Exploring Traits of Engineered Coral Entities to be Employed in Reef Restoration
Abstract
1. Introduction
2. Materials and Methods
2.1. Planulae Collection, Rearing, and Creating Chimeras
2.2. Monitoring
2.3. Statistical Analysis
3. Results
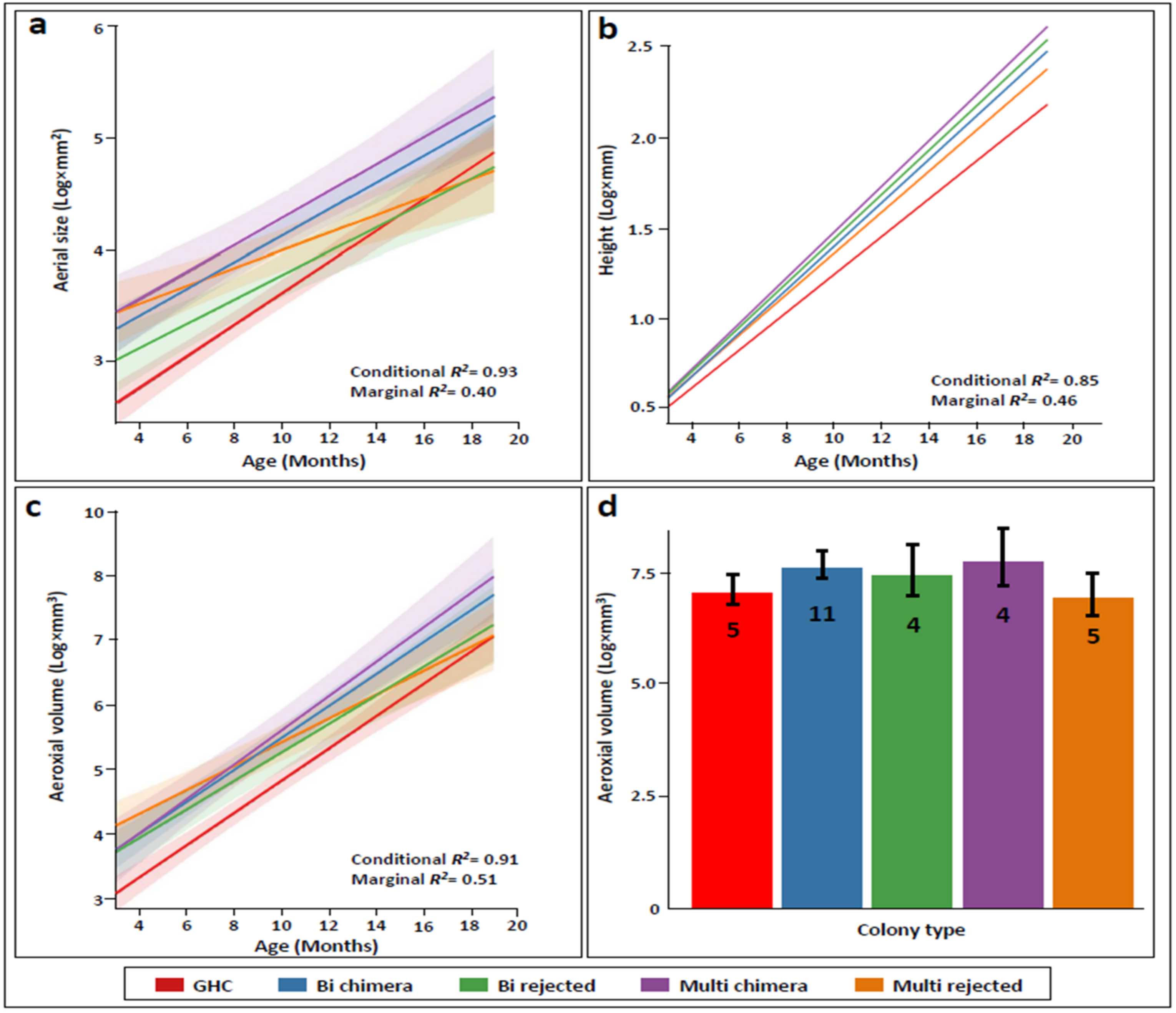
3.1. Aerial Size
3.2. Height
3.3. Aeroaxial Ecological Volume
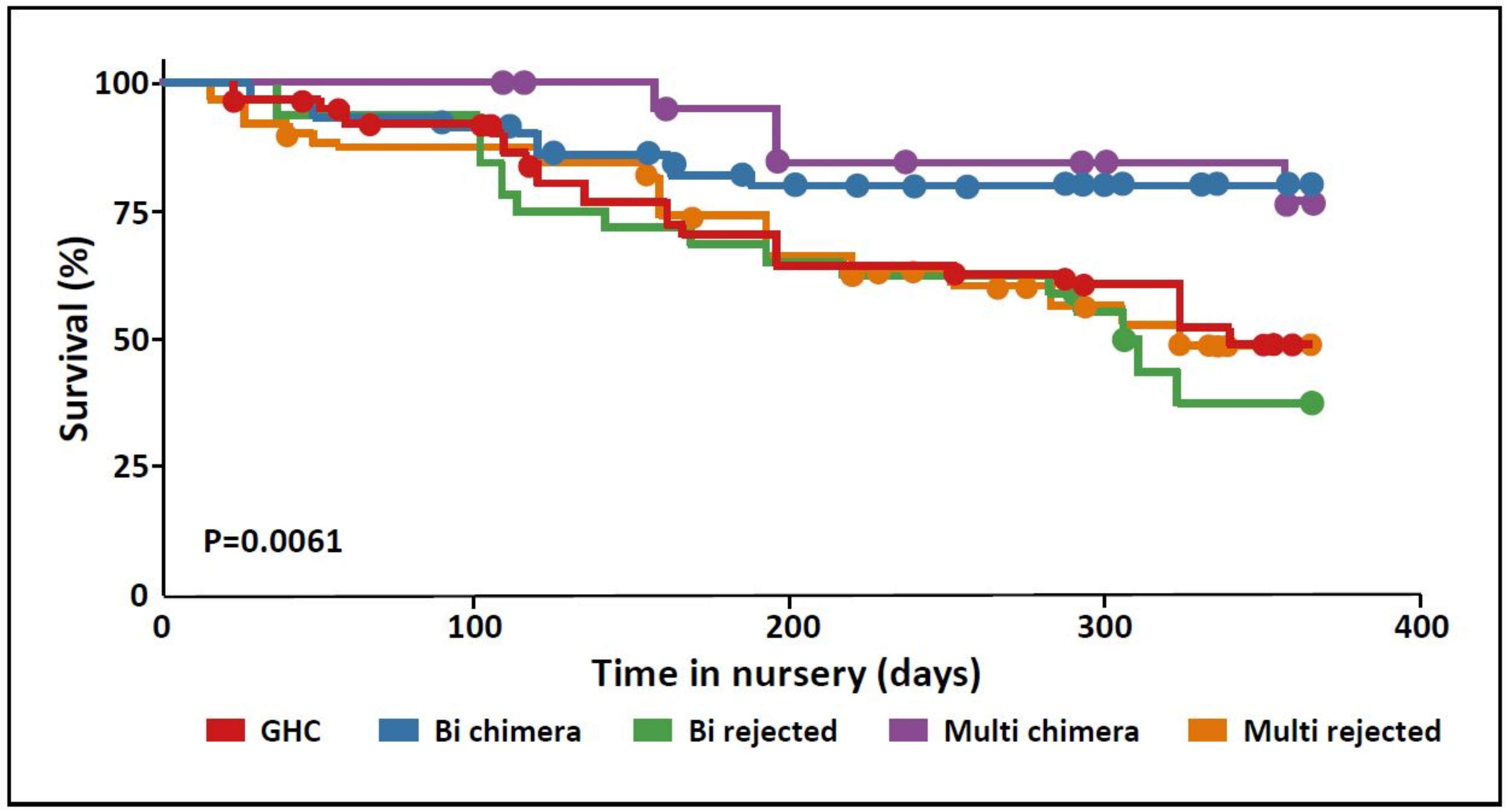
3.4. Survival
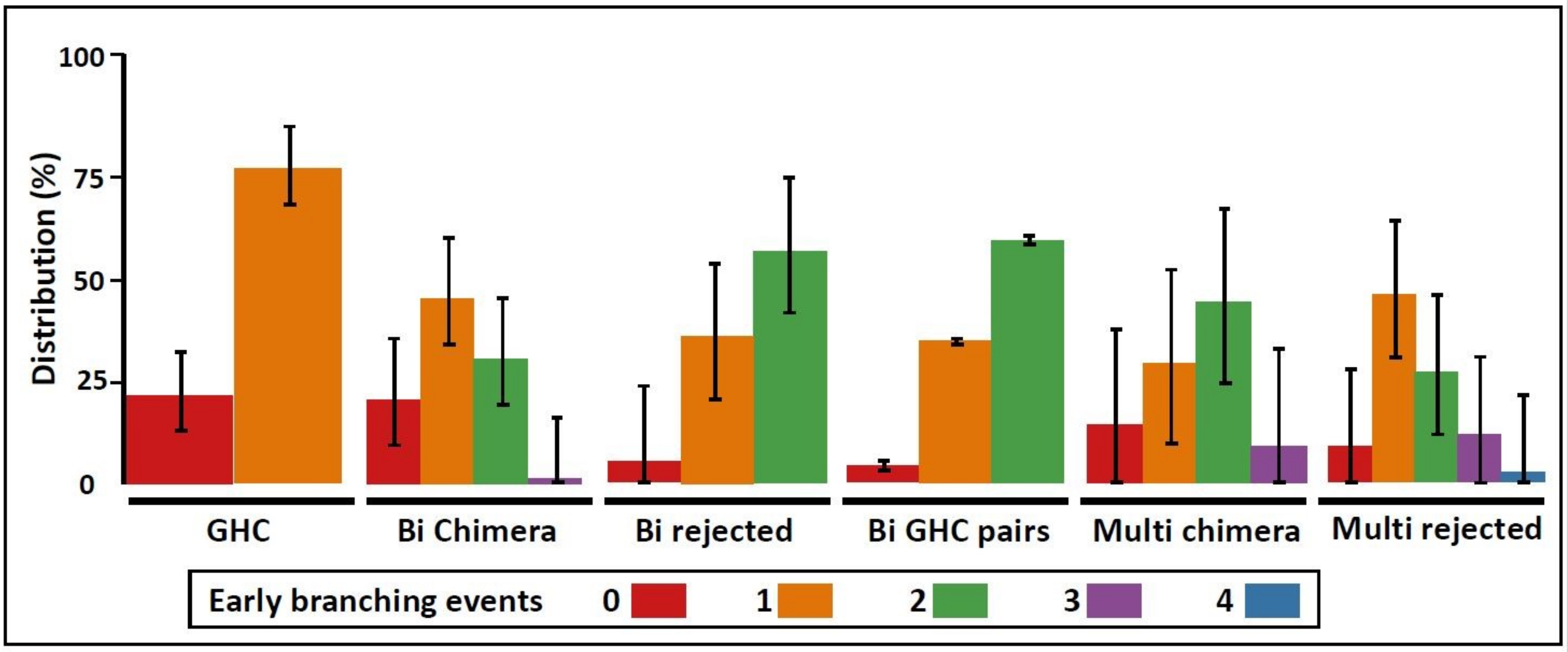
3.5. Astogenic Statuses for Early Branching Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rinkevich, B. Quo vadis chimerism? Chimerism 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Schweinsberg, M.; Weiss, L.C.; Striewski, S.; Tollrian, R.; Lampert, K.P. More than one genotype: How common is intracolonial genetic variability in scleractinian corals? Mol. Ecol. 2015, 24, 2673–2685. [Google Scholar] [CrossRef]
- Magor, B.G.; De Tomaso, A.; Rinkevich, B.; Weissman, I.L. Allorecognition in colonial tunicates: Protection against predatory cell lineages? Immunol. Rev. 1999, 167, 69–79. [Google Scholar] [CrossRef]
- Rinkevich, B.; Weissman, I.L. Variation in the outcomes following chimera formation in the colonial tunicate Botryllus schlosseri. Bull. Mar. Sci. 1989, 45, 213–227. [Google Scholar]
- Adams, K.M.; Nelson, J.L. Microchimerism: An investigative frontier in autoimmunity and transplantation. J. Am. Med. Assoc. 2004, 291, 1127–1131. [Google Scholar] [CrossRef]
- Buss, L.W. Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc. Natl. Acad. Sci. USA 1982, 79, 5337–5341. [Google Scholar] [CrossRef]
- Ross, C.N.; French, J.A.; Orti, G. Germ-line chimerism and paternal care in marmosets (Callithrix kuhlii). Proc. Natl. Acad. Sci. USA 2007, 104, 6278–6282. [Google Scholar] [CrossRef]
- Ilan, M.; Loya, Y. Ontogenetic variation in sponge histocompatibility responses. Biol. Bull. 1990, 179, 279–286. [Google Scholar] [CrossRef]
- Albarella, S.; De Lorenzi, L.; Catone, G.; Magi, G.E.; Petrucci, L.; Vullo, C.; D’Anza, E.; Parma, P.; Raudsepp, T.; Ciotola, F.; et al. Diagnosis of XX/XY blood cell chimerism at low percentage in horse. J. Equine Vet. Sci. 2018. [Google Scholar] [CrossRef]
- Noble, J.P.A.; Lee, D. First report of allogeneic fusion and allorecognition in tabulate corals. J. Paleontol. Soc. 1991, 65, 69–74. [Google Scholar] [CrossRef]
- Helm, C.; Schülke, I. Contact reactions and fusion of Late Jurassic ramose coral Thamnasteria dendroidea in a patch reef environment. Coral Reefs 2000, 19, 89–92. [Google Scholar] [CrossRef]
- Puill-Stephan, E.; Willis, B.L.; van Herwerden, L.; van Oppen, M.J.H. Chimerism in wild adult populations of the broadcast spawning coral Acropora millepora on the Great Barrier Reef. PLoS ONE 2009, 4, e7751. [Google Scholar] [CrossRef]
