EAT BREATHE EXCRETE REPEAT: Physiological Responses of the Mussel Mytilus galloprovincialis to Diclofenac and Ocean Acidification
Abstract
1. Introduction
2. Materials and Methods
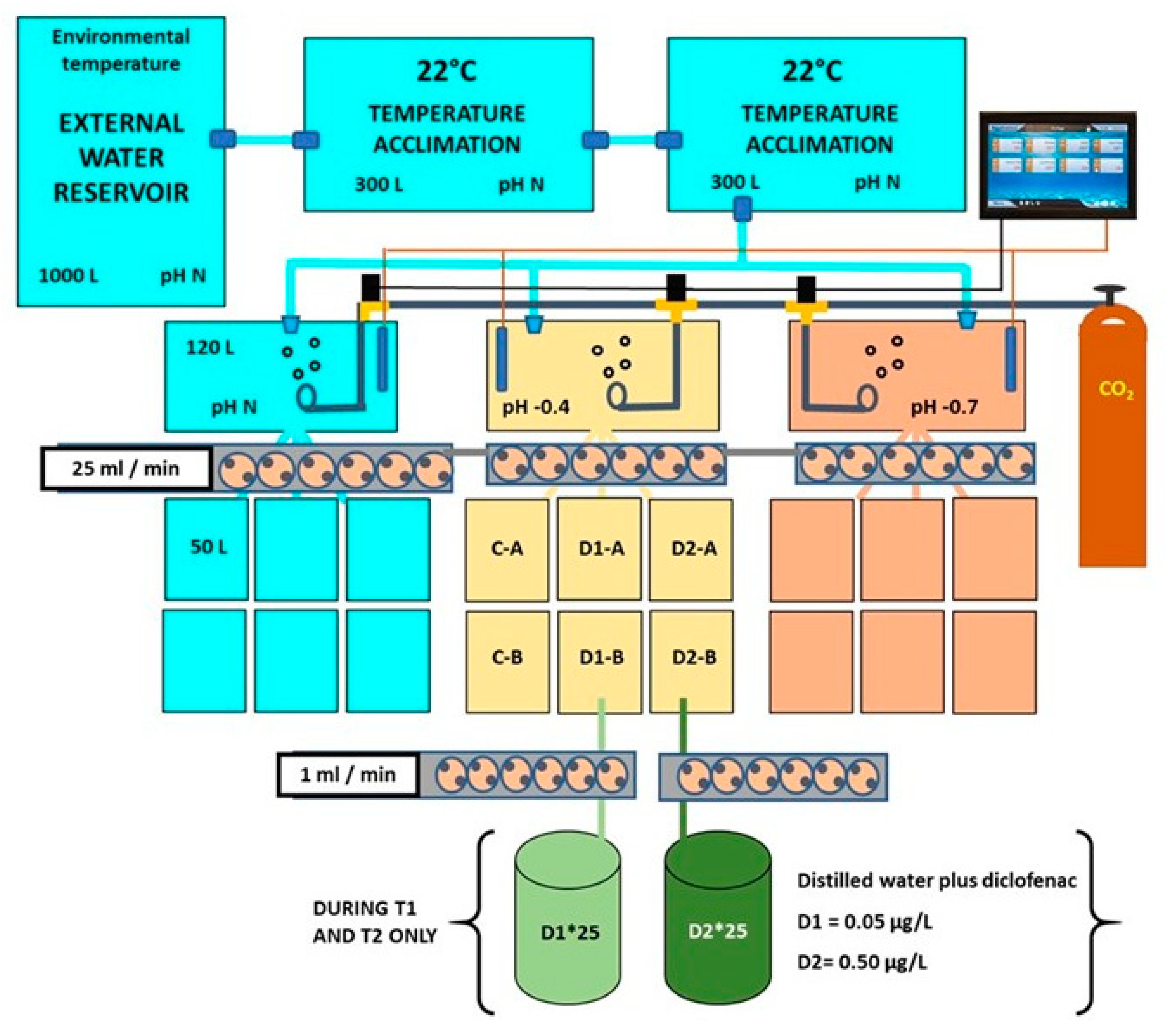
2.1. Experimental Setup for Bivalve Exposure
2.2. Measurements of Physiological Parameters
2.2.1. Clearance Rate (CR)
2.2.2. Respiration Rate (RR)
2.2.3. Excretion Rate (ER)
2.2.4. Statistical Analyses
3. Results
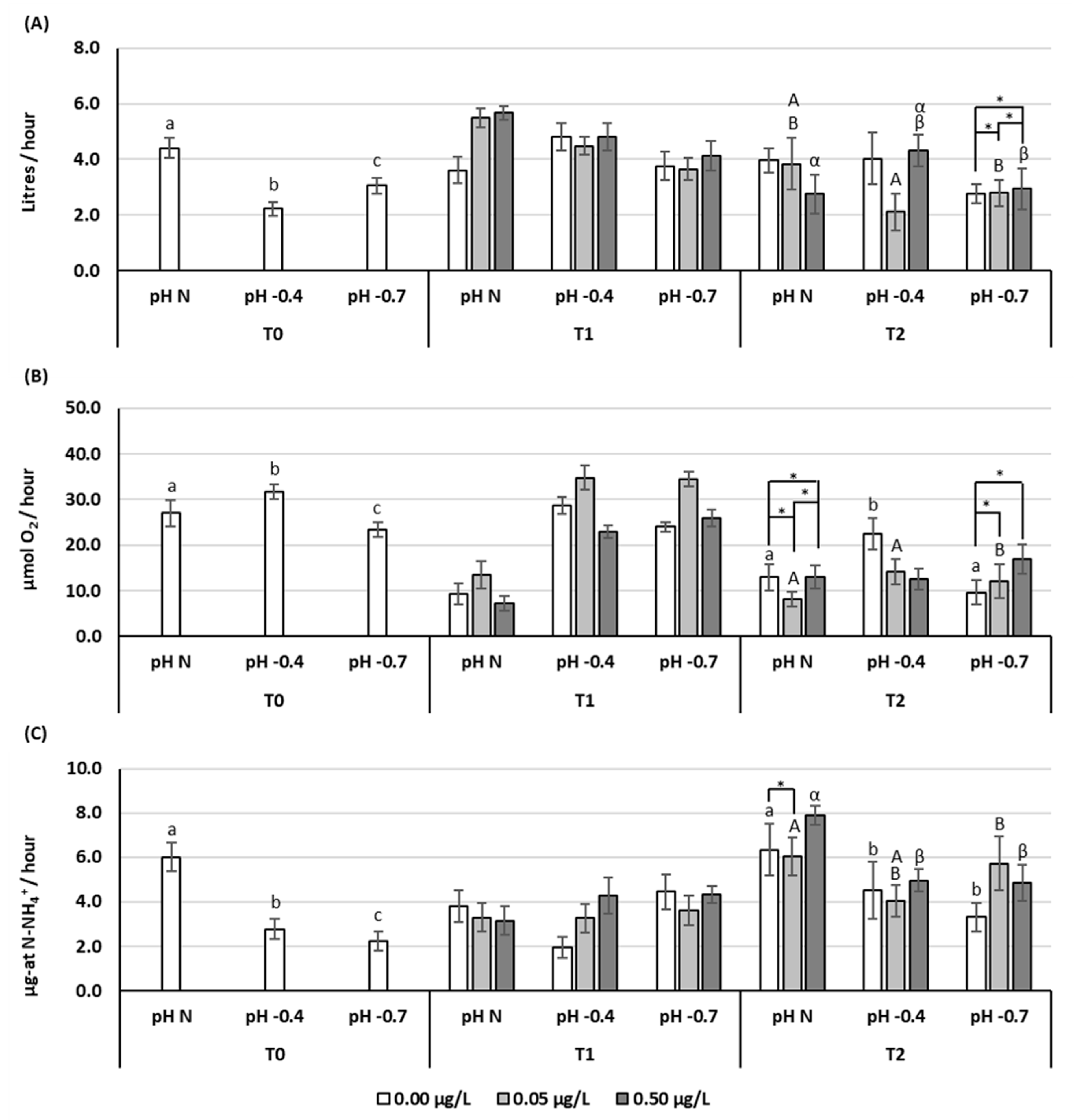
3.1. Clearance Rate (CR)
3.2. Respiration Rate (RR)
3.3. Excretion Rate (ER)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schiedek, D.; Sundelin, B.; Readman, J.W.; MacDonald, R.W. Interactions between climate change and contaminants. Mar. Pollut. Bull. 2009, 54, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M. Global change ecotoxicology: Identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res. 2012, 76, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, M. Climate change and ocean acidification—interactions with aquatic toxicology. Aquat. Toxicol. 2013, 126, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Dahms, H.U. The grand challenges in marine pollution research. Front. Mar. Sci. 2014, 1, 9. [Google Scholar] [CrossRef]
- Delorenzo, M.E. Impacts of climate change on the ecotoxicology of chemical contaminants in estuarine organisms. Curr. Zool. 2015, 61, 641–652. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Fabbri, E. Pharmaceuticals in the environment: Expected and unexpected effects on aquatic fauna. Ann. N. Y. Acad. Sci. 2015, 1340, 20–28. [Google Scholar] [CrossRef]
- Desbiolles, F.; Malleret, L.; Tiliacos, C.; Wong-Wah-Chung, P.; Laffont-Schwob, I. Occurrence and ecotoxicological assessment of pharmaceuticals: Is there a risk for the Mediterranean aquatic environment? Sci. Total Environ. 2018, 639, 1334–1348. [Google Scholar] [CrossRef]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef]
- Garrison, A.W.; Pope, J.D.; Allen, F.R. Analysis of Organic Compounds in Domestic Wastewater. In Identification and Analysis of Organic Pollutants in Water; Keith, C.H., Ed.; Ann Arbor Science: Ann Arbor, MI, USA, 1976; pp. 517–566. [Google Scholar]
