Party must take legislative, administrative, or policy measures to provide for legal certainty, clarity, and transparency. Regarding access to the aTK, Article 7 states that indigenous peoples and local communities (IPLCs) are the holders of the aTK, and access to it must be performed with a PIC or approval and involvement of the IPLCs. Each Party shall take legislative, administrative, or policy measures to guarantee that the benefits arising from the use of aTK are shared in a fair and equitable way with IPLCs (Article 5.5) and that their customary laws, community protocols, and procedures are considered (Article 12).
The NP states that each Party must designate an ABS National Focal Point (NFP) and one or more Competent National Authorities (CNA) (Article 13). The NFP is responsible for providing information: (1) regarding the procedures to obtain the PIC and establishment of MAT, including benefit-sharing; (2) on CNA; (3) on relevant indigenous and local communities; and (4) on relevant stakeholders. The CNA must, in accordance with national legislative, administrative, or policy measures, be responsible for granting access or, as applicable, issuing written evidence that access requirements have been met and be responsible for advising on applicable procedures and requirements for obtaining PIC and MAT. A single entity to fulfill the functions of both focal point and national authority may be designated by each Party.
The NP establishes in Article 14 an Access and Benefit-Sharing Clearing House (ABS Clearing House) responsible for sharing information related to ABS and, in particular, to information available by each Party relevant to the implementation of the NP. The ABS Clearing House should have available information provided by each Party regarding legislative, administrative, and policy measures on ABS, NFP, and CNA and permits (or their equivalent) issued at the time of access as evidence of the decision to grant PIC and of the establishment of MAT. The ABS Clearing House has been established as an online platform and can be freely consulted [
12].
According to Articles 15 and 16, Parties will take appropriate, effective, and proportionate legislative, administrative, or policy measures to provide that the GR and/or the aTK utilized within its jurisdiction have been accessed according to the PIC and that MAT have been established, as required by the domestic access and benefit-sharing legislation or regulatory requirements of the other Party. Each Party shall also take measures to monitor and to enhance transparency about the use of the GR. Those measures shall include the designation of one or more checkpoints to collect or receive information related to PIC, to the source of the GR, to the establishment of MAT, and/or to the use of the GR. Reports on the utilization will be placed on the ABS Clearing House as Checkpoint Communiqués.
When a permit, or equivalent, is issued and made available to the ABS Clearing House, it will constitute an international recognized certificate of compliance (IRCC) (Article 17) that will serve as evidence that the GR have been accessed in accordance with PIC and that MAT terms have been established, as required by the domestic ABS legislation or regulatory requirements of the Party providing the PIC.
4. The Nagoya Protocol in the European Union and Atlantic Area Countries
The following section compiles information regarding the application of the NP in the European Union (EU) and its Atlantic Area Member States, namely, Portugal, Spain, France, the United Kingdom and Northern Ireland, and Ireland. For each territory, entry into force date of the NP, applicable legislation, NFP and CNA, GR access measures, and official checkpoints are described.
4.1. European Union
In the EU, the NP entered into force on 12 October 2014. Therefore, as a Party and in order to apply the obligatory elements of the NP, the Regulation (EU) Nº 511/2014 (ABS Regulation) [
13] was adopted. On 13 October 2015, the EU adopted the Implementing Regulation (EU) 2015/1866 [
14], laying down detailed rules for the implementation of Regulation ABS with regard to the register of collection, monitor user compliance, and best practices. On 22 August 2016, the Guidance Document [
15] was approved concerning the field of application and the obligations resulting from Regulation ABS (
Table 1).
The EU has its own NFP, in which contacts are available at the ABS Clearing House website. Other orientation documents are being prepared to facilitate the interpretation of the Regulation (EU) nº 511/2014 in the main user sectors of the GR, namely, in the following areas: cosmetics, animal production, plants breeding, biologic control, pharmacy, biotechnology, and foodstuffs and animal feed.
The ABS Regulation entered into force on 9 June 2014. It is applicable from the commencement of the NP in the EU (12 October 2014), with the exception of Articles 4, 7, and 9 which are applicable from October 2015 (Article 17). It mainly establishes obligations (Article 4) for the users of GR and/or aTK in the EU, predicts the creation of a register of collections (Article 5), imposes the designation by each Member State of a CNA (Article 6), identifies control points for the users of GR (Article 7), and encourages the creation of best practices (Article 8).
Regarding user obligations, the exercise of the right of due diligence is imposed to ensure that access to GR and/or to aTK is in accordance with the applicable legal requirements and, if need be, that the benefits will be shared in a fair and equitable way, based on MAT. Hence, all users should seek, obtain, and maintain the information that is relevant for the access and fair and equitable share (e.g., IRCC or in its absence of relevant documents, as identified in Article 4) and transfer them to the subsequent users.
The ABS regulation predicts the establishment of an EU register of collections (of the GR) (Article 5), which contributes to reducing the risks of the use of the GR acquired illegally. The collections that intend to be included in the register of the EU should only be accepted if certain criteria are met (as defined in Article 5, paragraph 3). The users that acquire the GR from a collection, which is included in the register of collections within the EU, will be considered as having exercised due diligence to obtain all the necessary information (Article 4). The necessary information, and the request for inclusion in the register of collections, is detailed in the Implementing Regulation (EU) 2015/1866 (Annex I).
The users of GR are required to declare, at the control points identified in the ABS regulation (Article 7), that they complied with their due diligence obligation. Two checkpoints are established in the EU. The first checkpoint is related with all recipients of research funding where such research involves the utilization of GR and/or aTK. They are to be requested in order to declare that they exercised due diligence. The template for this declaration can be found in the Implementing Regulation (EU) 2015/1866 (Annex II). The second checkpoint concerns the final stage of product development, and the template is available in the Implementing Regulation (EU) 2015/1866 (Annex III). Specific events triggering submission of the declaration are defined in Articles 5 and 6 of the Implementing Regulation (EU) 2015/1866. For both checkpoints, the CNA designated by individual Member States under Regulation ABS transfer the information to the ABS Clearing House. The CNA controls, through an approach based on risk, if the users of the GR fulfill their obligations under the ABS Regulation (Articles 9 and 10). The Member States shall also ensure that any transgressions to the regulation by the users of the GR are punished by effective, proportionate, and dissuasive sanctions (Article 11).