- Hennige, S.J.; Morrison, C.L.; Form, A.U.; Büscher, J.; Kamenos, N.A.; Roberts, J.M. Self-recognition in corals facilitates deep-sea habitat engineering. Sci. Rep. 2014, 4, 6782. [Google Scholar] [CrossRef]
- Puill-Stephan, E.; van Oppen, M.J.H.; Pichavant-Rafini, K.; Willis, B.L. High potential for formation and persistence of chimeras following aggregated larval settlement in the broadcast spawning coral, Acropora millepora. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2012, 279, 699–708. [Google Scholar] [CrossRef]
- Rinkevich, B.; Shaish, L.; Douek, J.; Ben-Shlomo, R. Venturing in coral larval chimerism: A compact functional domain with fostered genotypic diversity. Sci. Rep. 2016, 6, 19493. [Google Scholar] [CrossRef]
- Maier, E.; Buckenmaier, A.; Tollrian, R.; Nürnberger, B. Intracolonial genetic variation in the scleractinian coral Seriatopora hystrix. Coral Reefs 2012, 31, 505–517. [Google Scholar] [CrossRef]
- Oury, N.; Gelin, P.; Magalon, H. Together stronger: Intracolonial genetic variability occurrence in Pocillopora corals suggests potential benefits. Ecol. Evol. 2020, 10, 5208–5218. [Google Scholar] [CrossRef]
- Fidler, A.E.; Bacq-Labreuil, A.; Rachmilovitz, E.; Rinkevich, B. Efficient dispersal and substrate acquisition traits in a marine invasive species via transient chimerism and colony mobility. PeerJ 2018, 6. [Google Scholar] [CrossRef]
- Cameron, K.A.; Harrison, P.L. Density of coral larvae can influence settlement, post-settlement colony abundance and coral cover in larval restoration. Sci. Rep. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Nozawa, Y.; Loya, Y. Genetic relationship and maturity state of the allorecognition system affect contact reactions in juvenile Seriatopora corals. Mar. Ecol. Prog. Ser. 2005, 286, 115–123. [Google Scholar] [CrossRef]
- Wijayanti, D.P.; Hidaka, M. Is genetic involve in the outcomes of contact reactions between parent and offspring and between siblings of the coral Pocillopora damicornis? ILMU Kelaut. Indones. J. Mar. Sci. 2018, 23, 69. [Google Scholar] [CrossRef]
- Linden, B.; Rinkevich, B. Elaborating an eco-engineering approach for stock enhanced sexually derived coral colonies. J. Exp. Mar. Bio. Ecol. 2017, 486, 314–321. [Google Scholar] [CrossRef]
- Linden, B.; Rinkevich, B. Creating stocks of young colonies from brooding coral larvae, amenable to active reef restoration. J. Exp. Mar. Bio. Ecol. 2011, 398, 40–46. [Google Scholar] [CrossRef]
- Chang, E.S.; Orive, M.E.; Cartwright, P. Nonclonal coloniality: Genetically chimeric colonies through fusion of sexually produced polyps in the hydrozoan Ectopleura larynx. Evol. Lett. 2018, 2, 442–455. [Google Scholar] [CrossRef]
- Toh, T.; Chou, L. Aggregated settlement of Pocillopora damicornis planulae on injury sites may facilitate coral wound healing. Bull. Mar. Sci. 2013, 89, 503–504. [Google Scholar] [CrossRef]