- Richardson, M.L.; Bowron, J.M. The fate of pharmaceutical chemicals in the aquatic environment. J. Pharm. Pharmacol. 1985, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Miao, X.S.; Koenig, B.G.; Struger, J. Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the Lower Great Lakes, Canada. Environ. Toxicol. Chem. 2003, 22, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Bayen, S.; Zhang, H.; Desai, M.M.; Ooi, S.K.; Kelly, B.C. Occurrence and distribution of pharmaceutically active and endocrine disrupting compounds in Singapore’s marine environment: Influence of hydrodynamics and physical-chemical properties. Environ. Pollut. 2013, 182, 1–8. [Google Scholar] [CrossRef]
- Birch, G.F.; Drage, D.S.; Thompson, K.; Eaglesham, G.; Mueller, J.F. Emerging contaminants (pharmaceuticals, personal care products, a food additive and pesticides) in waters of Sydney estuary, Australia. Mar. Pollut. Bull. 2015, 97, 56–66. [Google Scholar] [CrossRef]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in the marine environment: A review of its occurrence and effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 47, S78–S87. [Google Scholar] [CrossRef]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R.D. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Chaudhry, M.J.; Arshad, M.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, M.; Bebianno, M.J. Effects of non-steroidal anti-inflammatory drug (NSAID) diclofenac exposure in mussel Mytilus galloprovincialis. Aquat. Toxicol. 2014, 148, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.K.; Gusso-Choueri, P.K.; Maranho, L.A.; de Souza Abessa, D.M.; Mazur, W.A.; de Campos, B.G.; Guimaraes, L.L.; de Toledo, M.S.; Lebre, D.; Marques, J.R.; et al. A tiered approach to assess effects of diclofenac on the brown mussel Perna perna: A contribution to characterize the hazard. Water Res. 2018, 132, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Mezzelani, M.; Gorbi, S.; Fattorini, D.; d’Errico, G.; Consolandi, G.; Milan, M.; Bargelloni, L.; Regoli, F. Long-term exposure of Mytilus galloprovincialis to diclofenac, ibuprofen and ketoprofen: Insights into bioavailability, biomarkers and transcriptomic changes. Chemosphere 2018, 198, 238–248. [Google Scholar] [CrossRef]
