Evaluation of Pollutant Emissions into the Atmosphere during the Loading of Hydrocarbons in Marine Oil Tankers in the Arctic Region
Abstract
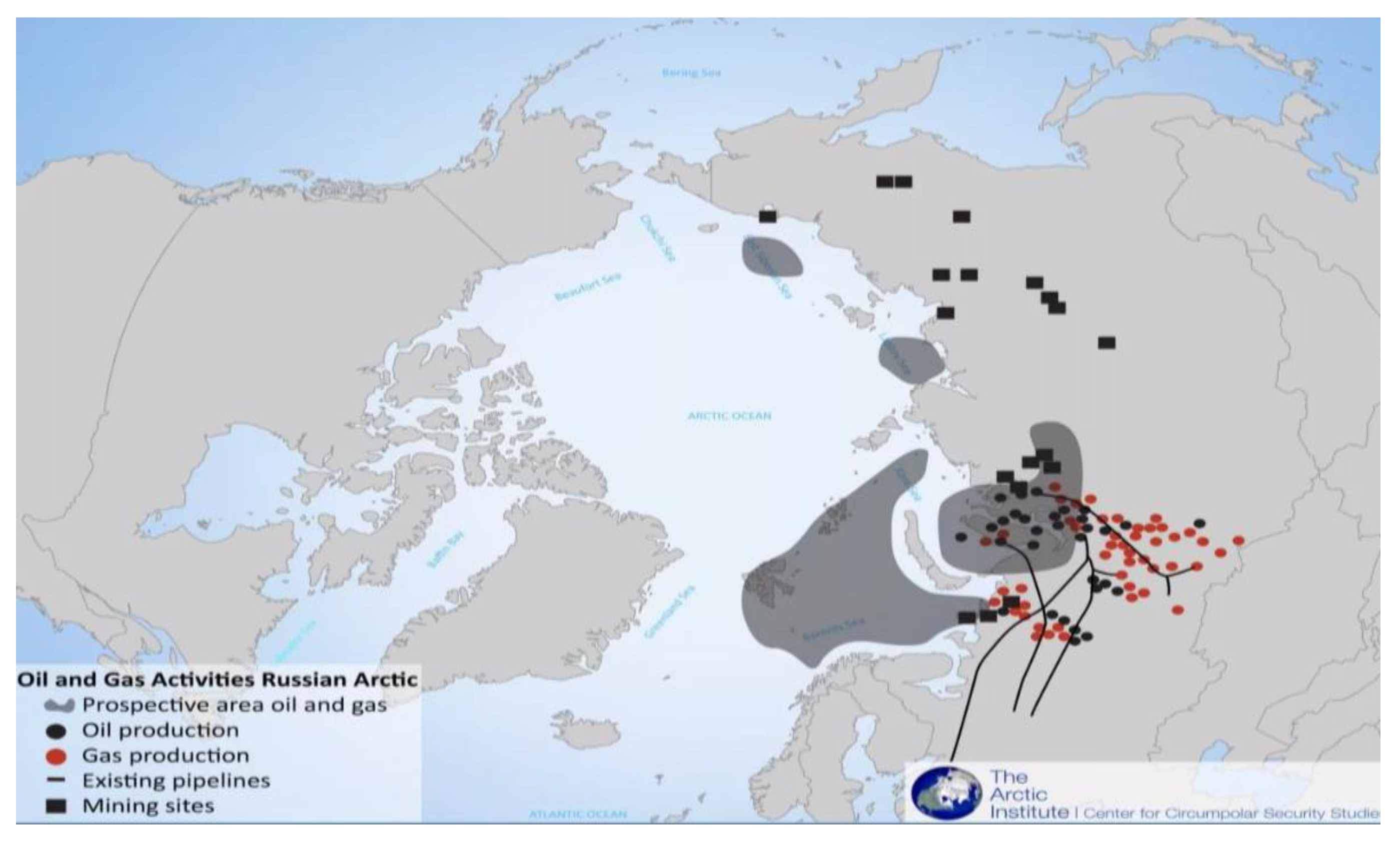
1. Introduction
2. Methodology
2.1. Analytical Method for Estimating VOC Emissions into the Atmosphere
2.2. Experimental Method for Estimating VOC Emissions into the Atmosphere
3. Case Study
3.1. Conceptfor Constructing a Mathematical Model of the Gasspace Dynamics of a Tanker
- (1)
- The inertance of the flow can be neglected due to the gas phase pipeline has a long length;
- (2)
- the gas constant is regarded as constant, R = const;
- (3)
- the law of mass transfer during evaporation is accepted exponentially.
