Investigation on the Emission Characteristics with a Wet-Type Exhaust Gas Cleaning System for Marine Diesel Engine Application
Abstract
1. Introduction
2. Experimental Apparatus and Method
2.1. Engine Test
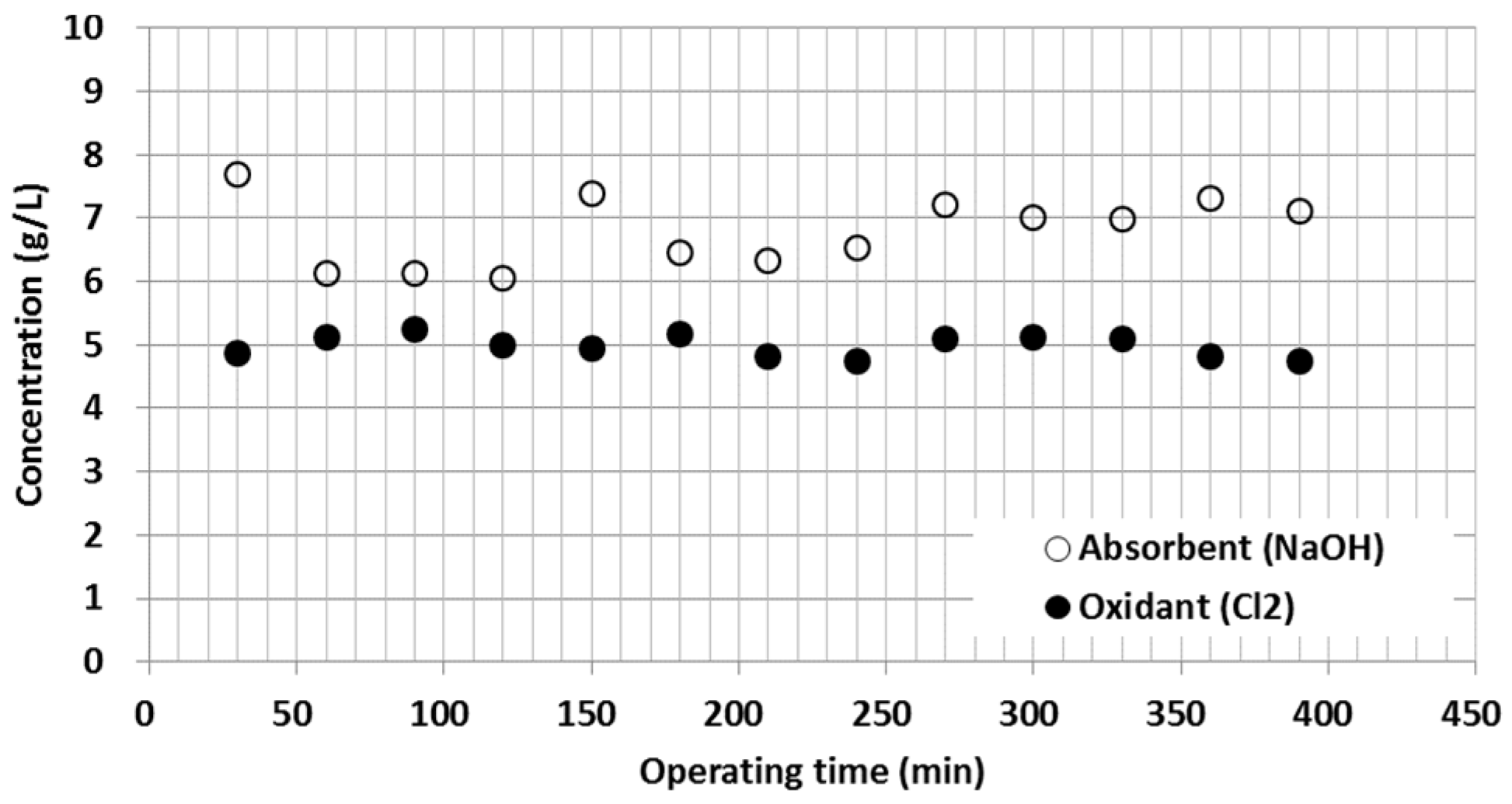
2.2. Oxidation and Reduction Agent
- (1)
- Inject 1 mL of the sample into 25 mL of ultrapure water.
