Potential Environmental Effects of Marine Renewable Energy Development—The State of the Science
Abstract
1. Introduction
2. Methods
- (1)
- Collision risk with turbine blades;
- (2)
- Effects of underwater noise on animals;
- (3)
- Effects of electromagnetic fields (EMF) on animals;
- (4)
- Changes in benthic and pelagic habitats;
- (5)
- Changes in oceanographic processes;
- (6)
- Entanglement of animals with mooring systems.
3. Results
3.1. Collision Risk around Turbines
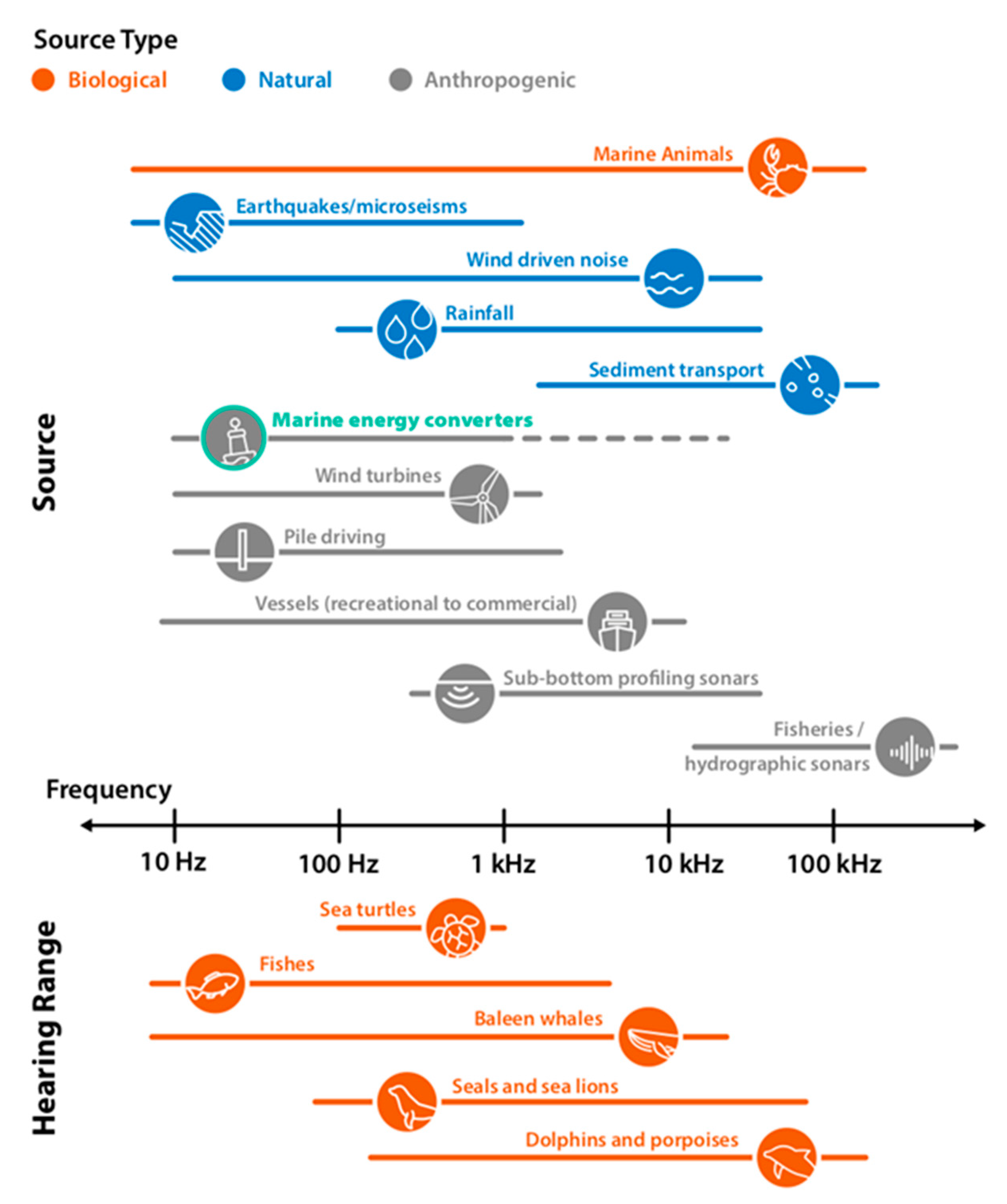
3.2. Risk to Marine and Riverine Animals from Underwater Noise Generated by MRE Devices
3.3. Risk to Animals from Electromagnetic Fields (EMFs) Emitted by MRE Cables
3.4. Changes in Benthic and Pelagic Habitats Caused by MRE Devices
3.5. Changes in Oceanographic Systems Associated with MRE
3.6. Entanglement Risk with MRE Mooring Systems and Subsea Cables
3.7. Social and Economic Data Collection for MRE
3.8. Management Strategies for Permitting MRE Projects
4. Discussion
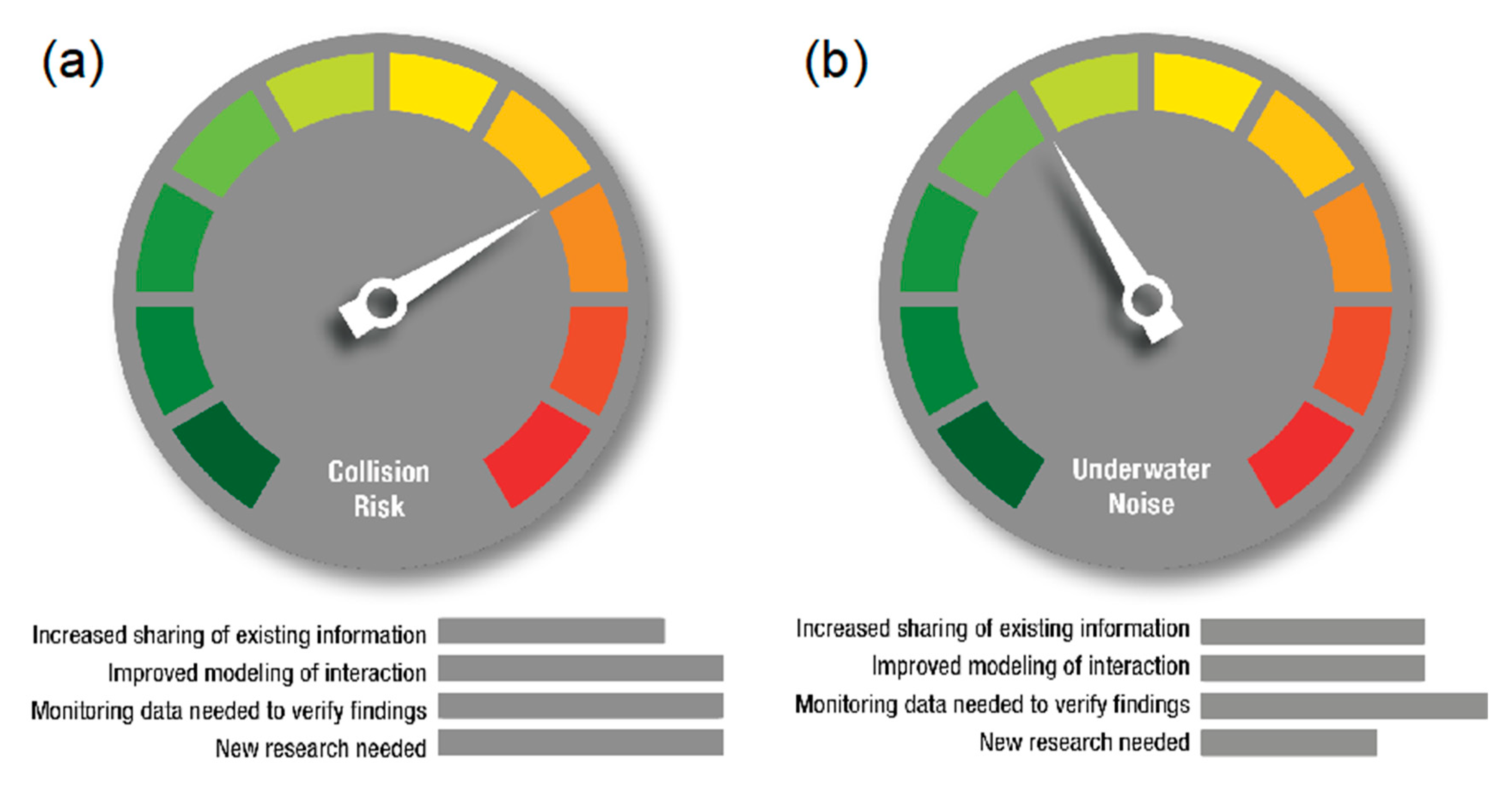
4.1. Additional Research Needed to Verify and Understand Results
4.2. Key Strategies for Accelerating MRE Development Responsibly
- (1)
- Data and information transfer. Carrying out a process of assessing and organizing datasets and knowledge from already permitted MRE projects and associated research studies, as well as lessons learned from other offshore industries, will make information readily accessible and transparent. Using open-source models and codes, as well as publicly accessible archives of data and model outputs, will make necessary data readily available. These steps can help all stakeholders, including MRE developers and regulators, to make informed decisions for siting and permitting [106].
- (2)
- Proportionate effort and costs. The effort to collect and analyze data around a specific stressor-receptor interaction should be related to the size of the MRE project and the likely risk to the specific animals or habitats of concern. Applying datasets and information from already permitted projects and appropriate research studies, augmented by site-specific data needed to validate their use, should help identify the adequate level of effort [106]. The accumulated evidence on stressor–receptor interactions can be applied to ensure that the highest risk interactions receive the appropriate attention and data collection efforts. Other interactions, for which the level of risk has been shown to be low, should be retired, or downgraded, to reduce data collection and effort.