- Munari, M.; Matozzo, V.; Gagné, F.; Chemello, G.; Riedl, V.; Finos, L.; Pastore, P.; Badocco, D.; Marin, M.G. Does exposure to reduced pH and diclofenac induce oxidative stress in marine bivalves? A comparative study with the mussel Mytilus galloprovincialis and the clam Ruditapes philippinarum. Environ. Pollut. 2018, 240, 925–937. [Google Scholar] [CrossRef]
- Munari, M.; Matozzo, V.; Chemello, G.; Riedl, V.; Pastore, P.; Badocco, D.; Marin, M.G. Seawater acidification and emerging contaminants: A dangerous marriage for haemocytes of marine bivalves. Environ. Res. 2019, 175, 11–21. [Google Scholar] [CrossRef]
- Liu, W.; He, M. Effects of Ocean Acidification on the metabolic rates of three species of bivalve from Southern Coast of China. Chin. J. Oceanol. Limnol. 2012, 30–32, 206–211. [Google Scholar] [CrossRef]
- Navarro, J.M.; Torres, R.; Acuna, K.; Duarte, C.; Manriquez, P.H.; Lardies, M.; Lagos, N.A.; Vargas, C.; Aguilera, V. Impact of medium-term exposure to elevated pCO2 levels on the physiological energetics of the mussel Mytilus chilensis. Chemosphere 2013, 90–93, 1242–1248. [Google Scholar] [CrossRef]
- Matoo, O.B.; Ivanina, A.V.; Ullstad, C.; Beniash, E.; Sokolova, I.M. Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp. Biochem. Phys. A 2013, 164, 545–553. [Google Scholar] [CrossRef]
- Freitas, R.; Almeida, A.; Calisto, V.; Velez, C.; Moreira, A.; Schneider, R.J.; Esteves, V.I.; Wrona, F.J.; Figueira, E.; Soares, A.M.V.M. The impacts of pharmaceutical drugs under ocean acidification: Newdata on single and combined long-term effects of carbamazepine on Scrobicularia plana. Sci. Total Environ. 2016, 541, 977–985. [Google Scholar] [CrossRef]
- Almeida, A.; Freitas, R.; Calisto, V.; Esteves, V.I.; Schneider, R.J.; Soares, A.M.V.M.; Figueira, E.; Campos, B.; Barata, C. Effects of carbamazepine and cetirizine under an ocean acidification scenario on the biochemical and transcriptome responses of the clam Ruditapes philippinarum. Environ. Pollut. 2018, 235, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Hartin, C.A.; Bond-Lamberti, B.; Patel, P.; Mundra, A. Ocean acidification over the next three centuries using a simple global climate carbon-cycle model: Projections and sensitivities. Biogeosciences 2016, 13, 4329–4342. [Google Scholar] [CrossRef]