The users’ associations, or other interested parties, may ask the European Commission for the recognition of a certain set of procedures, instruments, or mechanisms as a best practice. It is up to the authorities of Member States to verify whether the application by a user of a recognized good practice reduces the risk of noncompliance by the user and whether or not reducing the controls of compliance is justified (Article 8).
All Member States of the EU (and users of GR and/or aTK in the EU) are obliged to abide by these regulations, irrespective of whether they are individual parties to the protocol or not. Nevertheless, the objective of these regulations is to establish a compliance system among the EU Member States. Therefore, each Member State has the right to control access to the GR and/or aTK, as well as benefit-sharing, which is not established by the EU Regulations (see
Table 1).
4.1.1. Scope of the EU Regulations
Before accessing GR and/or aTK in the EU, it is important to understand if they fall into the scope of the EU Regulations, which includes geographic, temporal, material, and personal terms. Those terms are detailed in the Guidance Document in the scope of application and core obligations of Regulation ABS. It is important to note that all of the conditions described below will need to be fulfilled cumulatively for the EU ABS Regulations to apply. Nevertheless, even if the Regulation ABS is not applicable, users must pay attention to any ABS rules that may exist in the provider country.
Geographic Scope
The geographic scope is related to the origin of the GR and to their utilization in the EU. Therefore, in order to be in the scope of the EU ABS regulation: (a) the GR must have been subject to the sovereign rights of a state (exceptions are if the GR are obtained from areas beyond national jurisdiction, for example, the high seas and from areas covered by the Antarctic Treaty System); (b) provider countries must have ratified the NP and established access measures on the GR; and (c) utilization has to take place within the Union.
Temporal Scope
The GR must have been accessed and utilized as of 12 October 2014 (when the NP entered into force). If the GR were accessed before that date and were utilized after that date, they are not in the scope.
Material Scope
The material scope of the EU ABS Regulation is divided into GR, aTK, and utilization. According to the CBD, “genetic resources” are defined as genetic material of actual or potential value, being any material of plant, animal, microbial, or other origin containing functional units of heredity (Articles 3.1 and 2 of ABS Regulation/Article 3 of ABS Regulation/Article 2 of the CBD). The following are not within the scope of the EU ABS regulation: (a) GR that are governed by specialized international instruments and other international agreements, such as the International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA) and the WHO’s Pandemic Influenza Preparedness (PIP) Framework; (b) human GR; and (c) GR as traded commodities, since no research and development is performed on such GR. The aTK is defined in the EU ABS Regulation as traditional knowledge held by an indigenous or local community that is relevant for utilization of the GR, which is described in the MAT applying to the utilization of GR (Article 3(7) of the ABS Regulation). In order to be within the scope of the EU ABS Regulation, aTK needs to be related to the utilization of those resources, and it must be covered by relevant contractual agreements. Under the EU ABS Regulation (and as defined in the NP), “utilization” means to conduct research and development on the genetic and/or biochemical composition of GR, including through the application of biotechnology (as defined in Article 2 of the Convention (Article 3(5) of the Regulation). According to Article 2(d) of the NP, “biotechnology” is defined as any technological application that uses biological systems, living organisms, or derivatives thereof to make or modify products or processes for specific use. Therefore, derivatives are in the scope of the EU ABS Regulation, according to its definition (Article 2 (e) of the Protocol) as naturally occurring biochemical compounds resulting from the genetic expression or metabolism of biological or GR, even if they do not contain functional units of heredity.
Personal Scope
The EU ABS Regulation is applicable to all users. A “user” is defined in Article 3(4) of the EU ABS Regulation as any natural or legal person that utilizes GR and/or aTK. Thus, the obligation of due diligence applies to individuals, including researchers, and to organizations such as universities or other research organizations and companies that use GR and/or aTK. This means that a person who only stores, transfers, or trades material, without conducting research and/or development, does not fall under the scope of the EU ABS Regulation.
The EU has taken legislative, administrative, or policy measures to implement Article 5 of the NP with regard to fair and equitable benefit-sharing. These are implemented under Article 4(1) of the EU ABS Regulation that states that “users shall exercise due diligence to ascertain that GR and/or aTK which they utilize have been accessed in accordance with applicable access and benefit-sharing legislation or regulatory requirements and that benefits are fairly and equitable shared upon MAT, in accordance with any applicable legislation or regulatory requirements”.
4.2. Atlantic Area Countries
4.2.1. Portugal
The Portuguese Republic is a party to the CBD that was approved for ratification through the Decree nº 21/93 [
16] of 21 June. The NP was approved by Portugal through the Decree nº 7/2017 [
17] and entered into force on 10 July 2017. On 22 December 2016, a rectification to the Portuguese version of the Document of Orientation was approved.
In Portugal, Decree-Law 122/2017 [
18] establishes the measures at the national level for the implementation of the NP and the EU ABS legislation (
Table 1). It specifies some of the elements of Regulation ABS, namely, the identification of a CNA (responsible for compliance checks) and the establishment of the applicable sanctions, among others, to infractions of Articles 4 (Obligations of users) and 7 (Monitoring user compliance) of Regulation ABS. In conjunction with the existing EU ABS legislation, Decree-Law 122/2017 implements the obligation compliance measures of the NP in Portugal, reiterates some elements of Regulation ABS and Implementing Regulation (EU) 2015/1866 but does not establish access rules at the national level. The Portuguese CNA, established by Decree-Law 122/2017, is the Institute for Nature Conservation and Forests, I.P. (ICNF, I.P.), that works also as the NFP. Competent bodies of the Portuguese Autonomous Regions of Azores and Madeira are designated as Competent Regional Authorities. The Competent Regional Authority in Madeira is the Institute for Forestry and Nature Conservation, IP-RAM, whereas the process for designating the same in Azores is ongoing [
19] (
Table S1). In Portugal, the CNA (ICNF), as well as the IP-RAM, are responsible for carrying out the checkpoint functions on the basis of the geographical location of the submitter (where utilization takes place). The checkpoints established in Portugal are the same as those established at the EU level.