- Amar, K.O.; Chadwick, N.E.; Rinkevich, B. Coral kin aggregations exhibit mixed allogeneic reactions and enhanced fitness during early ontogeny. BMC Evol. Biol. 2008, 8, 126. [Google Scholar] [CrossRef]
- Amar, K.O.; Rinkevich, B. Mounting of erratic histoincompatible responses in hermatypic corals: A multi-year interval comparison. J. Exp. Biol. 2010, 213, 535–540. [Google Scholar] [CrossRef]
- Barki, Y.; Gateño, D.; Graur, D.; Rinkevich, B. Soft-coral natural chimerism: A window in ontogeny allows the creation of entities comprised of incongruous parts. Mar. Ecol. Prog. Ser. 2002, 231, 91–99. [Google Scholar] [CrossRef]
- Frank, U.; Oren, U.; Loya, Y.; Rinkevich, B. Alloimmune maturation in the coral Stylophora pistillata is achieved through three distinctive stages, 4 months post-metamorphosis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1997, 264, 99–104. [Google Scholar] [CrossRef]
- Hidaka, M. Tissue compatibility between colonies and between newly settled larvae of Pocillopora damicornis. Coral Reefs 1985, 4, 111–116. [Google Scholar] [CrossRef]
- Nozawa, Y.; Hirose, M. When does the window close? The onset of allogeneic fusion 2–3 years post-settlement in the scleractinian coral, Echinophyllia aspera. Zool. Stud. 2011, 50, 396. [Google Scholar]
- Jiang, L.; Lei, X.; Liu, S.; Huang, H. Fused embryos and pre-metamorphic conjoined larvae in a broadcast spawning reef coral. F1000Research 2015, 4. [Google Scholar] [CrossRef]
- Rivera, H.E.; Goodbody-Gringley, G. Aggregation and cnidae development as early defensive strategies in Favia fragum and Porites astreoides. Coral Reefs 2014, 33, 1079–1084. [Google Scholar] [CrossRef]
- Mizrahi, D.; Navarrete, S.A.; Flores, A.A.V. Groups travel further: Pelagic metamorphosis and polyp clustering allow higher dispersal potential in sun coral propagules. Coral Reefs 2014, 33, 443–448. [Google Scholar] [CrossRef]
- Mercier, A.; Sun, Z.; Hamel, J.-F. Internal brooding favours pre-metamorphic chimerism in a non-colonial cnidarian, the sea anemone Urticina felina. Proc. R. Soc. B Biol. Sci. 2011, 278, 3517–3522. [Google Scholar] [CrossRef]
- Hidaka, M.; Yurugi, K.; Sunagawa, S.; Kinzie, R.A. Contact reactions between young colonies of the coral Pocillopora damicornis. Coral Reefs 1997, 16, 13–20. [Google Scholar] [CrossRef]
- Rinkevich, B. Allorecognition and xenorecognition in reef corals: A decade of interactions. Hydrobiologia 2004, 530–531, 443–450. [Google Scholar] [CrossRef]
- Rinkevich, B. Conserved histocompatible machinery in marine invertebrates? Invertebr. Surviv. J. 2015, 12, 170–172. [Google Scholar]
- Rinkevich, B.; Weissman, I.L. Incidents of rejection and indifference in Fu/HC incompatible protochordate colonies. J. Exp. Zool. 1992, 263, 105–111. [Google Scholar] [CrossRef]