- Widdows, J. Physiological Measurements in: The Effects of Stress and Pollution on Marine Animals; Bayne, B.L., Brown, D.A., Burns, K., Dixon, D.R., Ivanovici, A., Livingstone, D.R., Lowe, D.M., More, M.N., Stebbing, A.R.D., Widdows, J., Eds.; Praeger Press: New York, NY, USA, 1985; pp. 3–45. [Google Scholar]
- Widdows, J.; Johnson, D. Physiological Energetics of Mytilus edulis: Scope for Growth. Mar. Ecol. Prog. Ser. 1988, 46, 113–121. [Google Scholar] [CrossRef]
- Widdows, J.; Nasci, C.; Fossato, V.U. Effects of pollution on the scope for growth of mussels (Mytilus galloprovincialis) from the Venice Lagoon, Italy. Mar. Environ. Res. 1997, 43, 69–79. [Google Scholar] [CrossRef]
- Widdows, J. Marine and Estuarine Invertebrate Toxicity Test. In Handbook of Ecotoxicology; Calow, P., Ed.; Blackwell Scientific: Oxford, UK, 1993; Volume 1, pp. 146–166. [Google Scholar]
- Solorzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar]
- Ericson, H.; Thorsén, G.; Kumbla, L. Physiological effects of diclofenac, ibuprofen and propranolol on baltic sea blue mussels. Aquat. Toxicol. 2010, 99, 223–231. [Google Scholar] [CrossRef]
- Beesley, A.; Lowe, D.M.; Pascoe, C.K.; Widdicombe, S. Effect of CO2 induced seawater acidification on the health of Mytilus edulis. Dim. Res. 2008, 37, 215–225. [Google Scholar]
- Thomsen, J.; Melzner, F. Moderate seawater acidification does not elicit long-term metabolic depression in the blue mussel Mytilus edulis. Mar. Biol. 2010, 157, 2667–2676. [Google Scholar] [CrossRef]
- Kurihara, H.; Kato, S.; Ishimatsu, A. Effects of increased seawater pCO2 on the early development of the oyster Crassostrea gigas. Aquat. Biol. 2007, 1, 91–98. [Google Scholar] [CrossRef]
- Dupont, S.; Thorndyke, M. Ocean Acidification and its Impact on the Early Life-History Stages of Marine Animals. In Impacts of Acidification on Biological, Chemical and Physical Systems in the Mediterranean and Black Seas; Briand, F., Ed.; CIESM Workshop Monographs; CIESM Publisher: Monaco, 2008; Volume 36, 124p. [Google Scholar]
- Dupont, S.; Thorndyke, M. Impact of CO2-driven ocean acidification on invertebrate early life-history—What we know, what we need to know and what we can do. Biogeosci. Discuss. 2009, 6, 109–131. [Google Scholar] [CrossRef]