Presently, access to the GR in Portugal is only regulated in the Autonomous Region of the Azores, where Regional Decree-Law 9/2012/A [
20] and Regional Regulatory Decree 20/2012/A [
21] establish a legal framework for the access to, the transfer of, and the sharing of benefits arising out of the utilization of natural (including genetic) resources (
Table 1). This regime establishes that the collection of natural resources for scientific purposes is subject to PIC [
19].
Chapter II of the Regional Decree-Law 9/2012/A and Chapter I of the Regional Regulatory Decree 20/2012/A establish fair and nonarbitrary rules and procedures on accessing GR (as provided in Article 6.3 (b)); provide information on how to apply for PIC (as provided in article 6.3 (c)), provide for a clear and transparent decision by a CNA (as provided in Article 6.3 (d)), and provide for the issuance at the time of access of a permit or its equivalent (as provided in Article 6.3 (e)). Currently, no permits or their equivalents are available through the ABS Clearing House website, since they will be submitted once the Competent Regional Authority of the Azores is formally designated [
19]. In addition, Chapter IV of the Regional Decree-Law 9/2012/A and Chapter I of the Regional Regulatory Decree 20/2012/A provide rules and procedures for requiring and establishing MAT (as provided in Article 6.3 (g)). The Azores government has made a form available online to request access authorization to the natural resources of the Azores and their collection of samples for scientific purposes [
22].
Portugal has taken legislative, administrative, or policy measures to implement Article 5 of the NP regarding fair and equitable benefit-sharing. These are implemented under Article 4(1) of the EU ABS Regulation, which states that users shall exercise due diligence, and under Article 6(3) of Decree-Law 122/2017.
Portugal requires that GR users provide information related to PIC, to the GR source, to the establishment of MAT, and/or to the utilization of GR at a designated checkpoint, as provided in Article 17.1 (a)(i) and (ii) of the NP. That is in accordance with Article 7(1) and 7(2) of Regulation ABS and in line with Article 8 of Decree-Law 122/2017. Annexes II and III of the Commission Implementing Regulation provide the specific information requested from users, including information related to PIC, to the source of GR, to the establishment of MAT and to the use of GR.
Portugal uses the EU-wide web-based tool, DECLARE, which enables users to submit the due diligence declarations required by Article 7 of Regulation ABS to the relevant competent authorities. DECLARE is also used to transfer nonconfidential information from the due diligence declarations to the ABS Clearing House. Decree-Law 122/2017 further promotes the use of cost-effective communication tools.
Portugal does not have indigenous and local communities, and for that reason, no provisions have been taken related to those (Articles 6, 7 and 12).
Portugal is encouraging the development of codes of conduct, model contractual clauses for MAT, guidelines, and best practices in line with Article 13 of Regulation ABS and Article 12 “cooperation and complementary measures“ of Decree-Law 122/2017, which determine that members of the Advisory Group on ABS (established by Article 5 of Decree-Law 122/2017) may establish protocols; agreements; or other forms of cooperation with entities (e.g., universities, public laboratories, museums, etc.) utilizing GR, among others (Art.12 (b)), in order to develop model contractual clauses (as provided in Article 19 of the NP) and develop codes of conduct and best practices (as provided in Article 19 of the NP).
The contribution of Portugal for the implementation and application of the NP has been proactive through the development of several seminars and meetings across the country [
19].
4.2.2. Spain
The Nagoya Protocol entered into force in Spain on 12 October 2014. In Spain, in addition to EU regulations, Law 42/2007 [
23] of December 13 of the Natural Heritage and Biodiversity, modified by Law 33/2015 [
24] of September 21 and the Royal Decree 124/2017 [
25] of February 24, regarding access to GR from wild taxa and control of their use, are within the realm of the ABS measures and of the NP (
Table 1).
Law 42/2007 establishes the basic legal regime for the conservation, sustainable use, improvement, and restoration of the Spanish natural heritage and biodiversity as part of the duty to conserve and to guarantee the rights of people to an adequate environment for their well-being, health, and development. Law 33/2015, as of September 21, has recently amended Law 42/2007 in order to guarantee the correct application of the international law and the incorporation of the regulations of the EU in the Spanish legal system. In this regard, it introduces modifications that are in line with the provisions of the NP and of the EU ABS Regulation. It is for this purpose that provisions have been established for the regulation of the access to Spanish GR from wild taxa and to the promotion of traditional knowledge for the conservation of natural heritage and biodiversity. A new article on the control of the use of GR has been added [
26]. Royal Decree 124/2017, as of 24 February, on the access to GR from wild taxa and the control of use; establishes specific procedures for the access to Spanish GR, in situ and ex situ, from wild taxa; and for the control of the use of the GR and aTK in Spain. This regulation excludes access to certain GR, including when they are exclusively for taxonomic purposes, or phytogenetic, and animal GR for agriculture and food [
27].
