Polychaete Invasion May Lead to Biogeochemical Change in Host Marine Environment
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Cinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Kristensen, E.; Delefosse, M.; Quintana, C.O.; Flindt, M.R.; Valdemarsen, T. Influence of benthic macrofauna community shifts on ecosystem functioning in shallow estuaries. Front. Mar. Sci. 2014, 1, 41. [Google Scholar] [CrossRef]
- Blank, M.; Laine, A.O.; Jürss, K.; Bastrop, R. Molecular identification key based on PCR/RFLP for three polychaete sibling species of the genus Marenzelleria, and the species’ current distribution in the Baltic Sea. Helgol. Mar. Res. 2008, 62, 129–141. [Google Scholar] [CrossRef]
- Delefosse, M.; Banta, G.T.; Canal-Vergés, P.; Penha-Lopes, G.; Quintana, C.O.; Valdemarsen, T.; Kristensen, E. Macrobenthic community response to the Marenzelleria viridis (Polychaeta) invasion of a Danish estuary. Mar. Ecol. Prog. Ser. 2012, 461, 83–94. [Google Scholar] [CrossRef][Green Version]
- Quintana, C.O.; Raymond, C.; Nascimento, F.J.A.; Bonaglia, S.; Forster, S.; Gunnarsson, J.S.; Kristensen, E. Functional performance of three invasive Marenzelleria species under contrasting ecological conditions within the Baltic Sea. Estuaries Coasts 2018, 41, 1766–1781. [Google Scholar] [CrossRef]
- Kristensen, E.; Hansen, T.; Delefosse, M.; Banta, G.; Quintana, C.O. Contrasting effects of the polychaetes Marezelleria viridis and Nereis diversicolor on benthic metabolism and solute transport in sandy coastal sediments. Mar. Ecol. Prog. Ser. 2011, 425, 125–139. [Google Scholar] [CrossRef]