- Duerden, J.E. Aggregated Colonies in Madreporarian Corals. Am. Nat. 1902, 36, 461–471. [Google Scholar] [CrossRef]
- Pancer, Z.; Gershon, H.; Rinkevich, B. Coexistence and possible parasitism of somatic and germ cell lines in chimeras of the colonial urochordate Botryllus schlosseri. Biol. Bull. 1995, 189, 106–112. [Google Scholar] [CrossRef]
- Stoner, D.S.; Rinkevich, B.; Weissman, I.L. Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proc. Natl. Acad. Sci. USA 1999, 96, 9148–9153. [Google Scholar] [CrossRef]
- Rinkevich, B.; Yankelevich, I. Environmental split between germ cell parasitism and somatic cell synergism in chimeras of a colonial urochordate. J. Exp. Biol. 2004, 207, 3531–3536. [Google Scholar] [CrossRef]
- Rinkevich, B.; Weissman, I.L. Chimeras vs. genetically homogeneous individuals: Potential fitness costs and benefits. Nord. Soc. Oikos 1992, 63, 119–124. [Google Scholar] [CrossRef]
- Boschma, H. On the Post Larval Development of the Coral Maeandra areolata; Carnegie Institution for Science: Washington, DC, USA, 1929. [Google Scholar]
- Chadwick-Furman, N.; Rinkevich, B. A complex allorecognition system in a reef-building coral: Delayed responses, reversals and nontransitive hierarchies. Coral Reefs 1994, 13, 57–63. [Google Scholar] [CrossRef]
- Rinkevich, B.; Frank, U.; Bak, R.P.M.; Müller, W.E.G. Alloimmune responses between Acropora hemprichi conspecifics: Nontransitive patterns of overgrowth and delayed cytotoxicity. Mar. Biol. 1994, 118, 731–737. [Google Scholar] [CrossRef]
- Frank, U.; Brickner, I.; Rinkevich, B.; Loya, Y.; Bak, R.P.M.; Achituv, Y.; Ilan, M. Allogeneic and xenogeneic interactions in reef-building corals may induce tissue growth without calcification. Mar. Ecol. Prog. Ser. 1995, 124, 181–188. [Google Scholar] [CrossRef]
- Raymundo, L.J.; Maypa, A.P. Getting bigger faster: Mediation of size-specific mortality via fusion in juvenile coral transplants. Ecol. Appl. 2004, 14, 281–295. [Google Scholar] [CrossRef]
- Rinkevich, B. The active reef restoration toolbox is a vehicle for coral resilience and adaptation in a changing world. J. Mar. Sci. Eng. 2019, 7, 201. [Google Scholar] [CrossRef]
- Rinkevich, B. Ecological engineering approaches in coral reef restoration. ICES J. Mar. Sci. 2020. [Google Scholar] [CrossRef]
- Rinkevich, B. Coral chimerism as an evolutionary rescue mechanism to mitigate global climate change impacts. Glob. Chang. Biol. 2019, 25, 1198–1206. [Google Scholar] [CrossRef]
- Shefy, D.; Shashar, N.; Rinkevich, B. The reproduction of the Red Sea coral Stylophora pistillata from Eilat: 4-decade perspective. Mar. Biol. 2018, 165, 27. [Google Scholar] [CrossRef]