In Spain, one CNA and nineteen Regional Competent Authorities were established with different roles (see
Table S1 for further details) [
28], while the Ministry for the Ecological Transition serves as the NFP for the NP. Article 5 of Royal Decree 124/2017 designates the competent authority regarding the access to GR from wild taxa. For the purposes of the provisions of Article 71.3 of Law 42/2007, in the scope of the General State Administration, the competent authority for the access to GR from wild taxa is the General Directorate of Quality and Environmental Evaluation and Natural Environment of the Ministry of Agriculture and Fisheries, Food and Environment, currently named Ministry for the Ecological Transition. It is responsible for providing the PIC and negotiating the MAT: (a) the Directorate-General for Sustainability of the Coast and Sea of the Ministry for the Ecological Transition, for marine GR; (b) the respective body of the General Administration of the State, for the GR that are in public property owned by the State; (c) the managing body of the ex situ conservation institution, for the GR in ex situ conservation institutions of a national character or ownership; and (d) the body that determines the autonomous community or autonomous communities where access to the GR was performed, in the case of terrestrial wild taxa GR from whose area of distribution covers more than one autonomous community. If the GR are not included in any of the above-mentioned scenarios, the competent authority will be the body designated by the autonomous community in the territory they are (see
Table S1). According to Article 13 of Royal Decree 124/2017, concerning the designation of the Spanish competent authorities for the application of EU ABS Regulation, the following bodies of the public administrations are competent authorities:
- (1)
General Directorate of Quality and Environmental Evaluation and Natural Environment of the Ministry of Agriculture and Fisheries, Food and Environment (currently Ministry for the Ecological Transition) (Functions described in Article 13.1 of Royal Decree 124/2017).
- (2)
Competent bodies that designate the autonomous communities within the scope of their competences. (Functions described in Article 13.2 of Royal Decree 124/2017) [
28].
Currently, Spain has made 52 permits (or their equivalents) available to the ABS Clearing House, constituting an IRCC, issued at the time of access as evidence of the decision to PIC; the establishment of MAT; and for the constitution of an IRCC [
29].
Spain has three checkpoints, two of them corresponding to the ones that are established by the EU legislation and one that is established in the Spanish legislation (Article 14.3, Royal Decree Law 124/2017). The third checkpoint is related to the users of GR and/or aTK when applying for a patent [
25,
28].
In Spain, the access to GR is regulated and subject to PIC (there is information on how to apply). Detailed information is described in Article 71 of Law 42/2007 and Articles 6, 7, and 8 of the Royal Decree 124/2017. The PIC, MAT, and access authorization are considered in Article 71 of Law 42/2007 and Articles 6 and 7 of the RD 124/2017. In addition, Article 7 of Royal Decree 124/2017 allows the Sectoral Conference on the Environment to approve guidelines for the establishment of MAT, including the distribution of benefits derived from the use of GR. This development is currently pending [
23,
25,
28].
Spain has taken legislative, administrative, or policy measures to implement Article 5 of the NP with regard to fair and equitable benefit-sharing. These are implemented under Article 4(1) of the EU ABS Regulation that states that users shall exercise due diligence under Article 71 of Law 42/2007 and Articles 6 and 7 of Royal Decree 124/2017, which refer to fair and equitable sharing [
23,
25,
28].
Spain requires that GR users provide information related to PIC, to the GR source, to the establishment of MAT, and/or to the utilization of GR at a designated checkpoint, as provided in Article 17.1 (a)(i) and (ii) of the NP. That is in accordance with Articles 7(1) and 7(2) of Regulation ABS. Annexes II and III of the Commission Implementing Regulation provide the specific information requested from users, including information related to PIC, to the source of GR, to the establishment of MAT, and to the use of GR. As previous mentioned, Spain has included an additional control point (Article 14.3 of Royal Decree Law 124/2017) related to users of GR and/or aTK when applying for a patent. The information required for these users is in accordance with the contents established in the annexes of the Commission Implementing Regulation [
25,
28].
In Spain, access requests and due diligence statements can be submitted electronically through the electronic headquarters of the Ministry for the Ecological Transition [
30] and not directly to DECLARE. In addition, Article 11 of Royal Decree 124/2017 creates the state information system on access and use of GR and/or aTK in Spain, with the objective of coordinating information regarding both access to Spanish GR and the use in Spain of GR and/or aTK [
25,
28].
Spain does not have indigenous and local communities and, for that reason, no provisions have been taken related to those (Articles 6, 7 and 12) [
28].
Spain is encouraging the development of codes of conduct, model contractual clauses for MAT, guidelines, and best practices, in line with Article 13 of Regulation ABS. For its part, Spain foresees it in the legislation; Article 7 of Royal Decree 124/2017, on access to GR from wild taxa and the control of utilization, dictates that the Sectoral Conference on the Environment will approve guidelines for the establishment of MAT, including the distribution of the benefits derived from the use of GR [
28].
The contribution of Spain for the implementation and application of the NP has been proactive. The creation of a Genetic Resources Section on the webpage of the Ministry for the Ecological Transition, the presentation of seminars about EU ABS Regulation with each of the sectors that use GR in Spain, elaboration of documents of frequently asked questions about EU ABS Regulation, and the elaboration of Users Manuals for requests of Due Diligence Declarations are among the measures taken [
28].
4.2.3. France
France became a party to the NP on 29 November 2016 and, in addition to the EU regulations, has developed legislative measures, namely, the Law on Biodiversity nº 2016-1087 [
31]. This is completed by the Decree nº 2017-848 [
32] (regarding ABS procedures related to GR and/or aTK) and an Administrative Order nº 0228 [
33] (that establishes the ABS standard contract of GR used from national territory, according to the
Code de l’environnement) (
Table 1). In addition, an Order of March 20, 2018 relating to the procedures for the registration of the collections in the EU collection register is also available at the ABS Clearing House website [
34]. Although France has eleven overseas territories, at the moment, specific measures are only being developed for French Polynesia (
Code de l’environnement de la Polynésie française) and New Caledonia (
Code de l’environnement de la Province Sud de Nouvelle-Calédonie), and draft texts are being prepared within the Province Nord and Loyauté Islands.