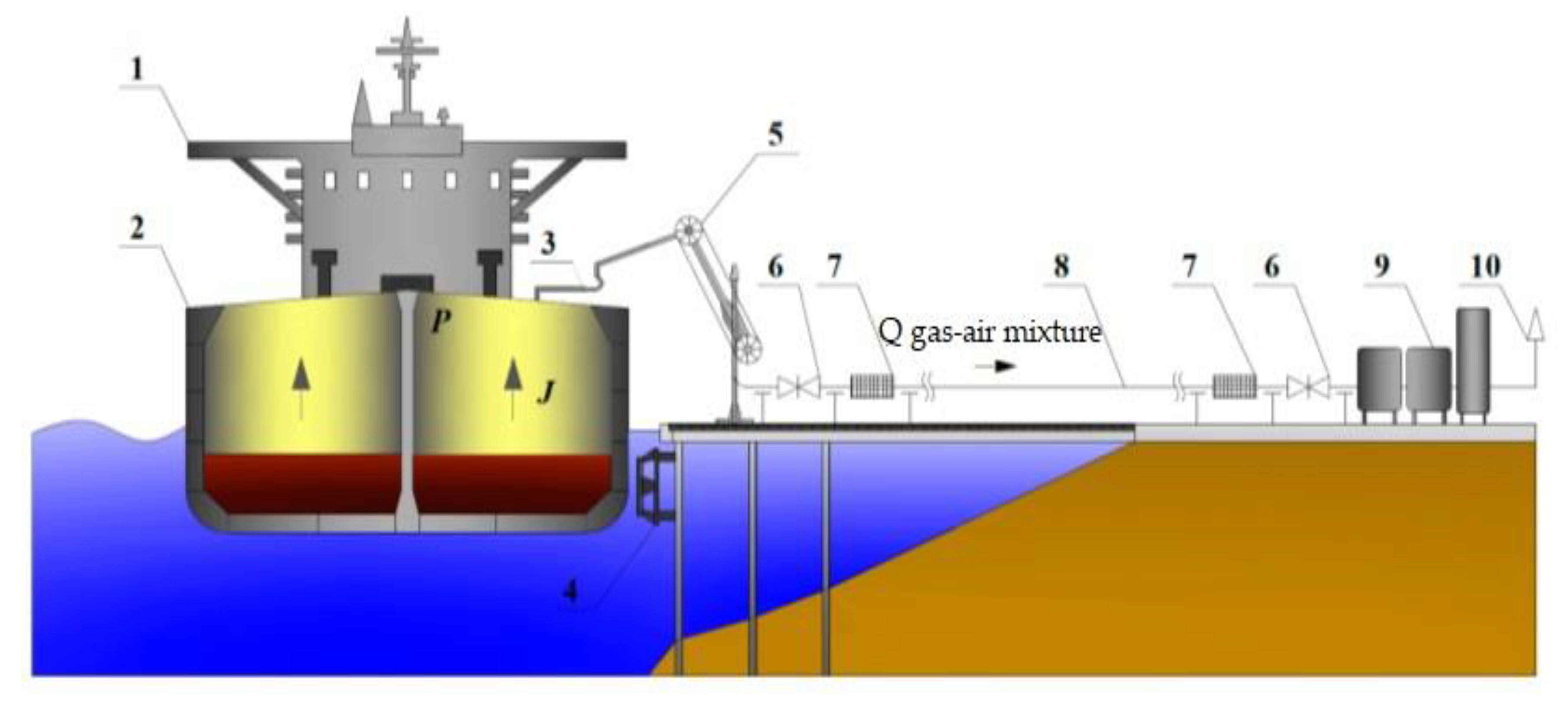
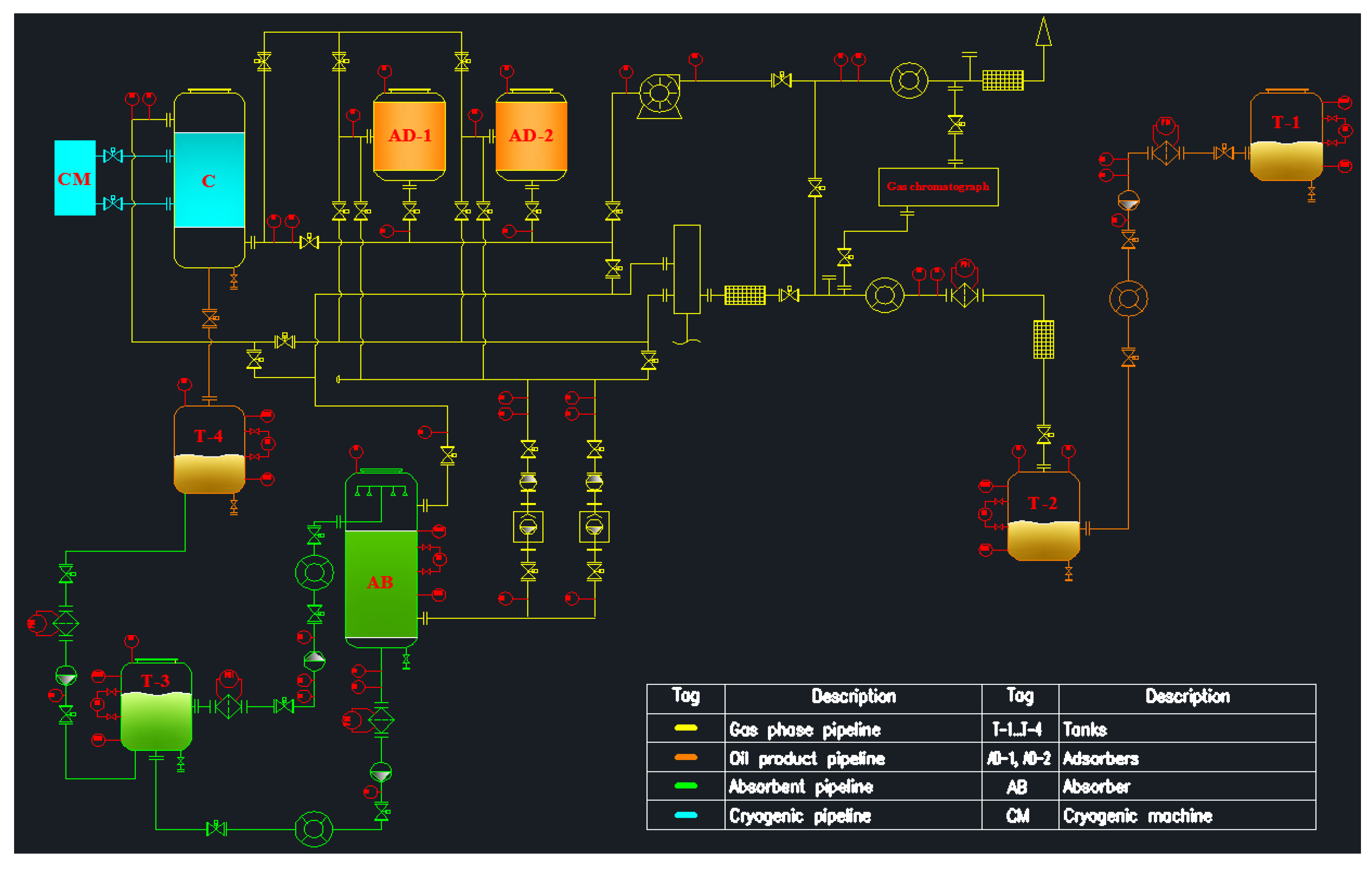
3.2. Laboratory Stand Concept for Vapor Recovery Unit
- −
- adsorption;
- −
- absorption;
- −
- condensation.