- (3)
- Applying principles of MSP and AM. The permitting and social acceptance of a proposed MRE project can be helped by applying information gathered through stakeholder engagement and data gathering associated with MSP in the region of a proposed MRE project [88]. Similarly, the application of AM as a means of iteratively learning more about potential environmental effects of MRE devices can assist with efficient and effective permitting processes and ensure that post-installation monitoring is useful and appropriate [107].
4.3. Trends in MRE Environmental Effects
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kroposki, B.; Johnson, B.; Zhang, Y.; Gevorgian, V.; Denholm, P.; Hodge, B.; Hannegan, B. Achieving a 100% Renewable Grid: Operating Electric Power Systems with Extremely High Levels of Variable Renewable Energy. IEEE Power Energy Mag. 2017, 15, 61–73. [Google Scholar] [CrossRef]
- Owusu, P.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- United Nations General Assembly. Report on the Work of the United Nations Open-Ended Informal Consultative Process on Oceans and the Law of the Sea at Its Thirteenth Meeting (A/67/120); United Nations: New York, NY, USA, 2012. [Google Scholar]
- Ocean Energy Systems. Annual Report, An Overview of Ocean Energy Activities in 2019; The Executive Committee of Ocean Energy Systems: Lisbon, Portugal, 2019; p. 152. [Google Scholar]
- Pörtner, H.; Gutt, J. Impacts of Climate Variability and Change on (Marine) Animals: Physiological Underpinnings and Evolutionary Consequences. Integr. Comp. Biol. 2016, 56, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, K.; De Young, C.; Soto, D.; Bahri, T. (Eds.) Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical Paper, No. 530; FAO: Rome, Italy, 2009; p. 212. [Google Scholar]
- LiVecchi, A.; Copping, A.; Jenne, D.; Gorton, A.; Preus, R.; Gill, G.; Robichaud, R.; Green, R.; Geerlofs, S.; Gore, S.; et al. Powering the Blue Economy; Exploring Opportunities for Marine Renewable Energy in Maritime Markets; U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy: Washington, DC, USA, 2019.
- Copping, A.; Green, R.; Cavagnaro, R.; Jenne, S.; Martinez, J.; Greene, D.; Yang, Y. Powering the Blue Economy—Ocean Observing Use Cases Report; Report PNNL-29585; Pacific Northwest National Laboratory: Richland, WA, USA, 2020. [Google Scholar]
- Lee, K.; Noh, J.; Khim, J. The Blue Economy and the United Nations’ sustainable development goals: Challenges and opportunities. Environ. Int. 2020, 137, 105528. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). Rethinking Innovation for a Sustainable Ocean Economy; OECD Publishing: Paris, France, 2019. [Google Scholar]
- World Bank. PROBLUE: 2019 Annual Report; World Bank: Washington, DC, USA, 2019; p. 36. [Google Scholar]
- Langhamer, O. The location of offshore wave power devices structures epifaunal assemblages. Int. J. Mar. Energy 2016, 16, 174–180. [Google Scholar] [CrossRef]
- Coates, D.; Kapasakali, D.; Vincx, M.; Vana verbeke, J. Short-term effects of fishery exclusion in offshore wind farms on macrofaunal communities in the Belgian part of the North Sea. Fish. Res. 2016, 179, 131–138. [Google Scholar] [CrossRef]
- Uihlein, A.; Magagna, D. Wave and tidal current energy—A review of the current state of research beyond technology. Renew. Sustain. Energy Rev. 2016, 58, 1070–1081. [Google Scholar] [CrossRef]
- Copping, A.; Freeman, M.; Gorton, A.; Hemery, L. Risk Retirement—Decreasing Uncertainty and Informing Consenting Processes for Marine Renewable Energy Development. J. Mar. Sci. Eng. 2020, 8, 172. [Google Scholar] [CrossRef]
- Gibbs, M.; Browman, H. Risk assessment and risk management: A primer for marine scientists. ICES J. Mar. Sci. 2015, 72, 992–996. [Google Scholar] [CrossRef]
- Copping, A.; Hemery, L. (Eds.) OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Ocean Energy Systems (OES): Richland, WA, USA, 2020. [Google Scholar]
- Boehlert, G.; Gill, A. Environmental and Ecological Effects of Ocean Renewable Energy Development: A Current Synthesis. Oceanography 2010, 23, 68–81. [Google Scholar] [CrossRef]
- Offshore Renewables Joint Industry Programme (ORJIP). ORJIP Ocean Energy: The Forward Look; an Ocean Energy Environmental Research Strategy for the UK; Aquatera Ltd.: Stromness, Orkney, 2017. [Google Scholar]