In France, distinctions have been made between the NFP and CNA tasks. The NFP is the Ministry of Environment (
Ministère de la Transition écologique et solidaire), which is responsible for Article 13.1 of the NP, mainly general ABS information, liaison with the Secretariat, international aspects of the NP – COP/MOP, and the Compliance Committee. The same Ministry is defined as the CNA, responsible for matters of: Due Diligence Declaration, access declaration to GR and/or aTK, access authorization request for the utilization of GR and/or aTK for commercial purposes, and and the standard contract for benefit-sharing [
35,
36]. The Ministry of Research (
Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation - MESRI) is also a CNA, responsible for: Due Diligence Declaration of research projects funded by external sources, register of collections at the European level, and the list of model species for research [
36]. The delegation of different responsibilities between the NFP and the CNA in France is an internal decision (see
Table S1) [
35].
The Law on Biodiversity (Art. L. 412-18 (II)) transposes Article 17 of the NP, whereas France requires PIC and MAT regulating the access to the GR. The Law on Biodiversity defined entities responsible for monitoring the utilization of the GR: The Ministry of Environment and Ministry of Research that gather due diligence declarations from all users, the National Institute of Industrial Property (INPI) – Patent Office for final stage of product development, and the Ministry of Research for research purposes. These three monitoring checkpoints shall convey relevant information to the Minister of Environment for the final compliance check, who is then responsible for providing the declarations to the ABS Clearing House and verifying the compliance of their own users [
35,
36,
37].
The Law of Biodiversity also regulates the ABS of GR and/or aTK (in situ and ex situ) and specifies a list for which provisions are granted and excluded. The access to GR and/or aTK is regulated by this law, and PIC is required. Digital data obtained from gene sequencing is frequently stored in publicly available databases, though it is not part of the scope of NP, nor of the EU ABS Regulation 511/2014. Nevertheless, France Interim National Report stresses the fact that their law only covers situations where there is a “tangible” link to the GR (in situ and ex situ collection) [
35].
France has taken legislative, administrative, or policy measures to implement Article 5 of the NP with regard to fair and equitable benefit-sharing. Nevertheless, it is important to mention that specific situations exist in the overseas territories. These are implemented under the French Law on Biodiversity nº 2016-1087, the ABS Implementing Decree Nº 2017-848, and the Administrative Order of September 13, 2017. Decree nº 2017-848 details specific procedures for the utilization of traditional knowledge [
35].
France requires that GR users provide information related to PIC, to the GR source, to the establishment of MAT, and/or to the utilization of GR at a designated checkpoint, as provided in Article 17.1 (a)(i) and (ii) of the NP. In accordance with Article 7(1) and 7(2) of Regulation ABS, users need to provide information as required by Article 17.1 of the NP. Annexes II and III of the Commission Implementing Regulation provide the specific information requested from users, including information related to PIC, to the source of GR, to the establishment of MAT, and to the use of GR. In addition, competent authorities in France perform checks on user compliance in accordance with a periodically revised risk-based plan [
35].
According to the
Ministère de la Transition écologique et solidaire, two paths can be followed with regard to granting the access to GR and/or aTK, though it does not substitute the European level platform, DECLARE. Thus, printed forms from
Centre d’Enregistrement et de Révision de Formulaires Administratifs (CERFA) or a dematerialized procedure were defined. CERFA printed forms exist as: an authorization for the use of GR and for aTK, a declaration on the use of GR, and as Due Diligence Declarations. These forms are to be sent to the
apa@developpement-durable.gouv.fr. The dematerialized procedure, a more recent update, can be done online. These forms exist for declarations on the use of GR or aTK and for an authorization for commercialization, whether it is for a legal or natural person [
37]. The administrative order nº 0288 implements the MAT as a standard contract that comprises the obligations of the user to share the benefits of the usage of GR and/or aTK with the local communities [
35].
European and French laws predict a respectful attitude from the user’s point of view regarding the origin of GR and/or aTK, meaning prior consent before utilization. In case of noncompliance, the French Law on Biodiversity foresees sanctions that can go from administrative to criminal level. The French Biodiversity Agency is an important entity, created at the time of the Law on Biodiversity to implement public measures in the areas of knowledge, preservation, management, and recovery of terrestrial biodiversity. French law considers it an obligation to use the benefits generated from the application of the ABS system, to use the revenues to finance projects of conservation and sustainability, and to support local communities when benefits arise from the aTK. In addition, a legal entity was created (
personne morale de droit public) aiming to support local communities to engage and clarify the ABS contract to them. At present, France has made 233 permits (or their equivalents) available to the ABS Clearing House, constituting an IRCC, issued at the time of access as evidence of the decision to PIC, the establishment of MAT, and for the constitution of an IRCC [
35,
38].
France has “indigenous and local communities”, in which definition is presented in the Law on Biodiversity, given the diversity of situations existent in the overseas territories. These communities do not have the established right to grant access to GR, since according to the French law, those are considered common heritage of the French nation. Furthermore, according to the CBD, the principle of the sovereign rights of the state over the GR applies. The French Law on Biodiversity (2016) [
31] and the Implementing Decree Nº 2017-848 [
32] ensure that the traditional knowledge is accessed with the communities’ approval and benefit-sharing is agreed in a written contract with the legal representative of the community [
35].
France encourages the development of codes of conduct, model contractual clauses for MAT, guidelines, and best practices in line with Article 13 of Regulation ABS. A standard contract has been developed in France to ensure legal certainty for users and to support the implementation of benefit-sharing [
35].
Finally, France has been an active player in supporting the development capacity for the implementation of the NP. It has contributed financially to the ABS GEF Fund Support for developing countries to ratify the NP, GIZ ABS Initiative, and two ABS projects through the French Environmental Fund (“Phytotrade Africa” project and “Sud Expert Plantes Développement Durable” (SEPDD) project – French Development Agency) [
35].