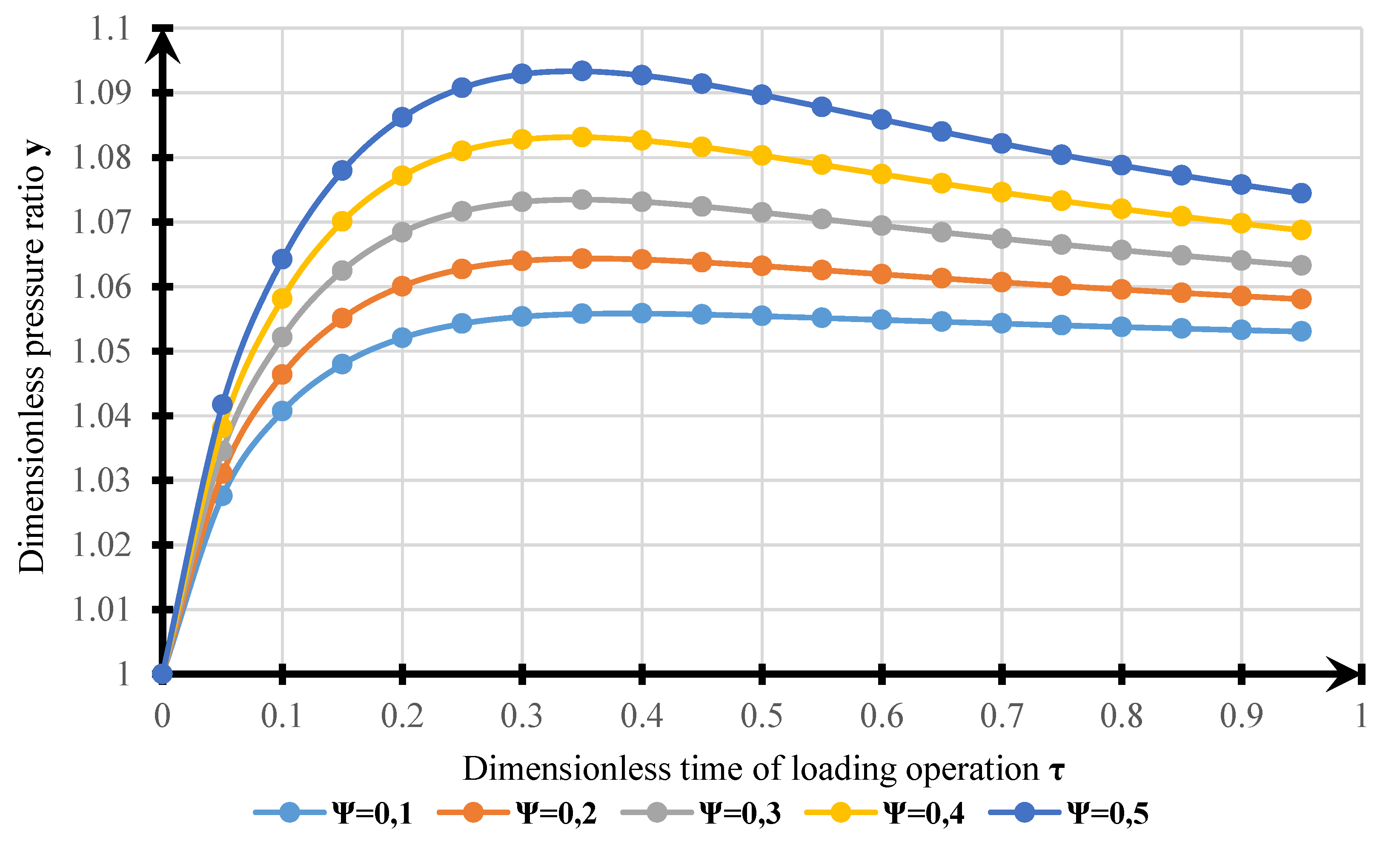
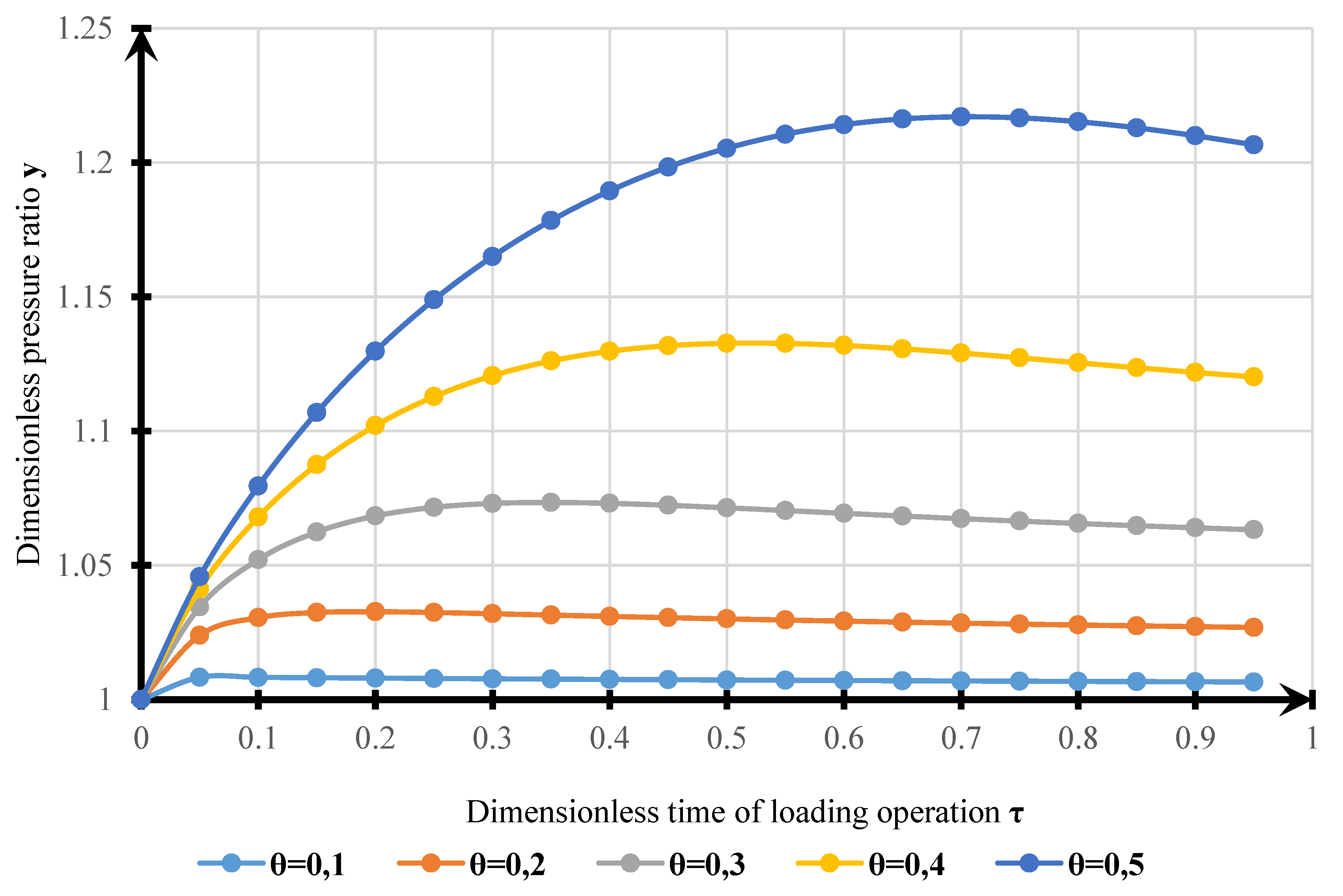
4. Results
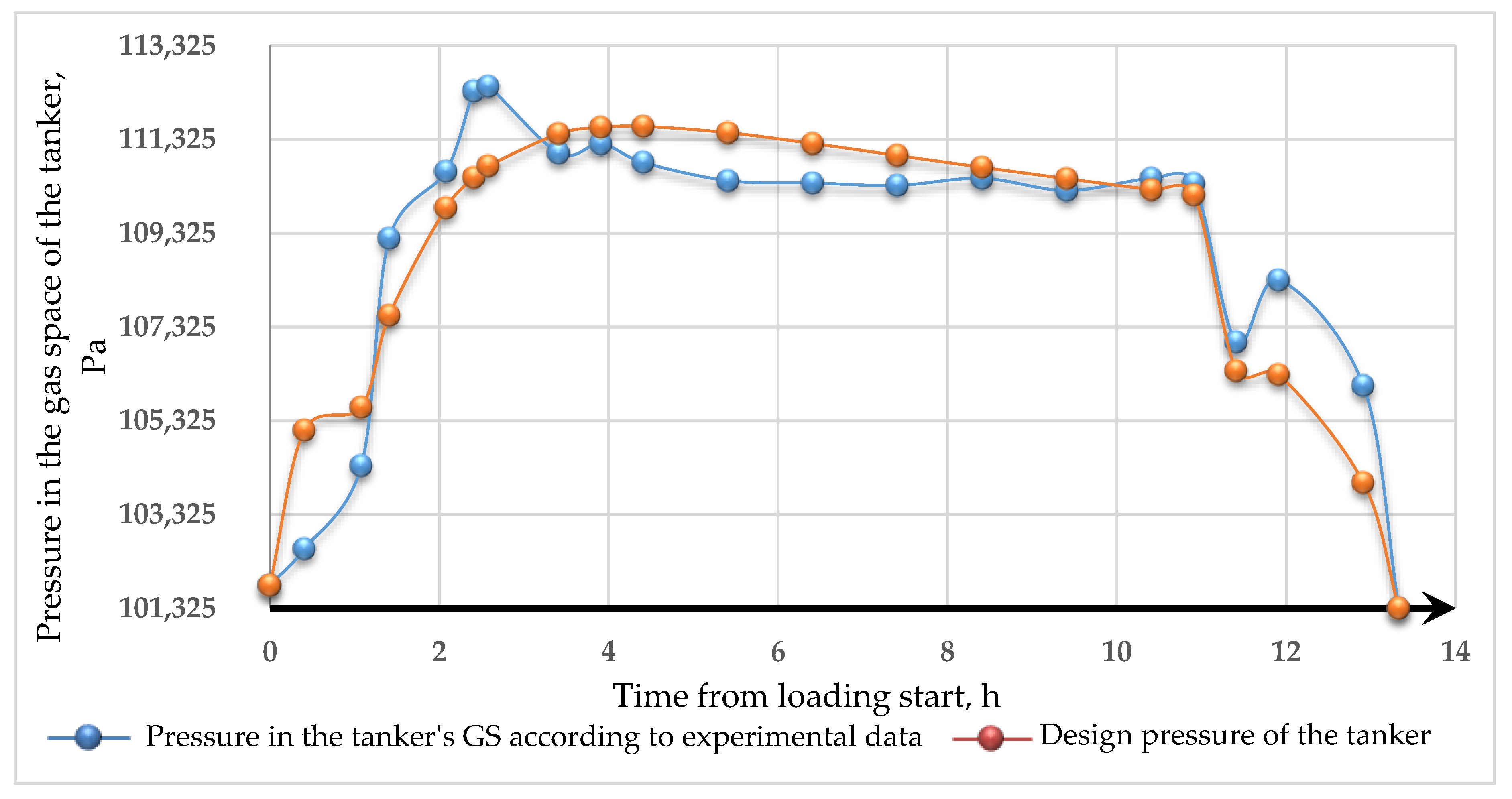
- The model obtained can be used for estimating VOC emissions when hydrocarbons are loaded into a tanker. The results of modeling the dynamics of pressure changes in the tanker’s GS with experimental data allowed us to determine the calculation error. The standard error of the model is 1215 Pa. Complex transitional processes explain the discrepancy of the model in changing the injection modes, and the account of re-existing processes can be made using smoothing functions.
- An experimental method for estimating vapor emissions can be achieved by creating a stand in the laboratory, using the technological scheme developed in this work.
5. Conclusions
- A mathematical model was constructed that adequately describes the dynamics of gas flows during tanker loading. The results obtained were compared with the experimental data on the example of loading the SOPHIE SCHULTE tanker. Most of the error scans explain transitional processes when loading the tanker. Transitional processes are of some scientific interest for further work in this direction.