- Copping, A. The State of Knowledge for Environmental Effects: Driving Consenting/Permitting for the Marine Renewable Energy Industry; Pacific Northwest National Laboratory (PNNL) for Ocean Energy Systems (OES): Lisbon, Portugal, 2018; p. 25. [Google Scholar]
- Lieber, L.; Nimmo-Smith, W.; Waggitt, J.; Kregting, L. Fine-scale hydrodynamic metrics underlying predator occupancy patterns in tidal stream environments. Ecol. Indic. 2018, 94, 397–408. [Google Scholar] [CrossRef]
- Waggitt, J.; Robbins, A.; Wade, H.; Masden, E.; Furness, R.; Jackson, A.; Scott, B. Comparative studies reveal variability in the use of tidal stream environments by seabirds. Mar. Policy 2017, 81, 143–152. [Google Scholar] [CrossRef]
- Matzner, S.; Trostle, C.; Staines, G.; Hull, R.; Avila, A.; Harker-Klimes, G. Triton: Igiugig Fish Video Analysis; PNNL-26576; Pacific Northwest National Laboratory: Richland, WA, USA, 2017. [Google Scholar]
- Band, B.; Sparling, C.; Thompson, D.; Onoufriou, J.; San Martin, E.; West, N. Refining Estimates of Collision Risk for Harbour Seals and Tidal Turbines. Scott. Mar. Freshw. Sci. 2016, 7, 17. [Google Scholar]
- Schmitt, P.; Culloch, R.; Lieber, L.; Molander, S.; Hammar, L.; Kregting, L. A tool for simulating collision probabilities of animals with marine renewable energy devices. PLoS ONE 2017, 12, e0188780. [Google Scholar] [CrossRef] [PubMed]
- Copping, A.; Grear, M. Applying a simple model for estimating the likelihood of collision of marine mammals with tidal turbines. Int. Mar. Energy J. 2018, 1, 27–33. [Google Scholar] [CrossRef]
- Onoufriou, J.; Brownlow, A.; Moss, S.; Hastie, G.; Thompson, D. Empirical determination of severe trauma in seals from collisions with tidal turbine blades. J. Appl. Ecol. 2017, 56, 1712–1724. [Google Scholar] [CrossRef]
- Joy, R.; Wood, J.; Sparling, C.; Tollit, D.; Copping, A.; McConnell, B. Empirical measures of harbor seal behavior and avoidance of an operational tidal turbine. Mar. Pollut. Bull 2018, 136, 92–106. [Google Scholar] [CrossRef]
- Bevelhimer, M.; Scherelis, C.; Colby, J.; Adonizio, M. Hydroacoustic Assessment of Behavioral Responses by Fish Passing Near an Operating Tidal Turbine in the East River, New York. Trans. Am. Fish. Soc. 2017, 146, 1028–1042. [Google Scholar] [CrossRef]
- Viehman, H.; Zydlewski, G. Fish Interactions with a Commercial-Scale Tidal Energy Device in the Natural Environment. Estuaries Coast 2015, 38, 241–252. [Google Scholar] [CrossRef]
- Robbins, A. Seabird Ecology in High-Energy Environments: Approaches to Assessing Impacts of Marine Renewables. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2017. [Google Scholar]
- Waggitt, J.; Cazenave, P.; Torres, R.; Williamson, B.; Scott, B. Quantifying pursuit-diving seabirds’ associations with fine-scale physical features in tidal stream environments. J. Appl. Ecol. 2016, 53, 1653–1666. [Google Scholar] [CrossRef]
- Isaksson, N.; Masden, E.; Williamson, B.; Costagliola-Ray, M.; Slingsby, J.; Houghton, J.; Wilson, J. Assessing the effects of tidal stream marine renewable energy on seabirds: A conceptual framework. Mar. Pollut. Bull. 2020, 157, 111314. [Google Scholar] [CrossRef] [PubMed]
- Southall, B.; Bowles, A.; Ellison, W.; Finneran, J.; Gentry, R.; Greene, C.; Kastak, D.; Ketten, D.; Miller, J.; Nachtigall, P.; et al. Marine Mammal Noise Exposure Criteria: Initial Scientific Recommendations. Aquat. Mamm. 2007, 33, 411–521. [Google Scholar] [CrossRef]
- Halvorsen, M.; Casper, B.; Woodley, C.; Carlson, T.; Popper, A. Threshold for onset of injury in Chinook salmon from exposure to impulsive pile driving sounds. PLoS ONE 2012, 7, e38968. [Google Scholar] [CrossRef]
- Walsh, J.; Bashir, I.; Garrett, J.; Thies, P.; Blondel, P.; Johanning, L. Monitoring the condition of Marine Renewable Energy Devices through underwater Acoustic Emissions: Case study of a Wave Energy Converter in Falmouth Bay, UK. Renew. Energy 2017, 102, 205–213. [Google Scholar] [CrossRef]
- National Marine Fisheries Service (NMFS). 2018 Revisions to: Technical Guidance for Assessing the Effects of Anthropogenic Sound on Marine Mammal Hearing (Version 2.0): Underwater Thresholds for Onset of Permanent and Temporary Threshold Shifts. NOAA Technical Memorandum NMFS-OPR-59; U.S. Department of Commerce, National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2018.