4.2.4. United Kingdom of Great Britain and Northern Ireland
In the United Kingdom (UK) and Northern Ireland (NI), the NP entered into force on 22 May 2016. In order to implement the EU Regulation and elements of the NP, the UK and NI developed The Nagoya Protocol (Compliance) Regulation 2015 No. 821 [
39].
The NFP is the Department for Environment Food and Rural Affairs, whereas the CNA is The Office for Product Safety and Standards (OPS&S), Department of Business, Energy & Industrial Strategy (DBEIS) (
Table S1). The UK and NI ratification of the NP does not extend to the fourteen overseas territories and three Crown Dependencies, such as Bermuda, Cayman Islands, and Gibraltar. The inexistence of access measures to GR means that there is not an established PIC or MAT. However, other aspects of UK law will be relevant for researchers when accessing GR from and within the UK: plant and animal health, property rights, and physical access rights. The fact that UK and NI do not have access measures on GR means that the collection can be done. However, under the Regulation ABS, due diligence must be performed and delivered to the Regulatory Delivery, currently named as The Office for Product Safety and Standards, which is defined as the official checkpoint. There are two checkpoints, one for research and other for stakeholders, which are at the last phase of product development, as it occurs in the EU. Declarations can be found in Implementing Regulation (EU) 2015/1866 (Annex II and III) and will be further officially submitted to the EU web-based application, DECLARE. The OPS&S will then provide the information to the ABS Clearing House, in order to emit the checkpoint communiqué. If confidential details are present, the ABS Clearing House will therefore contact the providers of the GR [
40,
41].
The legislative, administrative, or policy measures on fair and equitable benefit-sharing of Article 5 of the NP are provided by Article 4(1) of the EU ABS Regulation. However, the NP (Compliance) Regulation 2015 No. 821 defines the secretary of state as the competent authority for EU ABS Regulation purposes. The same is responsible for creating conditions of compliance on fair and equitable benefit-sharing and must monitor user compliance. Under noncompliance conditions, the OPS&S is responsible for carrying out checks on user compliance, in accordance with a periodically revised risk-based plan. Since the UK and NI do not have formal measures, their vision is to encourage the sharing of information as an association rather than providing an individual user treatment [
40,
41].
The UK and NI do not have indigenous or local communities; therefore, they do not have formal measures for ABS [
41].
The UK and NI encourage the development of codes of conduct, model contractual clauses for MAT, guidelines, and best practices in line with Article 13 of Regulation ABS. The UK is reviewing the best practice documents and developing guidance aimed to assist several sectors [
41].
The contributions of UK and NI for the implementation and application of NP have been proactive by the development of guidance and best practice documents, training sessions, and workshops. At the international level, the Darwin Initiative is supporting developing countries, such as Cameroon, Namibia, and South Africa. This initiative aims to develop a Community of Practice around ABS, and more information can be found on its website [
42]. Concurrently, work has been done by means of collaboration in technical and scientific research and development programs, such as the Biotechnology and Biological Sciences Research Council (BBSRC) and Medical Research Council (MRC) [
41].
It is important to mention that Brexit may lead to modifications in the context of the implementation of the EU legislation, as well as the use of the DECLARE platform. However, those are out of the scope of this revision. There is no information about Brexit available at the ABS Clearing House website. However, a draft of “The Nagoya Protocol (Compliance) (Amendment) (EU Exit) Regulations 2018” was published at the site of the UK government to ensure that regulations in the UK which implement the Nagoya Protocol will continue to be operable after the UK leaves the EU [
43]. The changes proposed are to: (1) clarify that the current responsibilities regard to “best practices” and “registered collections” will, in future, lie with the secretary of state; (2) “amend references to the Union, Member States and the Commission as appropriate to UK references”; and (3) “delete clauses that will no longer be appropriate, such as obligations to inform the Commission or other Member States” [
44].
4.2.5. Ireland
Ireland has been a signatory of the NP since 1 February 2012 and, in accordance with ABS Clearing House, it has not yet become a party [
45]. However, a recent regulation was published, S.I. No. 253 of 2019, creating a legislative framework that allows Ireland to proceed the ratification of the Nagoya Protocol [
46]. The Minister of Culture, Heritage and the Gaeltacht is assigned as the CNA. In addition, the S.I. No. 253 of 2019 regulates at the level of ABS Regulation Articles 4, 5, and 7 in terms of compliance and specifies procedures for notifications and sanctions in cases of noncompliance. Collection register is part of the regulation, whereas the collection holder must notify the can, and the Commission must be notified by the CAN [
47]. Further information can be found next to its ABS NFP, which is the Biodiversity Policy Department of Culture (
Table S1) [
45].