- Baird, A.H. The Ecology of Coral larvae: Settlement Patterns, Habitat Selection and the Length of the Larval Phase. Ph.D. Thesis, James Cook University of North Queensland, Townsville, Australia, 2001. [Google Scholar]
- Rinkevich, B. Conservation of coral reefs through active restoration measures: Recent approaches and last decade progress. Environ. Sci. Technol. 2005, 39, 4333–4342. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-Squares Means: The {R} Package {lsmeans}. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Therneau, T.M. A Package for Survival Analysis in R 2020 Version 3.2-7. Available online: https://cran.r-project.org/package=survival (accessed on 2 September 2020).
- Shaked, Y.; Genin, A. The Israel National Monitoring Program in the Northern Gulf of Aqaba 2018. Available online: https://iui-eilat.huji.ac.il/Research/NMPReports.aspx (accessed on 16 April 2019).
- Sheked, Y.; Genin, A. The Israel National Monitoring Program in the Northern Gulf of Aqaba 2019. Available online: https://iui-eilat.huji.ac.il/Research/NMPReports.aspx (accessed on 2 September 2020).
- Rinkevich, B. The Branching Coral Stylophora pistillata: Contribution of Genetics in Shaping Colony Landscape. Isr. J. Zool. 2002, 48, 71–82. [Google Scholar] [CrossRef]
- Goreau, N.; Goreau, T.; Hayes, R. Settling, survivorship and spatial aggregation in planulae and juveniles of the coral Porites porites (Pallas). Bull. Mar. Sci. 1981, 31, 424–435. [Google Scholar]
- Connell, J.H. Population ecology of reef-building corals. Biol. Geol. Coral Reefs 1973, 2, 205–245. [Google Scholar]
- Buss, L.W.; Jackson, J.B.C. Competitive networks: Nontransitive competitive relationships in cryptic coral reef environments. Am. Nat. 1979, 113, 223–234. [Google Scholar] [CrossRef]
- Rinkevich, B.; Loya, Y. Intraspecific competitive networks in the Red Sea coral Stylophora pistillata. Coral Reefs 1983, 1, 161–172. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Müller, I.; Zhan, R.; Maidhof, A. Intraspecific recognition system in scleractinian corals: Morphological and cytochemical description of the autolysis mechanism. J. Histochem. Cytochem. 1984, 32, 285–288. [Google Scholar] [CrossRef]
- Rinkevich, B.; Loya, Y. Intraspecific competition in a reef coral: Effects on growth and reproduction. Oecologia 1985, 66, 100–105. [Google Scholar] [CrossRef]
- Wallace, C.C.; Harrison, P.L. Reproduction, dispersal and recruitment of scleractinian corals. In Ecosystems of the World; Coral Reefs; Dubinsky, Z., Ed.; Elsevier Science Publishing Company, Inc.: Amsterdam, The Netherlands, 1990; Volume 25, pp. 133–207. [Google Scholar]
- Rinkevich, B.; Loya, Y. The reproduction of the Red Sea coral Stylophora pistillata. I. Gonads and planulae. Mar. Ecol. Prog. Ser. 1979, 1, 145–152. [Google Scholar] [CrossRef]