- Tetra Tech. Underwater Acoustic Modeling Report—Virginia Offshore Wind Technology Advancement Project (VOWTAP); Dominion Energy: Glen Allen, VA, USA, 2013. [Google Scholar]
- International Electrotechnical Commission (IEC). Technical Specification 62600-40: Marine Energy–Wave, Tidal and Other Water Current Converters—Part 40: Acoustic Characterization of Marine Energy Converters; IEC: Geneva, Switzerland, 2019. [Google Scholar]
- Hastie, G.; Russell, D.; Lepper, P.; Elliott, J.; Wilson, B.; Benjamins, S.; Thompson, D. Harbour seals avoid tidal turbine noise: Implications for collision risk. J. Appl. Ecol. 2018, 55, 684–693. [Google Scholar] [CrossRef]
- Robertson, F.; Wood, J.; Joslin, J.; Joy, R.; Polagye, B. Marine Mammal Behavioral Response to Tidal Turbine Sound (DOE-UW-06385); University of Washington: Seattle, WA, USA, 2018. [Google Scholar]
- Pine, M.; Schmitt, P.; Culloch, R.; Lieber, L.; Kregting, L. Providing ecological context to anthropogenic subsea noise: Assessing listening space reductions of marine mammals from tidal energy devices. Renew. Sustain. Energy Rev. 2019, 103, 49–57. [Google Scholar] [CrossRef]
- Polagye, B.; Basset, C. Risk to Marine Animals from Underwater Noise Generated by Marine Renewable Energy Devices. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 67–85. [Google Scholar]
- Newton, K.; Gill, A.; Kajiura, S. Electroreception in marine fishes: Chondrichthyans. J. Fish Biol. 2019, 95, 135–154. [Google Scholar] [CrossRef]
- Snyder, D.; Bailey, W.; Palmquist, K.; Cotts, B.; Olsen, K. Evaluation of Potential EMF Effects on Fish Species of Commercial or Recreational Fishing Importance in Southern New England (OCS Study BOEM 2019-049); Bureau of Ocean Energy Management, U.S. Department of the Interior: Sterling, VA, USA, 2019.
- Formicki, K.; Korzelecka-Orkisz, A.; Tański, A. Magnetoreception in fish. J. Fish Biol. 2019, 95, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Taormina, B.; Bald, J.; Want, A.; Thouzeau, G.; Lejart, M.; Desroy, N.; Carlier, A. A review of potential impacts of submarine power cables on the marine environment: Knowledge gaps, recommendations and future directions. Renew. Sustain. Energy Rev. 2018, 96, 380–391. [Google Scholar] [CrossRef]
- Albert, L.; Deschamps, F.; Jolivet, A.; Olivier, F.; Chauvaud, L.; Chauvaud, S. A current synthesis on the effects of electric and magnetic fields emitted by submarine power cables on invertebrates. Mar. Environ. Res. 2020, 159, 104958. [Google Scholar] [CrossRef]
- Hutchison, Z.; Sigray, P.; He, H.; Gill, A.; King, J.; Gibson, C. Electromagnetic Field (EMF) Impacts on Elasmobranch (shark, rays, and skates) and American Lobster Movement and Migration from Direct Current Cables; Bureau of Ocean Energy Management, U.S. Department of Interior: Sterling, VA, USA, 2018.
- Hutchison, Z.; Gill, A.; Sigray, P.; He, H.; King, J. Anthropogenic electromagnetic fields (EMF) influence the behaviour of bottom-dwelling marine species. Sci. Rep. 2020, 10, 4219. [Google Scholar] [CrossRef]
- Siegenthaler, A.; Niemantsverdriet, P.; Laterveer, M.; Heitkönig, I. Aversive responses of captive sandbar sharks Carcharhinus plumbeus to strong magnetic fields. J. Fish Biol. 2016, 89, 1603–1611. [Google Scholar] [CrossRef]
- Gill, A.; Desender, M. Risk to Animals from Electro-magnetic Fields Emitted by Electric Cables and Marine Renewable Energy Devices. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 86–103. [Google Scholar]
- Normandeau Associates Inc.; Exponent Inc.; Tricas, T.; Gill, A. Effects of EMFs from Undersea Power Cables on Elasmobranchs and other Marine Species (OCS Study BOEMRE 2011-09); Bureau of Ocean Energy Management Pacific OCS Region, U.S. Department of the Interior: Camarillo, CA, USA, 2011.