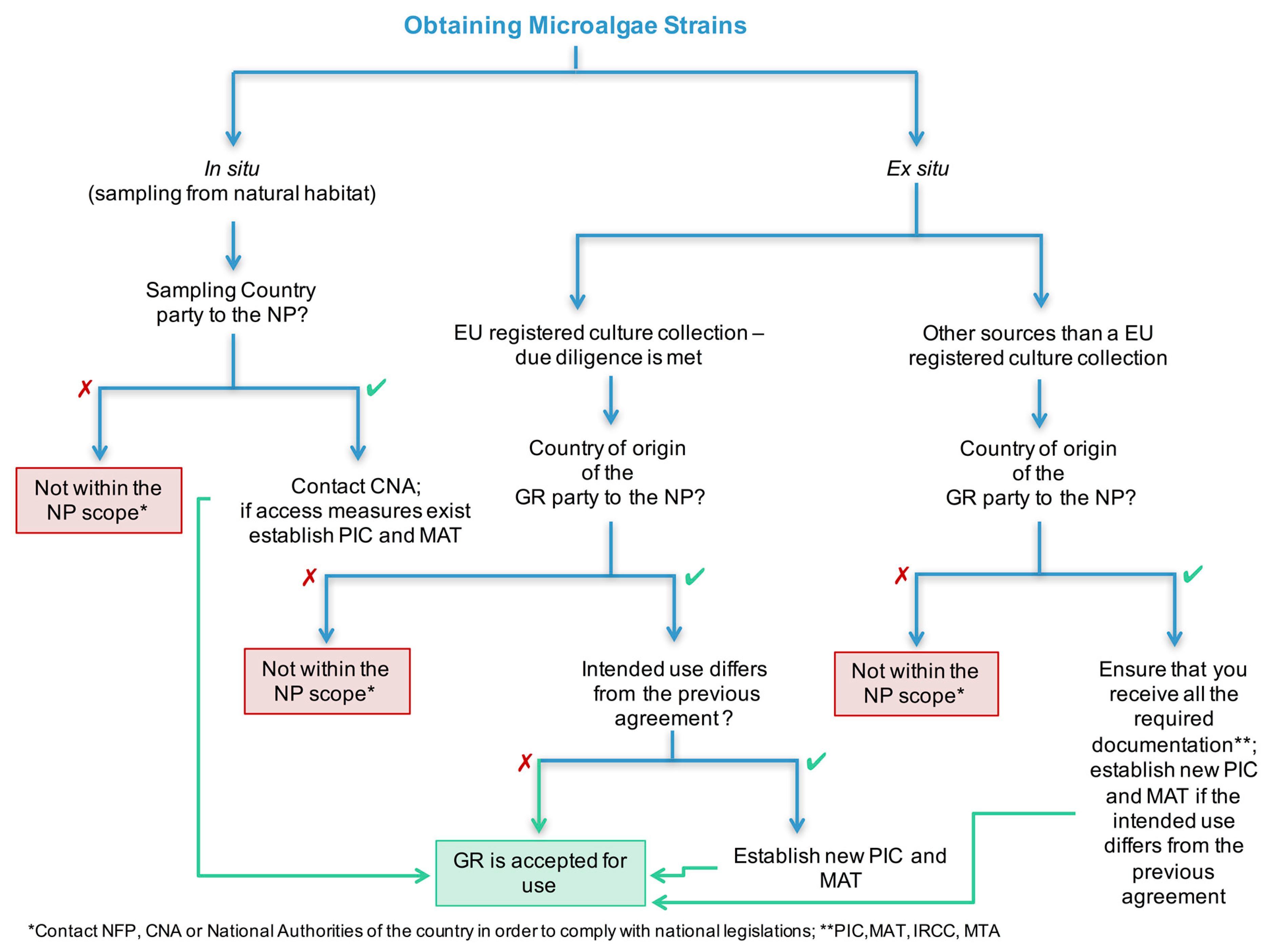
4.3. Dealing with GR: A Practical Guide for EU Atlantic Area Countries Users
Atlantic Area countries are currently investing in microalgae-based products [
5]. The legal complexity surrounding the application of the NP and ABS measures warrants urgent coordination of many factors with different countries. For that reason, we thought about proposing a simple practical guide that can be used in the EU microalgae research and development sector when dealing with practical situations regarding the use of the GR. However, the target group for this guide may be expanded.
The proposed decision tree (
Figure 1) is based on the specific terms of the scope of ABS regulations. NP may apply if the GR: (1) are defined as “any material of plant, animal, microbial or other origin containing functional units of heredity – of actual or potential value”, (2) were accessed after 12 October 2014, and (3) will be utilized in the meaning of “conducting R&D on the genetic or biochemical composition of the GR, including through the application of biotechnology” [
8,
9,
13]. In this sense, microalgae clearly meet the definition of GR as applied by the CBD and the NP. Nevertheless, it is important to state that there is lack of consensus about whether a genetic sequence data should be considered a GR under this definition [
48]. Secondly, the NP will only apply if the microalgae have been accessed after 12 October 2014. Further, the use of microalgae under the NP will only apply if defined as previously mentioned. Nevertheless, the definition of the term R&D is not always clear and may be subject to discussion [
49].
The microalgae research and development sector the origin of the GR commonly applies to field samples, culture collections, or other distinct sources (
Figure 1). In this way, researchers that are planning to conduct sampling campaigns (GR obtained in situ) will need to ascertain whether the provider country is a party to the NP and if ABS measures are in place. As concerns the reviewed EU Atlantic Area countries, only Ireland is not a party to the NP. If the provider country is party to the NP, the CNA must be contacted, and the terms for legal access and use of the GR must be established. Other common situations in the microalgae sector are to receive strains from different types of sources (GR obtained ex situ), such as culture collections (EU registered or not) or other users. EU registered culture collections bring advantages to the user at the same time due diligence is met for both parts (
Figure 1). Compliance can be eased through the report of the culture collection and strain number, avoiding the user’s quest for information next to the original depositor to fill the PIC and MAT. The EU register process is a long-term investment, due to the high investment and time-consuming phases. At the moment, only one culture collection, the Leibniz Institute German Collection of Microorganisms and Cell Cultures GmbH (DSMZ), has obtained the EU register status under the NP [
50]. Moreover, culture collections can opt to obtain a partial register of their collection, i.e., for more relevant strains or even for strains with all legal information. Nevertheless, it is important to understand that if the country of the origin of the GR is party to the NP, and if the intended use of GR is not in accordance with the previously established agreements, the actual intended use must be negotiated and novel PIC and MAT must be obtained before the GR is accepted for use (
Figure 1). Nonregistered EU collections have dedicated many efforts in maintaining and providing strains to users all around the globe. However, they face a complicated scenario, in which to collect the data that provide traceability are constrained by: unawareness of the depositors to the NP matters, reduced number of countries with IRCC, and a lack of ABS-associated documents and prevailing national legislations on non-NP parties [
15,
51]. In the case where the GR source is other than a registered EU collection and its origin is from a country that is party to the NP, it is important to ensure that all the necessary documentation is being received (PIC, MAT, IRCC, and MTA) and if the country ABS requirements, if any, have been followed, before considering to use the GR.