- Oren, U.; Benayahu, Y.; Lubinevsky, H.; Loya, Y. Colony integration during regeneration in the stony Coral Favia favus. Ecology 2001, 82, 802–813. [Google Scholar] [CrossRef]
- Christiansen, N.A.; Ward, S.; Harii, S.; Tibbetts, I.R. Grazing by a small fish affects the early stages of a post-settlement stony coral. Coral Reefs 2009, 28, 47–51. [Google Scholar] [CrossRef]
- Penin, L.; Michonneau, F.; Baird, A.H.; Connolly, S.R.; Pratchett, M.S.; Kayal, M.; Adjeroud, M. Early post-settlement mortality and the structure of coral assemblages. Mar. Ecol. Prog. Ser. 2010, 408, 55–64. [Google Scholar] [CrossRef]
- Rinkevich, B. Do reproduction and regeneration in damaged corals compete for energy allocation? Mar. Ecol. Prog. Ser. 1994, 143, 197–302. [Google Scholar] [CrossRef]
- Mueller, W.A.; Rinkevich, B. Cell Communication-mediated nonself-recognition and -intolerance in representative species of the animal kingdom. J. Mol. Evol. 2020, 88, 482–500. [Google Scholar] [CrossRef]
- Simon-Blecher, N.; Achituv, Y.; Rinkevich, B. Protochordate concordant xenotransplantation settings reveal outbreaks of donor cells and divergent life span traits. Dev. Comp. Immunol. 2004, 28, 983–991. [Google Scholar] [CrossRef]
- Rinkevich, B.; Weissman, I.L. Allogeneic resorption in colonial protochordates: Consequences of nonself recognition. Dev. Comp. Immunol. 1992, 16, 275–286. [Google Scholar] [CrossRef]
- Rinkevich, B.; Weissman, I.L. Chimeras in colonial invertebrates: A synergistic symbiosis or somatic-cell and germ-cell parasitism? Symbiosis 1987, 4, 117–134. [Google Scholar]
- Jaraud, A.; Bosse, P.; de Citres, C.D.; Tiret, L.; Cache, V.; Abitbol, M. Feline chimerism revealed by DNA profiling. Anim. Genet. 2020, 4, 631–633. [Google Scholar] [CrossRef]
- Lipsker, D.; Flory, E.; Wiesel, M.; Hanau, D. Between light and dark, the chimera comes out. Arch. Dermatol. 2008, 144, 327–330. [Google Scholar] [CrossRef][Green Version]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing Ocean, Marine Ecosystems, and Dependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Bolnick, D.I.; Amarasekare, P.; Araújo, M.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef]
- Sully, S.; Burkepile, D.E.; Donovan, M.K.; Hodgson, G.; van Woesik, R. A global analysis of coral bleaching over the past two decades. Nat. Commun. 2019, 10, 1264. [Google Scholar] [CrossRef]
- Bruno, J.F.; Selig, E.R. Regional decline of coral cover in the Indo-Pacific: Timing, extent, and subregional comparisons. PLoS ONE 2007, 2, e711. [Google Scholar] [CrossRef]
- Bruno, J.F.; Côté, I.M.; Toth, L.T. Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Ann. Rev. Mar. Sci. 2019, 11, 307–334. [Google Scholar] [CrossRef]