- Quintana, C.O.; Hansen, T.; Delefosse, M.; Banta, G.; Kristensen, E. Burrow ventilation and associated porewater irrigation by the polychaete Marenzelleria viridis. J. Exp. Mar. Biol. Ecol. 2011, 397, 179–187. [Google Scholar] [CrossRef]
- Kristiansen, K.D.; Kristensen, E.; Jensen, M.H. The influence of water column hypoxia on the behaviour of manganese and iron in sandy coastal marine sediment. Estuar. Coast. Shelf Sci. 2002, 55, 645–654. [Google Scholar] [CrossRef]
- Woodin, S.A.; Marinelli, R.L.; Lindsay, S.M. Process-specific cues for recruitment in sedimentary environments: Geochemical signals? J. Mar. Res. 1998, 56, 535–558. [Google Scholar] [CrossRef]
- Mascaró, O.; Valdemarsen, T.; Holmer, M.; Pérez, M.; Romero, J. Experimental manipulation of sediment organic content and water column aeration reduces Zostera marina (eelgrass) growth and survival. J. Mar. Exp. Biol. Ecol. 2009, 373, 26–34. [Google Scholar] [CrossRef]
- Jørgensen, B.B. A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments. Geomicrobiol. J. 1978, 1, 29–47. [Google Scholar] [CrossRef]
- Quintana Fossing, H.; Jørgensen, B.B. Measurement of bacterial sulfate reduction in sediments: Evaluation of a single-step chromium reduction method. Biogeochemistry 1989, 8, 205–222. [Google Scholar]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Banta, G.T.; Holmer, M.; Jensen, M.H.; Kristensen, E. Effects of two polychate worms, Nereis diversicolor and Arenicola marina, on aerobic and anaerobic decomposition in sandy marine sediment. Aquat. Microb. Ecol. 1999, 19, 189–204. [Google Scholar] [CrossRef]
- Muus, B.J. The Fauna in Danish Estuaries and Lagoons: Distribution and Ecology of Dominating Species in the Shallow Reaches of a Mesohaline Zone; Høst & Søn: Copenhagen, Denmark, 1967; 316p. [Google Scholar]