- Anderson, J.; Clegg, T.; Véras, L.; Holland, K. Insight into shark magnetic field perception from empirical observations. Sci. Rep. 2017, 7, 11042. [Google Scholar] [CrossRef] [PubMed]
- Wyman, M.; Klimley, A.; Battleson, R.; Agosta, T.; Chapman, E.; Haverkamp, P.; Pagel, M.; Kavet, R. Behavioral responses by migrating juvenile salmonids to a subsea high-voltage DC power cable. Mar. Biol. 2018, 165, 134. [Google Scholar] [CrossRef]
- Copping, A.; Sather, N.; Hanna, L.; Whiting, J.; Zydlewski, G.; Staines, G.; Gill, A.; Hutchison, I.; O’Hagan, A.; Simas, T.; et al. Annex IV 2016 State of the Science Report: Environmental Effects of Marine Renewable Energy Development Around the World; Ocean Energy Systems (OES): Lisbon, Portugal, 2016. [Google Scholar]
- Dannheim, J.; Bergström, L.; Birchenough, S.; Brzana, R.; Boon, A.; Coolen, J.; Dauvin, J.; De Mesel, I.; Derweduwen, J.; Gill, A.; et al. Benthic effects of offshore renewables: Identification of knowledge gaps and urgently needed research. ICES J. Mar. Sci. 2019, 77, 1092–1108. [Google Scholar] [CrossRef]
- Kramer, S.; Hamilton, C.; Spencer, G.; Ogston, H. Evaluating the Potential for Marine and Hydrokinetic Devices to Act as Artificial Reefs or Fish Aggregating Devices. Based on Analysis of Surrogates in Tropical, Subtropical, and Temperate U.S. West Coast and Hawaiian Coastal Waters; OCS Study BOEM 2015-021; U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy: Golden, CO, USA, 2015.
- Williamson, B.; Fraser, S.; Williamson, L.; Nikora, V.; Scott, B. Predictable changes in fish school characteristics due to a tidal turbine support structure. Renew. Energy 2019, 141, 1092–1102. [Google Scholar] [CrossRef]
- Lieber, L.; Nimmo-smith, W.; Waggitt, J.; Kregting, L. Localised anthropogenic wake generates a predictable foraging hotspot for top predators. Commun. Biol. 2019, 2, 123. [Google Scholar] [CrossRef]
- Adams, T.; Miller, R.; Aleynik, D.; Burrows, M. Offshore marine renewable energy devices as stepping stones across biogeographical boundaries. J. Appl. Ecol. 2014, 51, 330–338. [Google Scholar] [CrossRef]
- Alexander, K.; Meyjes, S.; Heymans, J. Spatial ecosystem modelling of marine renewable energy installations: Gauging the utility of Ecospace. Ecol. Model. 2016, 331, 115–128. [Google Scholar] [CrossRef]
- Greaves, D.; Iglesias, G. (Eds.) Wave and Tidal Energy; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Kraus, C.; Carter, L. Seabed recovery following protective burial of subsea cables—Observations from the continental margin. Ocean Eng. 2018, 157, 251–261. [Google Scholar] [CrossRef]
- Sheehan, E.; Cartwright, A.; Witt, M.; Attrill, M.; Vural, M.; Holmes, L. Development of epibenthic assemblages on artificial habitat associated with marine renewable infrastructure. ICES J. Mar. Sci. 2018, 77, 1178–1189. [Google Scholar] [CrossRef]
- O’Carroll, J.; Kennedy, R.; Creech, A.; Savidge, G. Tidal Energy: The benthic effects of an operational tidal stream turbine. Mar. Environ. Res. 2017, 129, 277–290. [Google Scholar] [CrossRef]
- Iglesias, G.; Carballo, R. Wave farm impact: The role of farm-to-coast distance. Renew. Energy 2014, 69, 375–385. [Google Scholar] [CrossRef]
- Robins, P.; Neill, S.; Lewis, M. Impact of tidal-stream arrays in relation to the natural variability of sedimentary processes. Renew. Energy 2014, 72, 311–321. [Google Scholar] [CrossRef]
- De Dominicis, M.; O’Hara Murray, R.; Wolf, J. Multi-scale ocean response to a large tidal stream turbine array. Renew. Energy 2017, 114, 1160–1179. [Google Scholar] [CrossRef]
- Nash, S.; O’Brien, N.; Olbert, A.; Hartnett, M. Modelling the far field hydro-environmental impacts of tidal farms—A focus on tidal regime, inter-tidal zones and flushing. Comput. Geosci. 2014, 71, 20–27. [Google Scholar] [CrossRef]
- Nuernberg, M.; Tao, L. Experimental study of wake characteristics in tidal turbine arrays. Renew. Energy 2018, 127, 168–181. [Google Scholar] [CrossRef]
- Ouro, P.; Runge, S.; Luo, Q.; Stoesser, T. Three-dimensionality of the wake recovery behind a vertical axis turbine. Renew. Energy 2019, 133, 1066–1077. [Google Scholar] [CrossRef]
- Chatzirodou, A.; Karunarathna, H.; Reeve, D. 3D modelling of the impacts of in-stream horizontal-axis Tidal Energy Converters (TECs) on offshore sandbank dynamics. Appl. Ocean Res. 2019, 91, 101882. [Google Scholar] [CrossRef]
- Schuchert, P.; Kregting, L.; Pritchard, D.; Savidge, G.; Elsäßer, B. Using Coupled Hydrodynamic Biogeochemical Models to Predict the Effects of Tidal Turbine Arrays on Phytoplankton Dynamics. J. Mar. Sci. Eng. 2018, 6, 58. [Google Scholar] [CrossRef]