- The developed model can be used for estimating VOCs emissions. It also allows the evaluation of the capacity of existing pipeline systems for the removal of the gas phase and the design of new installations for the recovery of oil vapor. This would reduce emissions of VOCs and increase the security of the Arctic region’s ecosystem.It is also important to create a laboratory stand for an experimental method for estimating VOCs emissions and the effectiveness of the VRU.
- The technological scheme of a possible laboratory stand for vapor recovery was developed, which can evaluate the efficiency of the VRU of adsorption, absorption, and condensation technologies.
Author Contributions
Funding
Conflicts of Interest
References
- The Council of the European Union. Council directive 1999/13/EC on the limitation of emissions of volatile organic compounds due to the use of organic solvents in certain activities and installations. Off. J. Eur. Commun. 1999, 4, 118–139. [Google Scholar]
- Buhaug, Ø.; Corbett, J.; Endresen, Ø.; Eyring, V.; Faber, J.; Hanayama, S. Second IMO GHG Study 2009; International Maritime Organization: London, UK, 2009. [Google Scholar]
- Spirig, C.; Neftel, A.; Ammann, C.; Dommen, J.; Grabmer, W.; Thielmann, A.; Schaub, A.; Beauchamp, J.D.; Wisthaler, A.; Hansel, A. Eddy covariance flux measurements of biogenic VOCs during ECHO 2003 using proton transfer reaction mass spectrometry. Atmos. Chem. Phys. 2005, 5, 465–481. [Google Scholar] [CrossRef]
- Bergin, M.S.; Russell, A.G.; Milford, J.B. Quantification of individual VOC reactivity using a chemically detailed, three-dimensional photochemical model. Environ. Sci. Technol. 1995, 29, 3029–3037. [Google Scholar] [CrossRef] [PubMed]
- Luecken, D.J.; Mebust, M.R. Technical challenges involved in implementation of VOC reactivity-based control of ozone. Environ. Sci. Technol. 2008, 42, 1615–1622. [Google Scholar] [CrossRef]
- Avery, R.J. Reactivity-based VOC control for solvent products: More efficient ozone reduction strategies. Environ. Sci. Technol. 2006, 40, 4845–4850. [Google Scholar] [CrossRef]
- Guidelines for the Development of a VOC Management Plan; IMO: London, UK, 2009.