- Sanders, M.B.; Bean, T.P.; Hutchinson, T.H.; Le Quesne, W.J.F. Juvenile king scallop, Pecten maximus, is potentially tolerant to low levels of ocean acidification when food is unrestricted. PLoS ONE 2013, 8, e74118. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Reiriz, M.J.; Range, P.; Alvarez-Salgado, X.A.; Espinosa, J.; Labarta, U. Tolerance of juvenile Mytilus galloprovincialis to experimental seawater acidification. Mar. Ecol. Prog. Ser. 2012, 454, 65–74. [Google Scholar] [CrossRef]
- Guinotte, J.M.; Fabry, V.J. Ocean acidification and its potential effects on marine ecosystems. Ann. N. Y. Acad. Sci. 2008, 1134, 320–342. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.L.; Spicer, J.I.; Widdicombe, S. Ocean acidification may increase calcification rates, but at a cost. Proc. R. Soc. B. 2008, 275, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.; Caldeira, K.; Elderfield, H.; Hoegh-Guldberg, O.; Liss, P.; Riebesell, U.; Shepherd, J.; Turley, C.; Watson, A. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide. In The Royal Society Policy Document 12; The Cloyvedon Press: Cardiff, UK, 2005. [Google Scholar]
- Michaelidis, B.; Ouzounis, C.; Paleras, A.; Portner, H.O. Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 2005, 293, 109–118. [Google Scholar] [CrossRef]
- Willson, L.L.; Burnett, L.E. Whole animal and gill tissue oxygen uptake in the Eastern oyster, Crassostrea virginica: Effects of hypoxia, hypercapnia, air exposure, and infection with the protozoan parasite Perkinsus marinus. J. Exp. Mar. Biol. Ecol. 2000, 246, 223–240. [Google Scholar] [CrossRef]
- Lannig, G.; Eilers, S.; Pörtner, H.O.; Sokolova, I.M.; Bock, C. Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas—Changes in metabolic pathways and thermal response. Mar. Drugs 2010, 8, 2318–2339. [Google Scholar] [CrossRef]
- Fernández-Reiriz, M.J.; Range, P.; Alvarez-Salgado, X.A.; Labarta, U. Physiological energetics of juvenile clams Ruditapes decussatus in a high CO2 coastal ocean. Mar. Ecol. Prog. Ser. 2011, 433, 97–105. [Google Scholar]
- Han, N.K.; Lee, S.W.; Wang, S.Y. The effect of temperature on the energy budget of the Manila clam, Ruditapes philippinarum. Aquacult. Int. 2008, 16, 143–152. [Google Scholar] [CrossRef]
- Bussell, J.A.; Gidman, E.A.; Causton, D.R.; Gwynn-Jones, D.; Malham, S.K.; Jones, M.L.M.; Reynolds, B.; Seed, R. Changes in the immune response and metabolic fingerprint of the mussel Mytilus edulis (Linnaeus) in response to lowered salinity and physical stress. J. Exp. Mar. Biol. Ecol. 2008, 358, 78–85. [Google Scholar] [CrossRef]