- Aller, R.C.; Aller, J.Y.; Zhu, Q.; Heilbrun, C.; Klingensmith, I.; Kaushik, A. Worm tubes as conduits for the electrogenic microbial grid in marine sediments. Sci. Adv. 2019, 5, eaaw3651. [Google Scholar] [CrossRef]
- Bertics, V.; Ziebis, W. Bioturbation and role of microniches for sulfate reduction in coastal marine sediments. Environ. Microbiol. 2010, 12, 3022–3024. [Google Scholar] [CrossRef]
- Schiedek, D. Marenzelleria cf. viridis (Polychaete: Spionidae)—Ecophysiological adaptations to a life in the coastal waters of the Baltic Sea. Aquat. Ecol. 1997, 31, 199–210. [Google Scholar] [CrossRef]
- Vasquez-Cardenas, D.; Quintana, C.O.; Meysman, F.J.R.; Kristensen, E.; Boschker, H.T.S. Species-specific effects of two bioturbating polychaetes on sediment chemoautotrophic bacteria. Mar. Ecol. Prog. Ser. 2016, 549, 55–68. [Google Scholar] [CrossRef]
- Dauer, D. Functional morphology and feeding behavior of Marenzelleria viridis (Polychaete: Spionidae). Bull. Mar. Sci. 1997, 60, 512–516. [Google Scholar]
- Hedman, J.E.; Gunnarsson, J.S.; Samuelson, G.; Gilbert, F. Particle reworking and solute transport by the sediment-living polychaetes Marenzelleria neglecta and Hediste diversicolorI. J. Exp. Mar. Biol. Ecol. 2011, 402, 294–301. [Google Scholar] [CrossRef]
- Preisler, A.; de Beer, D.; Lichtschlag, A.; Lavik, G.; Boetius, A.; Jørgensen, B.B. Biological and chemical sulfide oxidation in a Beggiatoa inhabited marine sediment. ISME J. 2007, 1, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Groenendaal, M. Tolerance of the lugworm (Arenicola marina) to sulphide. Neth. J. Sea Res. 1980, 14, 200–207. [Google Scholar] [CrossRef]