- Gill, D.A.; Mascia, M.B.; Ahmadia, G.N.; Glew, L.; Lester, S.E.; Barnes, M.; Craigie, I.; Darling, E.S.; Free, C.M.; Geldmann, J.; et al. Capacity shortfalls hinder the performance of marine protected areas globally. Nature 2017, 543, 665–669. [Google Scholar] [CrossRef]
- Bates, A.E.; Cooke, R.S.C.; Duncan, M.I.; Edgar, G.J.; Bruno, J.F.; Benedetti-Cecchi, L.; Côté, I.M.; Lefcheck, J.S.; Costello, M.J.; Barrett, N.; et al. Climate resilience in marine protected areas and the ‘Protection Paradox’. Biol. Conserv. 2019, 236, 305–314. [Google Scholar] [CrossRef]
- Rinkevich, B. Rebuilding coral reefs: Does active reef restoration lead to sustainable reefs? Curr. Opin. Environ. Sustain. 2014, 7, 28–36. [Google Scholar] [CrossRef]
- Rinkevich, B. Management of coral reefs: We have gone wrong when neglecting active reef restoration. Mar. Pollut. Bull. 2008, 56, 1821–1824. [Google Scholar] [CrossRef]
- Mitsch, W.J. What is ecological engineering? Ecol. Eng. 2012, 45, 5–12. [Google Scholar] [CrossRef]
- Epstein, N.; Rinkevich, B. Applying forest restoration principles to coral reef rehabilitation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 387–395. [Google Scholar] [CrossRef]
- Shafir, S.; Van Rijn, J.; Rinkevich, B. A mid-water coral nursery. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2006; pp. 1674–1679. [Google Scholar]
- Amar, K.O.; Rinkevich, B. A floating mid-water coral nursery as larval dispersion hub: Testing an idea. Mar. Biol. 2007, 151, 713–718. [Google Scholar] [CrossRef]
- Shafir, S.; Rinkevich, B. The underwater silviculture approach for reef restoration: An emergent aquaculture theme. In Aquaculture Research Trends; Schwartz, S.H., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; pp. 279–295. ISBN 9781604562170. [Google Scholar]
- Golomb, D.; Shashar, N.; Rinkevich, B. Coral carpets—A novel ecological engineering tool aimed at constructing coral communities on soft sand bottoms. Ecol. Eng. 2020, 145, 105743. [Google Scholar] [CrossRef]
- Horoszowski-Fridman, Y.; Izhaki, I.; Rinkevich, B. Long-term heightened larval production in nursery-bred coral transplants. Basic Appl. Ecol. 2020, 47, 12–21. [Google Scholar] [CrossRef]
- Rinkevich, B. Restoration strategies for coral reefs damaged by recreational activities: The use of sexual and asexual recruits. Soc. Ecol. Restor. 1995, 3, 241–251. [Google Scholar] [CrossRef]
- Rinkevich, B. Steps towards the evaluation of coral reef restoration by using small branch fragments. Mar. Biol. 2000, 136, 807–812. [Google Scholar] [CrossRef]
- Shafir, S.; Van Rijn, J.; Rinkevich, B. Nubbing of coral colonies: A novel approach for the development of inland broodstocks. Aquar. Sci. Conserv. 2001, 3, 183–190. [Google Scholar] [CrossRef]