| Sampling Time | Conditions (pH and Diclofenac) | pHT | TA | DIC | pCO2 | Ωcal | Ωarg | |
|---|---|---|---|---|---|---|---|---|
| T0 | N pH | 7.98 ± 0.01 | 2884.42 ± 52.45 | 2665.50 ± 50.58 | 631.47 ± 22.02 | 5.718 ± 0.11 | 3.76 ± 0.07 | |
| pH −0.4 | 7.76 ± 0.01 | 2800.33 ± 12.39 | 2681.52 ± 8.95 | 1080.65 ± 20.59 | 3.62 ± 0.08 | 2.38 ± 0.05 | ||
| pH −0.7 | 7.34 ± 0.05 | 2894.53 ± 40.94 | 2923.77 ± 32.32 | 3248.09 ± 376.73 | 1.56 ± 0.17 | 1.02 ± 0.11 | ||
| T1-T2 | N pH | 0.00 µg/L | 8.14 ± 0.01 | 2848.91 ± 5.40 | 2539.97 ± 4.86 | 399.19 ± 8.67 | 7.71 ± 0.15 | 5.07 ± 0.10 |
| 0.05 µg/L | 8.14 ± 0.01 | 2840.80 ± 2.45 | 2529.65 ± 7.58 | 391.60 ± 10.54 | 7.76 ± 0.17 | 5.10 ± 0.11 | ||
| 0.50 µg/L | 8.14 ± 0.01 | 2841.81 ± 6.30 | 2531.97 ± 5.32 | 395.75 ± 5.85 | 7.73 ± 0.09 | 5.09 ± 0.06 | ||
| pH −0.4 | 0.00 µg/L | 7.71 ± 0.03 | 2837.30 ± 7.66 | 2734.99 ± 17.47 | 1262.87 ± 113.96 | 3.34 ± 0.24 | 2.20 ± 0.16 | |
| 0.05 µg/L | 7.73 ± 0.02 | 2825.60 ± 5.93 | 2713.87 ± 8.83 | 1167.10 ± 62.20 | 3.49 ± 0.14 | 2.30 ± 0.09 | ||
| 0.50 µg/L | 7.74 ± 0.02 | 2820.23 ± 6.24 | 2708.33 ± 10.45 | 1143.96 ± 47.08 | 3.49 ± 0.10 | 2.29 ± 0.07 | ||
| pH −0.7 | 0.00 µg/L | 7.39 ± 0.02 | 2879.65 ± 11.71 | 2886.92 ± 15.69 | 2734.47 ± 106.67 | 1.72 ± 0.07 | 1.13 ± 0.04 | |
| 0.05 µg/L | 7.42 ± 0.01 | 2883.54 ± 12.98 | 2882.98 ± 15.51 | 2566.18 ± 85.56 | 1.81 ± 0.06 | 1.19 ± 0.04 | ||
| 0.50 µg/L | 7.43 ± 0.01 | 2878.18 ± 9.64 | 2872.24 ± 12.93 | 2504.41 ± 81.93 | 1.87 ± 0.06 | 1.23 ± 0.04 | ||
| Sampling Time | Factors | All Variables in | CR | RR | ER |
|---|---|---|---|---|---|
| T0 | pH | F(2,44) = 4.591 p(MC) = 0.011 | F(2,44) = 6.266 p(MC) = 0.005 | F(2,44) = 5.301 p(MC) = 0.006 | F(2,44) = 8.321 p(MC) = 0.004 |
| T1 | pH | F(2,44) = 38.919 p(MC) < 0.001 | F(2,44) = 2.832 p(MC) = 0.073 | F(2,44) = 43.027 p(MC) < 0.001 | F(2,44) = 5.773 p(MC) = 0.007 |
| Diclofenac | F(2,44) = 2.070 p(MC) = 0.127 | F(2,44) = 1.651 p(MC) = 0.199 | F(2,44) = 1.780 p(MC) = 0.183 | F(2,44) = 5.854 p(MC) = 0.007 | |
| pH*diclofenac | F(4,44) = 2.185 p(MC) = 0.073 | F(4,44) = 1.514 p(MC) = 0.212 | F(4,44) = 2.248 p(MC) = 0.082 | F(4,44) = 1.732 p(MC) = 0.162 | |