- van der Molen, J.; Ruardij, P.; Greenwood, N. Potential environmental impact of tidal energy extraction in the Pentland Firth at large spatial scales: Results of a biogeochemical model. Biogeosciences 2016, 13, 2593–2609. [Google Scholar] [CrossRef]
- Garavelli, L. Encounters of Marine Animals with Marine Renewable Energy Device Mooring Systems and Subsea Cables. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 147–153. [Google Scholar]
- Sparling, C.; Coram, A.; McConnell, B.; Thompson, D.; Hawkins, K.; Northridge, S. Wave and Tidal Consenting Position Paper Series: Marine Mammal Impacts; Natural Environment Research Council (NERC): Swindon, UK, 2013. [Google Scholar]
- Benjamins, S.; Harnois, V.; Smith, H.; Johanning, L.; Greenhill, L.; Carter, C.; Wilson, B. Understanding the Potential for Marine Megafauna Entanglement Risk from Marine Renewable Energy Developments; Scottish Natural Heritage Commissioned Report No. 791; NatureScot: Inverness, UK, 2014. [Google Scholar]
- Moore, M.; Bogomolni, A.; Bowman, R.; Hamilton, P.; Harry, C.; Knowlton, A.; Landry, S.; Rotstein, D.; Touhey, K. Fatally entangled right whales can die extremely slowly. In Proceedings of the OCEANS 2006, Washington, DC, USA, 18–21 September 2006. [Google Scholar]
- Robbins, J.; Knowlton, A.; Landry, S. Apparent survival of North Atlantic right whales after entanglement in fishing gear. Biol. Conserv. 2015, 191, 421–427. [Google Scholar] [CrossRef]
- Regeneris Consulting Ltd.; Cardiff University. The Economic Impact of the Development of Marine Energy in Wales; Regeneris Consulting Ltd.: Manchester, UK, 2013. [Google Scholar]
- Bonar, P.; Bryden, I.; Borthwick, A. Social and ecological impacts of marine energy development. Renew. Sustain. Energy Rev. 2015, 47, 486–495. [Google Scholar] [CrossRef]
- Dalton, G.; Allan, G.; Beaumont, N.; Georgakaki, A.; Hacking, N.; Hooper, T.; Kerr, S.; O’Hagan, A.; Reilly, K.; Ricci, P.; et al. Economic and socio-economic assessment methods for ocean renewable energy: Public and private perspectives. Renew. Sustain. Energy Rev. 2015, 45, 850–878. [Google Scholar] [CrossRef]
- Kerr, S.; Watts, L.; Colton, J.; Conway, F.; Hull, A.; Johnson, K.; Jude, S.; Kannen, A.; MacDougall, S.; McLachlan, C.; et al. Establishing an agenda for social studies research in marine renewable energy. Energy Policy 2014, 67, 694–702. [Google Scholar] [CrossRef]
- Freeman, M. Social and Economic Data Collection for Marine Renewable Energy. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A., Hemery, L., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 155–175. [Google Scholar]
- Ehler, C. Conclusions: Benefits, lessons learned, and future challenges of marine spatial planning. Mar. Policy 2008, 32, 840–843. [Google Scholar] [CrossRef]
- Dineshbabu, A.; Thomas, S.; Rohit, P.; Maheswarudu, G. Marine spatial planning for resource conservation, fisheries management and for ensuring fishermen security—Global perspectives and Indian initiatives. Curr. Sci. 2019, 116, 561–567. [Google Scholar] [CrossRef]
- O’Hagan, A. Marine Spatial Planning and Marine Renewable Energy. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A., Hemery, L., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 215–241. [Google Scholar]
- Williams, B. Adaptive management of natural resources—Framework and issues. J. Eviron. Manag. 2011, 92, 1346–1353. [Google Scholar] [CrossRef]
- Bennet, F.; Culloch, R.; Tait, A. Guidance on Effective Adaptive Management and Post-Consent Monitoring Strategies; RiCORE Project: Aberdeen, UK, 2016. [Google Scholar]
- Magagna, D.; Greaves, D.; Conley, D.; O’Hagan, A.; Holmes, B.; Witt, M.; Simas, T.; Olivares, C.; Leitão, J.; Mouslim, H.; et al. How Experiences of the Offshore Wind Industry Can Aid Development of the Wave Energy Sector: Lessons Learnt From EIA Studies. In Proceedings of the 22nd International Offshore and Polar Engineering Conference, Rhodes, Greece, 17–22 June 2012. [Google Scholar]
- Jansujwicz, J.; Johnson, T. Understanding and Informing Permitting Decisions for Tidal Energy Development Using Adaptive Management Framework. Estuaries Coast 2015, 38, 253–265. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine (NAS). Guidelines for Managing Geotechnical Risks in Design–Build Projects; Research Report 884; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Hasselman, D.; Barclay, D.; Cavagnaro, R.; Chandler, C.; Cotter, E.; Gillespie, D.; Hastie, G.; Horne, J.; Joslin, J.; Long, C.; et al. Environmental Monitoring Technologies and Techniques for Detecting Interactions of Marine Animals with Turbines. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development Around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 176–213. [Google Scholar]