- Ayre, D.J.; Hughes, T.P. Climate change, genotypic diversity and gene flow in reef-building corals. Ecol. Lett. 2004, 7, 273–278. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Souter, P.; Howells, E.J.; Heyward, A.; Berkelmans, R. Novel genetic diversity through somatic mutations: Fuel for adaption of reef corals? Diversity 2011, 3, 405–423. [Google Scholar] [CrossRef]
- Baums, I.B. A restoration genetics guide for coral reef conservation. Mol. Ecol. 2008, 17, 2796–2811. [Google Scholar] [CrossRef]
- Reusch, T.B.H.; Ehlers, A.; Hammerli, A.; Worm, B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl. Acad. Sci. USA 2005, 102, 2826–2831. [Google Scholar] [CrossRef]
- Hughes, A.R.; Stachowicz, J.J. Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc. Natl. Acad. Sci. USA 2004, 101, 8998–9002. [Google Scholar] [CrossRef]
- Crutsinger, G.M.; Collins, M.D.; Fordyce, J.A.; Gompert, Z.; Nice, C.C.; Sanders, N.J. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 2006, 313, 966–968. [Google Scholar] [CrossRef]
- Letters, E. Additive and interactive effects of plant genotypic diversity on arthropod communities and plant fitness. Ecol. Lett. 2006, 9, 24–34. [Google Scholar] [CrossRef]
- Drury, C.; Greer, J.B.; Baums, I.; Gintert, B.; Lirman, D. Clonal diversity impacts coral cover in Acropora cervicornis thickets: Potential relationships between density, growth, and polymorphisms. Ecol. Evol. 2019, 9, 4518–4531. [Google Scholar] [CrossRef]
- Carne, L.; Kaufman, L.; Scavo, K. Measuring success for Caribbean acroporid restoration: Key results from ten years of work in southern Belize. In Proceedings of the 13th International Coral Reef Symposium, Honolulu, HI, USA, 19–24 June 2016; pp. 352–368. [Google Scholar]
- Linden, B.; Vermeij, M.J.A.; Rinkevich, B. The coral settlement box: A simple device to produce coral stock from brooded coral larvae entirely in situ. Ecol. Eng. 2019, 132, 115–119. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shefy, D.; Shashar, N.; Rinkevich, B. Exploring Traits of Engineered Coral Entities to be Employed in Reef Restoration. J. Mar. Sci. Eng. 2020, 8, 1038. https://doi.org/10.3390/jmse8121038
Shefy D, Shashar N, Rinkevich B. Exploring Traits of Engineered Coral Entities to be Employed in Reef Restoration. Journal of Marine Science and Engineering. 2020; 8(12):1038. https://doi.org/10.3390/jmse8121038
Chicago/Turabian StyleShefy, Dor, Nadav Shashar, and Baruch Rinkevich. 2020. "Exploring Traits of Engineered Coral Entities to be Employed in Reef Restoration" Journal of Marine Science and Engineering 8, no. 12: 1038. https://doi.org/10.3390/jmse8121038
APA StyleShefy, D., Shashar, N., & Rinkevich, B. (2020). Exploring Traits of Engineered Coral Entities to be Employed in Reef Restoration. Journal of Marine Science and Engineering, 8(12), 1038. https://doi.org/10.3390/jmse8121038