- Levin, L.A.; Ekau, W.; Gooday, A.J.; Jorisson FMiddelburg, J.J.; Naqvi, S.W.; Neira, C.; Rabalais, N.N.; Zhang, J. Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 2009, 6, 2063–2098. [Google Scholar] [CrossRef]
- Pedersen, M.Ø.; Kristensen, E. Sensitivity of Ruppia maritima and Zostera marina to sulfide exposure around roots. J. Exp. Mar. Biol. Ecol. 2015, 468, 138–145. [Google Scholar] [CrossRef]

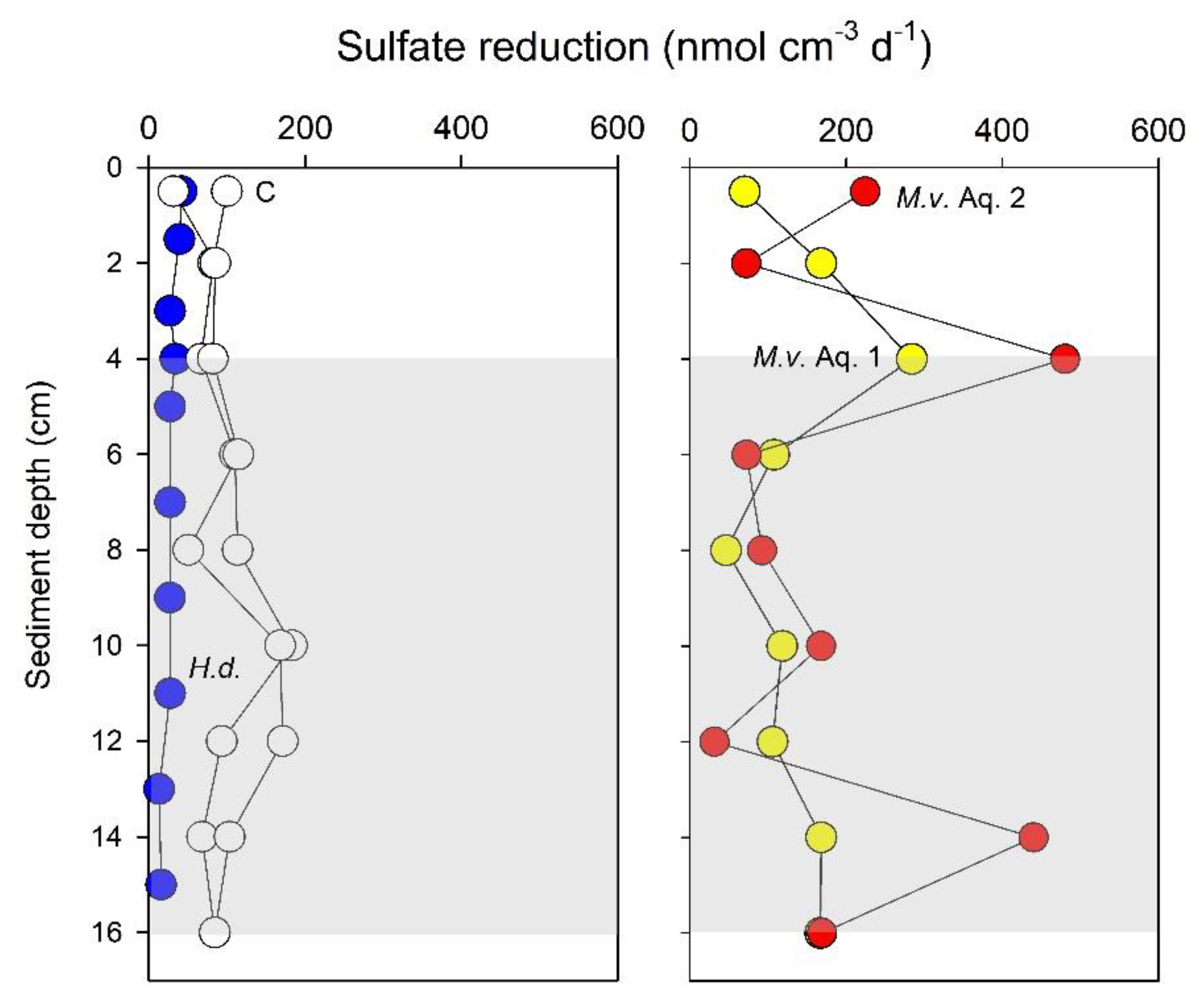
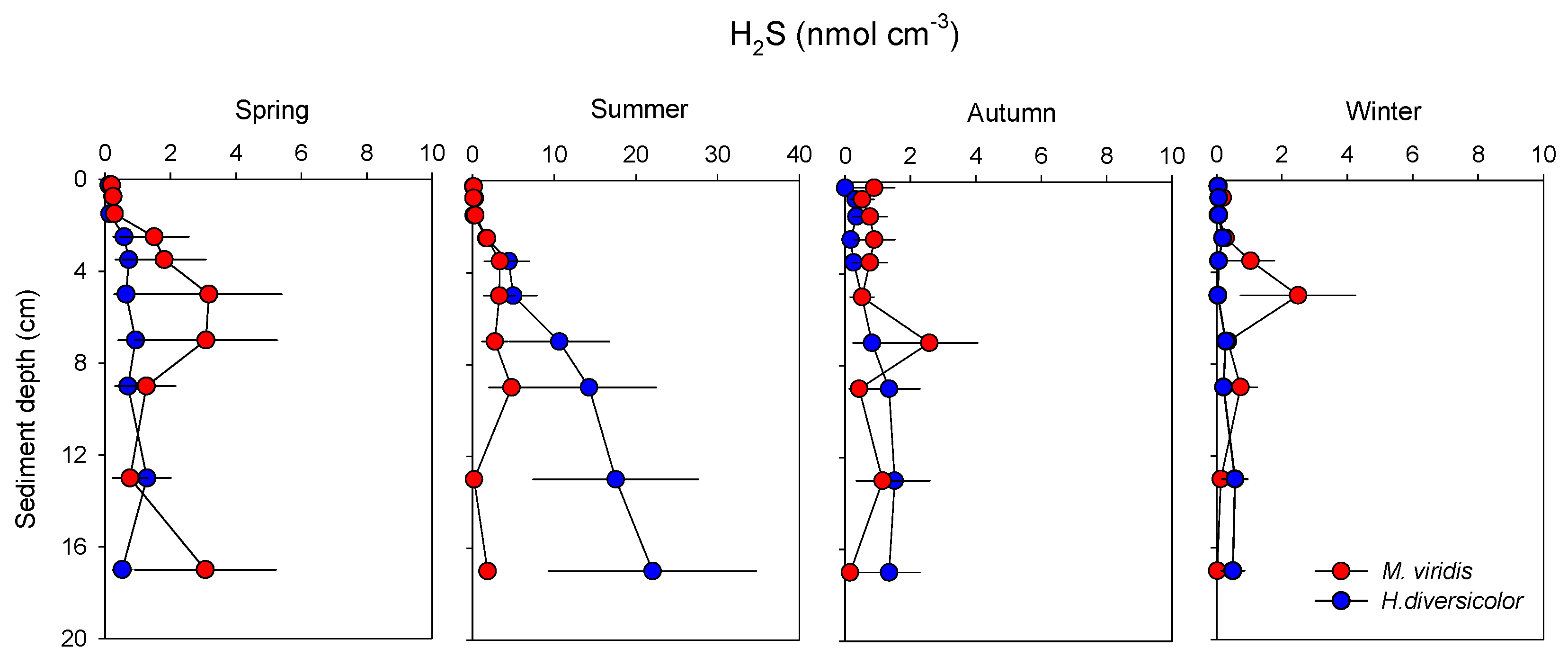
| Temperature (°C) | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| M. viridis | 11.0 | 18.0 | 11.7 | 5.0 |
| H. diversicolor | 11.5 | 30.0 | 14.2 | 6.5 |
| H2S (µmol m−2) | ||||
| M. viridis | 29.6 and 44.3 | 53.7 ± 10.9 | 24.8 ± 7.4 | 17.1 ± 6.5 |
| H. diversicolor | 12.0 ± 3.9 | 130.1 ± 45.6 | 12.4 ± 1.6 | 2.6 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana, C.O.; Kristensen, E. Polychaete Invasion May Lead to Biogeochemical Change in Host Marine Environment. J. Mar. Sci. Eng. 2020, 8, 940. https://doi.org/10.3390/jmse8110940
Quintana CO, Kristensen E. Polychaete Invasion May Lead to Biogeochemical Change in Host Marine Environment. Journal of Marine Science and Engineering. 2020; 8(11):940. https://doi.org/10.3390/jmse8110940
Chicago/Turabian StyleQuintana, Cintia O., and Erik Kristensen. 2020. "Polychaete Invasion May Lead to Biogeochemical Change in Host Marine Environment" Journal of Marine Science and Engineering 8, no. 11: 940. https://doi.org/10.3390/jmse8110940
APA StyleQuintana, C. O., & Kristensen, E. (2020). Polychaete Invasion May Lead to Biogeochemical Change in Host Marine Environment. Journal of Marine Science and Engineering, 8(11), 940. https://doi.org/10.3390/jmse8110940