Therefore, users must be aware of the due diligence documentation, namely, PIC, MAT, IRCC, and Material Transfer Agreement (MTA), when applicable [
13,
15,
49]. First, PIC must be obtained from the NCA detailing what, how, and by whom is collected the GR. The expected use of the GR, as well as the circumstances that may require renegotiations of the existent PIC (e.g., changes in the proposed use), must also be described. Nevertheless, if the provider country does not have access rules to the GR and no need of PIC, the users are advised to document that no conditions were requested to access the GR. Secondly, conditions for access and use the GR (e.g., benefit-sharing) must be negotiated through the establishment of MAT, with the NCA of the provider country. The MAT certificate usually describes restrictions of use, transfer to third parties, reporting requirements, and data and benefit-sharing obligations. In order to demonstrate that the PIC and the MAT have been established with the NCA of the provider country, the IRCC must be obtained from the ABS Clearing House website, kept, and transferred through the users. If the IRCC is not available (e.g., GR obtained ex situ), users must acquire documentation on: the characterization, date, and place of access of the GR; provide sources and subsequent users of the GR; and the presence/absence of rights/obligations related to benefit-sharing and relevant access permits, agreements, and contracts. At last, when stated in the MAT, an MTA regarding the transfer of ownership, ensuring adequate transmission of documentation and reinforcement of ABS compliance, should be signed for transferring the GR. This will provide legal certainty regarding the transference of the GR, as well as the required information stated in the EU ABS regulation (Article 4). In addition, a record of all the shared benefits related to the GR must be kept by the users [
13,
49].
In sum, the provider country status, concerning the NP, and access date are relevant information to consider. In addition, NFP, CNA, or other national authorities are essential in the process of complying with ABS regulation. The ABS Clearing House website can be accessed by any user to check the status of the country relative to the NP, as well as other relevant information’s (e.g., IRCC). Throughout the whole process, it is recommended that the user keep the records of contacts with any authority, in order to support their effort of due diligence.
5. Discussion and Conclusions
The NP intends to encourage the conservation of the biological diversity and the sustainable use of its components by promoting the utilization of GR and/or aTK through the reinforcement of fair and equitable sharing of benefits. Nevertheless, the implementation of the NP at the EU level seems to be a difficult task, since there are several factors that make the employment of the same particularly challenging [
52]. It is out of the scope of this review to discuss the difficulties in the implementation of the NP; still, we intended to compare the legal regimes that prevail in the EU, particularly in its Atlantic Area countries.
In order to harmonize the application of the NP in its Member States, the EU has developed specific legislation, namely Regulation (EU) Nº 511/2014 (ABS Regulation) and Regulation (EU) 2015/1866. All Member States, as well as users of GR and/or aTK in the EU, are bound by these regulations, regardless of whether or not they are individually party to the NP. Spain was the first country, among the reviewed ones, to become a party to the NP, whereas Portugal was the last one. In this sense, different countries had developed national legislation in the NP framework at different dates, and for that, complying with national regimes is a task that is still ongoing. For example, Ireland has not yet become a party to the NP and is in the process of the development of national legislation to implement EU ABS Regulation. One important fact is that EU Member States are free to decide to control, or not, the access to their GR, since EU regulations do not establish access measures. This is one of the first points where the EU Member States reveal different views on the use of GR, since some have chosen to adopt access measures, while others have not. Concerning the reviewed EU Atlantic Area countries, the UK and NI have chosen not to control access to GR. On the contrary, France, Spain, and the Autonomous Regions of Azores in Portugal adopted access measures to GR. This means that access to GR within the EU will follow different procedures and distinct measures will apply, depending on the GR provider country. For example, a user that intends to collect a GR in France, where access measures are in place, will have to contact its CNA and establish the necessary documentation, while a user that intends to do the same in the UK and NI will have a less bureaucratic burden, in the aim of the NP legislation. Another important distinction between the countries is that some present more than one competent authority. A good example of this is Spain, which has one CNA and nineteen Regional Competent Authorities, all with different roles. It is expected that the workflow of the legal procedures for obtaining a GR from different EU Member States follow different steps, and the users must be aware of that. Some EU Atlantic Area countries also have additional checkpoints to the ones that are mandatory in EU regulations, as is the case for Spain and France. Therefore, it is important to ensure that users are well-informed of their own country’s requirements for monitoring and reporting the use of GR from other parties to the NP.
The ABS Clearing House is a good platform to obtain and exchange information on the ABS. Nevertheless, on many of the countries’ webpages, the available information is scarce and/or not yet updated. In addition, when information regarding national legislation is available, it is not always translated from the original language and does not contain the precise steps as to how to act in accordance with the laws of each country. All of these facts contribute to the difficult process of obtaining accurate information.
The high diversity of existent regulations and competent authorities dealing with the NP and ABS matters at regional, national, and international levels makes the due diligence process heavy and difficult. As a consequence, GR and/or aTK users may prefer to obtain those in countries that do not exert their sovereign rights, avoiding time-consuming tasks and therefore rejecting the CBD principles and leaving behind many GR with potential interests. Culture collections are one of the most common sources of microalgae, especially as concerns academic research. Those are keepers of biodiversity and should be valued for the service they provide. Conversely, the NP at the EU level reduces the importance of the ones that are not EU-registered at the same time that it burdens their path for register. In this way, support should be provided to these collections in order to allow a more efficient transition/register according to the EU standards and reduce the underutilization of this biological diversity.
The NP is a complex treaty, in which the implementation of and compliance with requires detailed awareness of the relevant EU and country-level legislations. Several challenges still remain in the implementation and operationalization related with the NP. It is expected that, in the future, the NP will become an instrument that accurately supports the fair and equitable sharing of benefits arising from the use of GR worldwide.