- Tcvetkov, P.; Cherepovitsyn, A.; Fedoseev, S. The Changing Role of CO2 in the Transition to a Circular Economy: Review of Carbon Sequestration Projects. Sustainability 2019, 11, 5834. [Google Scholar] [CrossRef]
- Litvinenko, V. Russian developments of equipment and technology of deep hole drilling in ice. In Innovation-Based Development of the Mineral Resources Sector: Challenges and Prospects, Proceedings of the 11th Russian-German Raw Materials Conference, Potsdam, Germany, 7–8 November 2018; Taylor & Francis Group: London, UK, 2018. [Google Scholar]
- Ilinova, A.; Cherepovitsyn, A.; Evseeva, O. Stakeholder Management: An Approach in CCS Projects. Resources 2018, 7, 83. [Google Scholar] [CrossRef]
- Mariya, A.P.; Alexey, V.A. Reutilization Prospects of Diamond Clay Tailings at the Lomonosov Mine. Northwest. Russ. Miner. 2020, 10, 517. [Google Scholar] [CrossRef]
- Litvinenko, V.S. Digital Economy as a Factor in the Technological Development of the Mineral Sector. Nat. Resour. Res. 2020, 29, 1521–1541. [Google Scholar] [CrossRef]
- Pashkevich, M.A.; Petrova, T.A. Recyclability of ore beneficiation wastes at the Lomonosov Deposit. J. Ecol. Eng. 2019, 20, 27–33. [Google Scholar] [CrossRef]
- Abramovich, B.; Sychev, Y. Problems of ensuring energy security for enterprises from the mineral resources sector. J. Min. Inst. 2016, 217, 132–139. [Google Scholar]
- Romasheva, N.V.; Kruk, M.N.; Cherepovitsyn, A.E. Propagation perspectives of CO2 sequestration in the world. Int. J. Mech. Eng. Technol. 2018, 9, 1877–1885. [Google Scholar]
- Yang, G.; Zhai, X.Q. Optimal design and performance analysis of solar hybrid CCHP system considering influence of building type and climate condition. Energy 2019, 174, 647–663. [Google Scholar] [CrossRef]
- Tcvetkov, P.; Cherepovitsyn, A.; Makhovikov, A. Economic assessment of heat and power generation from small-scale liquefied natural gas in Russia. Energy Rep. 2020, 6, 391–402. [Google Scholar] [CrossRef]
- Mulder, T. VOC recovery systems. Hydrocarb. Eng. 2007, 12, 37–40. [Google Scholar]
- Gilman, J.B.; Lerner, B.M.; Kuster, W.C.; De Gouw, J.A. Source signature of volatile organic compounds from oil and natural gas operations in northeastern Colorado. Environ. Sci. Technol. 2013, 47, 1297–1305. [Google Scholar] [CrossRef]
- Helmig, D.; Thompson, C.R.; Evans, J.; Boylan, P.; Hueber, J.; Park, J.H. Highly elevated atmospheric levels of volatile organic compounds in the Uintah Basin, Utah. Environ. Sci. Technol. 2014, 48, 4707–4715. [Google Scholar] [CrossRef]
- Koss, A.; Yuan, B.; Warneke, C.; Gilman, J.B.; Lerner, B.M.; Veres, P.R.; Thompson, C.R. Observations of VOC emissions and photochemical products over US oil-and gas-producing regions using high-resolution H3O+ CIMS (PTR-ToF-MS). Atmos. Meas. Tech. 2017, 10, 2941. [Google Scholar] [CrossRef]
- Warneke, C.; Geiger, F.; Edwards, P.M.; Dube, W.; Pétron, G.; Kofler, J.; Lerner, B. Volatile organic compound emissions from the oil and natural gas industry in the Uinta Basin, Utah: Point sources compared to ambient air composition. Atmos. Chem. Phys. Discuss. 2014, 14, 11895–11927. [Google Scholar] [CrossRef]