| T2 | pH | F(2,44) = 13.544 p(MC) < 0.001 | F(2,44) = 2.577 p(MC) = 0.086 | F(2,44) = 14.453 p(MC) < 0.001 | F(2,44) = 10.961 p(MC) < 0.001 |
| Diclofenac | F(2,44) = 5.903 p(MC) = 0.004 | F(2,44) = 1.650 p(MC) = 0.206 | F(2,44) = 6.747 p(MC) = 0.003 | F(2,44) = 1.002 p(MC) = 0.996 | |
| pH*diclofenac | F(4,44) = 6.947 p(MC) < 0.001 | F(4,44) = 3.748 p(MC) = 0.012 | F(4,44) = 7.438 p(MC) < 0.001 | F(4,44) = 3.939 p(MC) = 0.010 |
| T1 | pH N vs. pH −0.4 | pH N vs. pH −0.7 | pH −0.4 vs. pH −0.7 | |
| Respiration rate | 0.258 | <0.001 | <0.001 | |
| Excretion rate | 0.008 | 0.860 | 0.002 | |
| 0.00 µg/L vs. 0.05 µg/L | 0.00 µg/L vs. 0.50 µg/L | 0.05 µg/L vs. 0.50 µg/L | ||
| Excretion rate | 0.630 | 0.004 | 0.018 | |
| T2 | pH N vs. pH −0.4 | pH N vs. pH −0.7 | pH −0.4 vs, pH −0.7 | |
| Respiration rate | 0.049 | 0.008 | <0.001 | |
| Excretion rate | <0.001 | <0.001 | 0.482 | |
| 0.00 µg/L vs. 0.05 µg/L | 0.00 µg/L vs. 0.50 µg/L | 0.05 µg/L vs. 0.50 µg/L | ||
| Respiration rate | 0.002 | 0.048 | 0.101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munari, M.; Matozzo, V.; Riedl, V.; Pastore, P.; Badocco, D.; Marin, M.G. EAT BREATHE EXCRETE REPEAT: Physiological Responses of the Mussel Mytilus galloprovincialis to Diclofenac and Ocean Acidification. J. Mar. Sci. Eng. 2020, 8, 907. https://doi.org/10.3390/jmse8110907
Munari M, Matozzo V, Riedl V, Pastore P, Badocco D, Marin MG. EAT BREATHE EXCRETE REPEAT: Physiological Responses of the Mussel Mytilus galloprovincialis to Diclofenac and Ocean Acidification. Journal of Marine Science and Engineering. 2020; 8(11):907. https://doi.org/10.3390/jmse8110907
Chicago/Turabian StyleMunari, Marco, Valerio Matozzo, Verena Riedl, Paolo Pastore, Denis Badocco, and Maria Gabriella Marin. 2020. "EAT BREATHE EXCRETE REPEAT: Physiological Responses of the Mussel Mytilus galloprovincialis to Diclofenac and Ocean Acidification" Journal of Marine Science and Engineering 8, no. 11: 907. https://doi.org/10.3390/jmse8110907
APA StyleMunari, M., Matozzo, V., Riedl, V., Pastore, P., Badocco, D., & Marin, M. G. (2020). EAT BREATHE EXCRETE REPEAT: Physiological Responses of the Mussel Mytilus galloprovincialis to Diclofenac and Ocean Acidification. Journal of Marine Science and Engineering, 8(11), 907. https://doi.org/10.3390/jmse8110907