- Sparling, C.; Seitz, A.; Masden, E.; Smith, K. Collision Risk for Animals around Turbines. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 29–65. [Google Scholar]
- Wilson, B.; Marmo, B.; Lepper, P.; Risch, D.; Benjamins, S.; Hastie, G.; Carter, C. Good noise, bad noise: A tricky case of balancing risk of physical injury against acoustic disturbance for marine mammals and tidal energy devices. J. Acoust. Soc. Am. 2017, 141, 3921. [Google Scholar] [CrossRef]
- Bevelhimer, M.; Pracheil, B.; Fortner, A.; Saylor, R.; Deck, K. Mortality and injury assessment for three species of fish exposed to simulated turbine blade strike. Can. J. Fish. Aquat. Sci. 2019, 76, 2350–2363. [Google Scholar] [CrossRef]
- Grear, M.; Motley, M.; Crofts, S.; Witt, A.; Summers, A.; Ditsche, P. Mechanical properties of harbor seal skin and blubber—A test of anisotropy. Zoology 2018, 126, 137–144. [Google Scholar] [CrossRef]
- Hemery, L. Changes in Benthic and Pelagic Habitats Caused by Marine Renewable Energy Devices. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 105–125. [Google Scholar]
- Wilding, T.; Gill, A.; Boon, A.; Sheehan, E.; Dauvin, J.; Pezy, J.; O’Beirn, F.; Janas, U.; Rostin, L.; De Mesel, I. Turning off the DRIP (‘Data-rich, information-poor’)—Rationalising monitoring with a focus on marine renewable energy developments and the benthos. Renew. Sustain. Energy Rev. 2017, 74, 848–859. [Google Scholar] [CrossRef]
- du Feu, R.; Funke, S.; Kramer, S.; Hill, J.; Piggott, M. The trade-off between tidal-turbine array yield and environmental impact: A habitat suitability modelling approach. Renew. Energy 2019, 143, 390–403. [Google Scholar] [CrossRef]
- Linder, H.; Horne, J. Evaluating statistical models to measure environmental change: A tidal turbine case study. Ecol. Indic. 2018, 84, 765–792. [Google Scholar] [CrossRef]
- Whiting, J.; Chang, G. Changes in Oceanographic Systems Associated with Marine Renewable Energy Devices. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 127–145. [Google Scholar]
- Kreitmair, M.; Adcock, T.; Borthwick, A.; Draper, S.; van den Bremer, T. The effect of bed roughness uncertainty on tidal stream power estimates for the Pentland Firth. R. Soc. Open. Sci. 2020, 7, 1. [Google Scholar] [CrossRef]
- Waldman, S.; Weir, S.; O’Hara Murray, R.; Woolf, D.; Kerr, S. Future policy implications of tidal energy array interactions. Mar. Policy 2019, 108, 103611. [Google Scholar] [CrossRef]
- Copping, A.; Freeman, M.; Gorton, A.; Hemery, L. Risk Retirement and Data Transferability for Marine Renewable Energy. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 263–279. [Google Scholar]
- Le Lièvre, C. Adaptive Management Related to Maritime Renewable Energy. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems (OES): Lisbon, Portugal, 2020; pp. 243–261. [Google Scholar]
- Copping, A.; Kramer, S. A snapshot of risk for environmental effects of marine renewable energy development. In Proceedings of the 6th Annual Marine Energy Technology Symposium, Washington, DC, USA, 30 April–2 May 2017. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copping, A.E.; Hemery, L.G.; Overhus, D.M.; Garavelli, L.; Freeman, M.C.; Whiting, J.M.; Gorton, A.M.; Farr, H.K.; Rose, D.J.; Tugade, L.G. Potential Environmental Effects of Marine Renewable Energy Development—The State of the Science. J. Mar. Sci. Eng. 2020, 8, 879. https://doi.org/10.3390/jmse8110879
Copping AE, Hemery LG, Overhus DM, Garavelli L, Freeman MC, Whiting JM, Gorton AM, Farr HK, Rose DJ, Tugade LG. Potential Environmental Effects of Marine Renewable Energy Development—The State of the Science. Journal of Marine Science and Engineering. 2020; 8(11):879. https://doi.org/10.3390/jmse8110879
Chicago/Turabian StyleCopping, Andrea E., Lenaïg G. Hemery, Dorian M. Overhus, Lysel Garavelli, Mikaela C. Freeman, Jonathan M. Whiting, Alicia M. Gorton, Hayley K. Farr, Deborah J. Rose, and Levy G. Tugade. 2020. "Potential Environmental Effects of Marine Renewable Energy Development—The State of the Science" Journal of Marine Science and Engineering 8, no. 11: 879. https://doi.org/10.3390/jmse8110879
APA StyleCopping, A. E., Hemery, L. G., Overhus, D. M., Garavelli, L., Freeman, M. C., Whiting, J. M., Gorton, A. M., Farr, H. K., Rose, D. J., & Tugade, L. G. (2020). Potential Environmental Effects of Marine Renewable Energy Development—The State of the Science. Journal of Marine Science and Engineering, 8(11), 879. https://doi.org/10.3390/jmse8110879