- Hanna, S.R.; Drivas, P.J. Modeling VOC emissions and air concentrations from the Exxon Valdez oil spill. Air Waste 1993, 43, 298–309. [Google Scholar] [CrossRef]
- Simpson, I.J.; Blake, N.J.; Barletta, B.; Diskin, G.S.; Fuelberg, H.E.; Gorham, K.; Weinheimer, A.J. Characterization of trace gases measured over Alberta oil sands mining operations: 76 speciated C2-C10 volatile organic compounds (VOCs), CO2, CH4, CO, NO, NO2, NOy, O3 and SO2. Atmos. Chem. Phys. 2010, 10, 11931–11954. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Li, K.; Hollebone, B.P.; Brown, C.E.; Landriault, M. Determination of Volatile Organic Compounds over Crude Oils Using Novel Automatic Liquid Sampler-Headspace (ALS-HS) Gas Chromatography/Mass Spectrometry. In Proceedings of the Thirtieth AMOP Technical Seminar, Environment Canada, Ottawa, ON, Canada, 5–7 June 2007; pp. 49–59. [Google Scholar]

| Parameter | Value | Unit |
|---|---|---|
| Deadweight | 115,583 | tons |
| Dimensions | 241 × 44 | m |
| Method for inerting GS | exhaust gases | – |
| Pressure of saturated oil vapors on the raid | 52.1 | kPa |
| № | The Current Time from Beginning of Loading, h | Experimental Data on Pressure in the Tanker’s GS, Pa | Pressure in the Tanker’s GS Calculated by Equation (12), Pa |
|---|---|---|---|
| 1 | 0 | 101,815 | 101,815 |
| 2 | 0.41 | 102,595 | 105,124 |
| 3 | 1.08 | 104,365 | 105,612 |
| 4 | 1.41 | 109,215 | 107,570 |
| 5 | 2.08 | 110,645 | 109,866 |
| 6 | 2.41 | 112,355 | 110,511 |
| 7 | 2.58 | 112,455 | 110,761 |
| 8 | 3.41 | 111,035 | 111,443 |
| 9 | 3.91 | 111,225 | 111,580 |
| 10 | 4.41 | 110,835 | 111,602 |
| 11 | 5.41 | 110,445 | 111,468 |
| 12 | 6.41 | 110,395 | 111,236 |
| 13 | 7.41 | 110,345 | 110,982 |
| 14 | 8.41 | 110,495 | 110,730 |
| 15 | 9.41 | 110,235 | 110,487 |
| 16 | 10.41 | 110,495 | 110,253 |
| 17 | 10.91 | 110,385 | 110,139 |
| 18 | 11.41 | 107,005 | 106,389 |
| 19 | 11.91 | 108,325 | 106,309 |
| 20 | 12.91 | 106,075 | 104,007 |
| 21 | 13.33 | 101,325 | 101,325 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fetisov, V.; Pshenin, V.; Nagornov, D.; Lykov, Y.; Mohammadi, A.H. Evaluation of Pollutant Emissions into the Atmosphere during the Loading of Hydrocarbons in Marine Oil Tankers in the Arctic Region. J. Mar. Sci. Eng. 2020, 8, 917. https://doi.org/10.3390/jmse8110917
Fetisov V, Pshenin V, Nagornov D, Lykov Y, Mohammadi AH. Evaluation of Pollutant Emissions into the Atmosphere during the Loading of Hydrocarbons in Marine Oil Tankers in the Arctic Region. Journal of Marine Science and Engineering. 2020; 8(11):917. https://doi.org/10.3390/jmse8110917
Chicago/Turabian StyleFetisov, Vadim, Vladimir Pshenin, Dmitrii Nagornov, Yuri Lykov, and Amir H. Mohammadi. 2020. "Evaluation of Pollutant Emissions into the Atmosphere during the Loading of Hydrocarbons in Marine Oil Tankers in the Arctic Region" Journal of Marine Science and Engineering 8, no. 11: 917. https://doi.org/10.3390/jmse8110917
APA StyleFetisov, V., Pshenin, V., Nagornov, D., Lykov, Y., & Mohammadi, A. H. (2020). Evaluation of Pollutant Emissions into the Atmosphere during the Loading of Hydrocarbons in Marine Oil Tankers in the Arctic Region. Journal of Marine Science and Engineering, 8(11), 917. https://doi.org/10.3390/jmse8110917