- (2)
- Add 1 g of potassium iodide and 1 mL of acetic acid.
- (3)
- Add two to three drops of 1% starch solution. The solution then turns reddish brown.
- (4)
- Add 0.1 N sodium thiosulfate to the reddish-brown solution and measure the amount added until it becomes colorless.
- (5)
- Determine the effective chlorine concentration as follows:where C is the titration amount of 0.1 N sodium thiosulfate and S is the sample injection amount.
- (1)
- Add 1 mL of sample to 25 mL of ultrapure water.
- (2)
- Add two to three drops of phenolphthalein. The solution turns red.
- (3)
- Add 0.1 M hydrochloric acid to the red solution and measure the amount injected until it becomes colorless.
- (4)
- Determine the concentration of sodium hydroxide as follows:where C is the titration amount of 0.1 M hydrochloric acid and S is the sample injection amount.
3. Results and Discussion
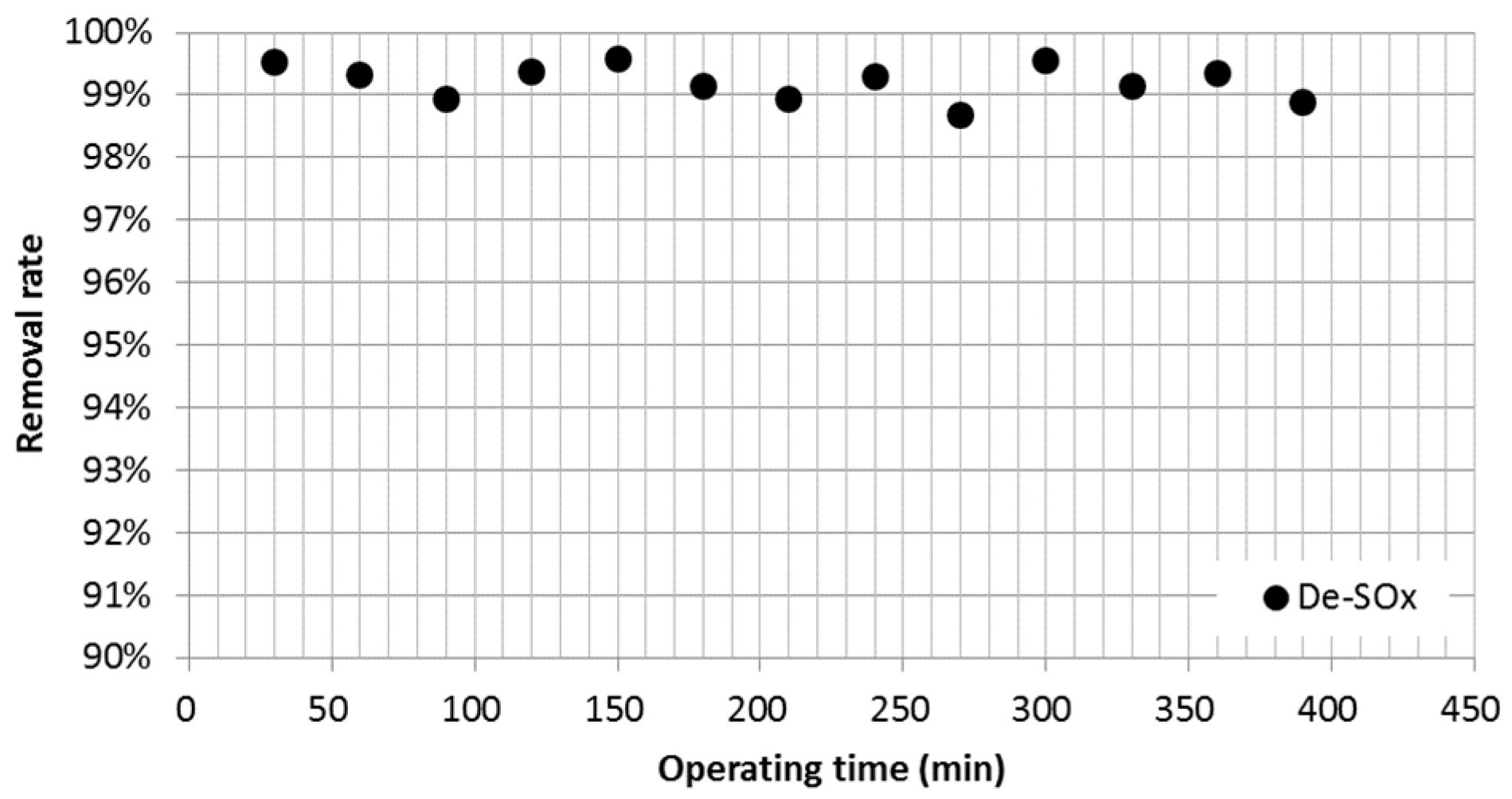
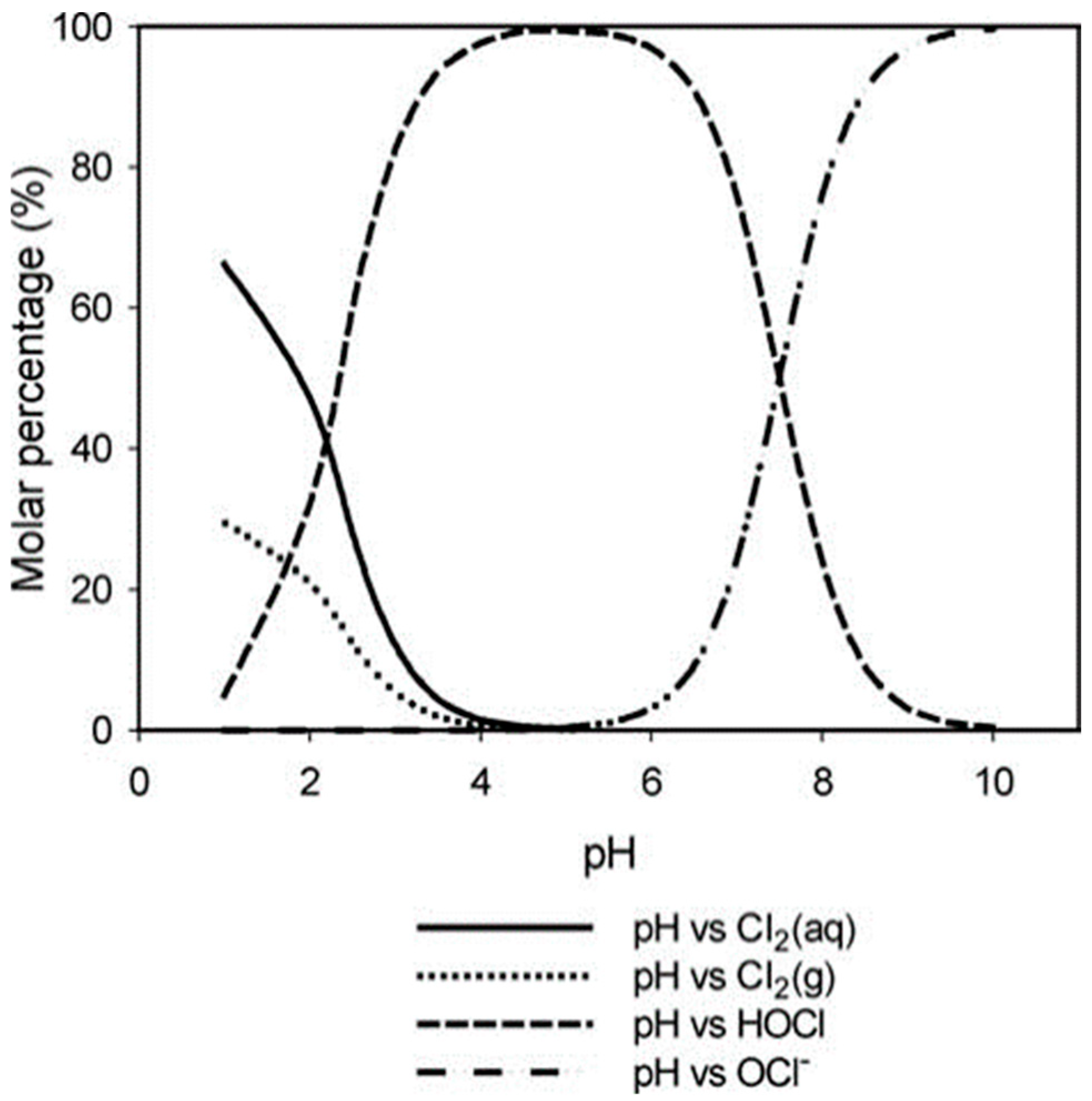
3.1. Sulfur Oxide (SOx) Emissions
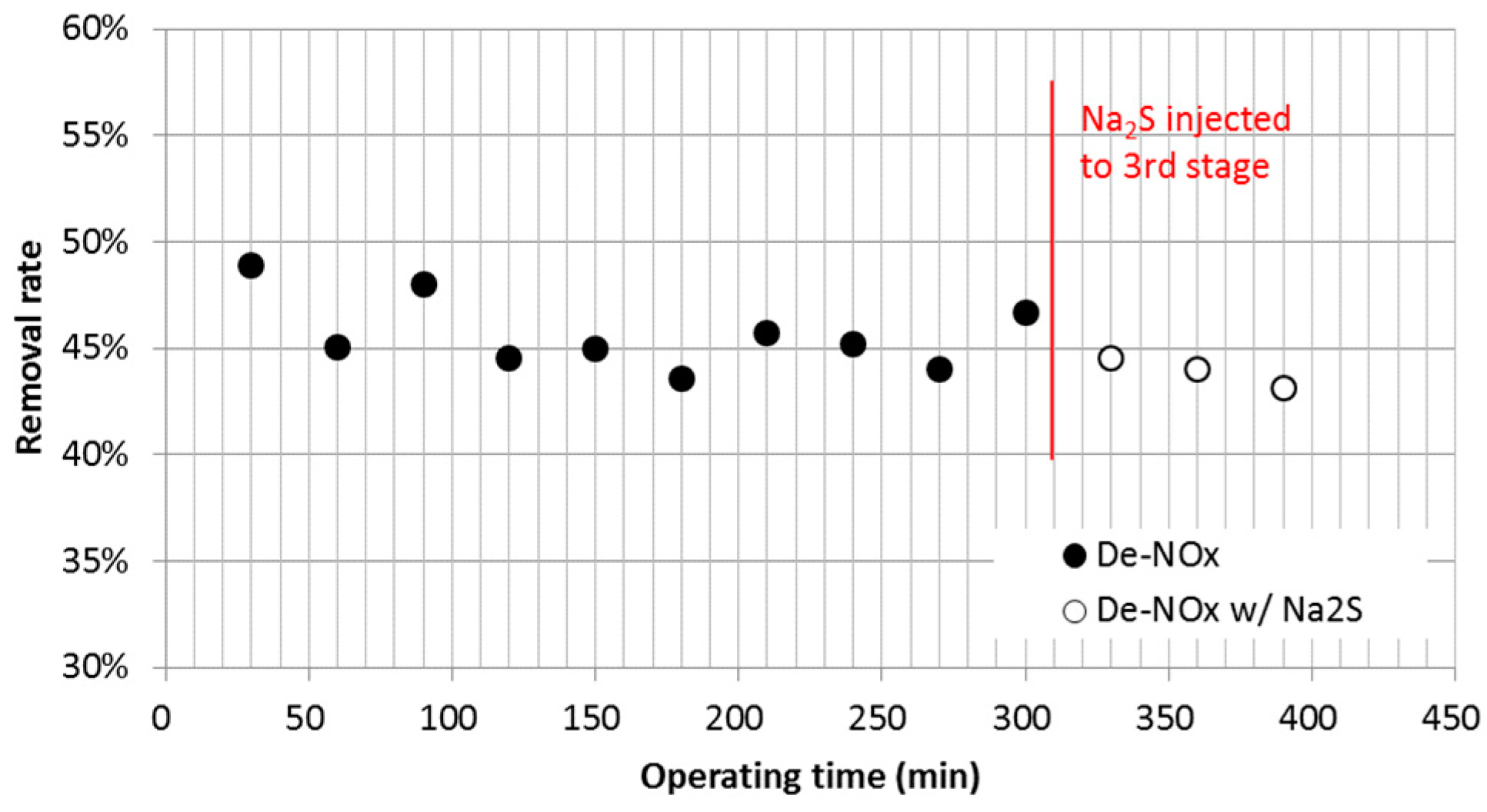
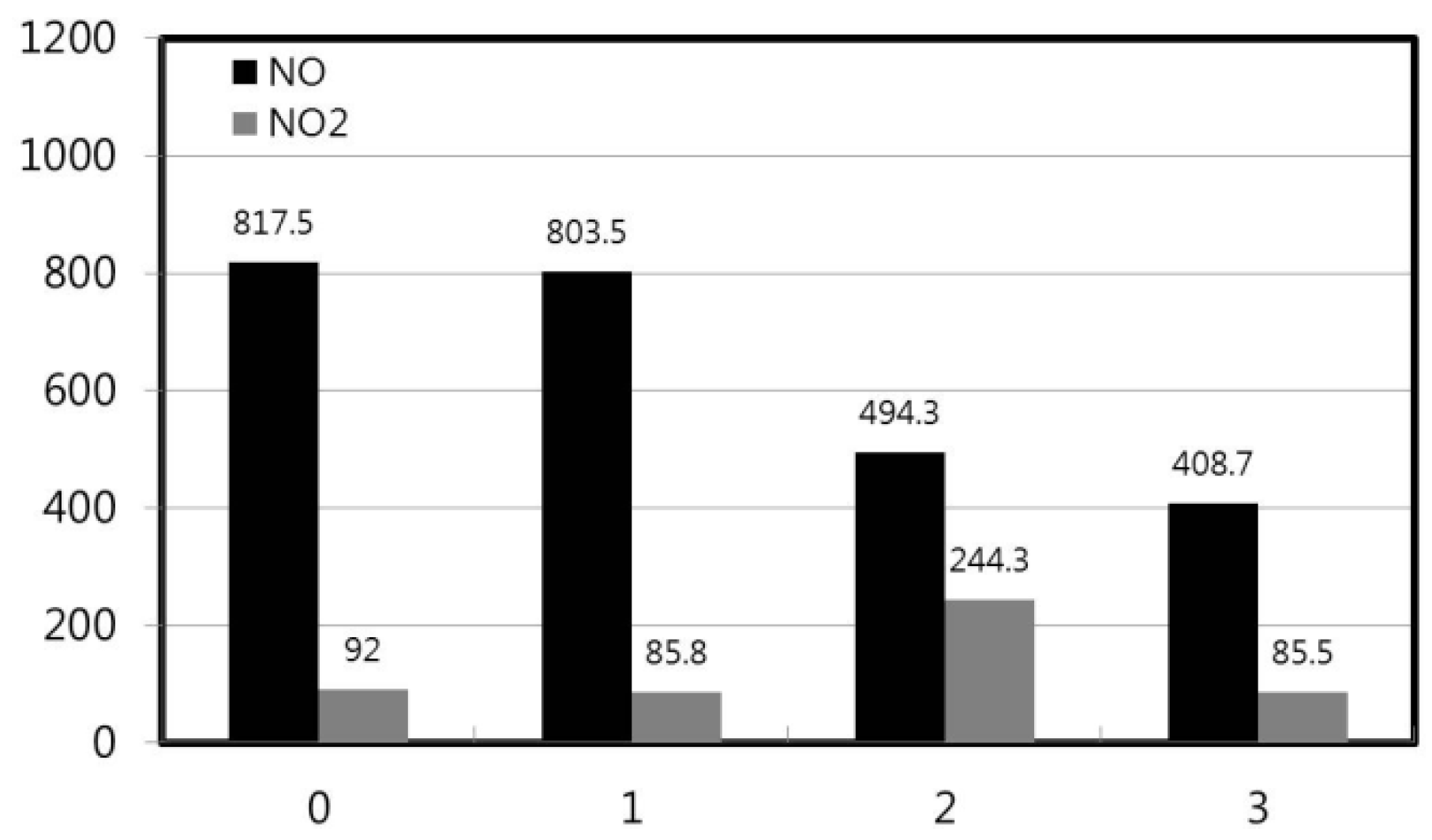
3.2. Nitric Oxide (NOx) Emissions
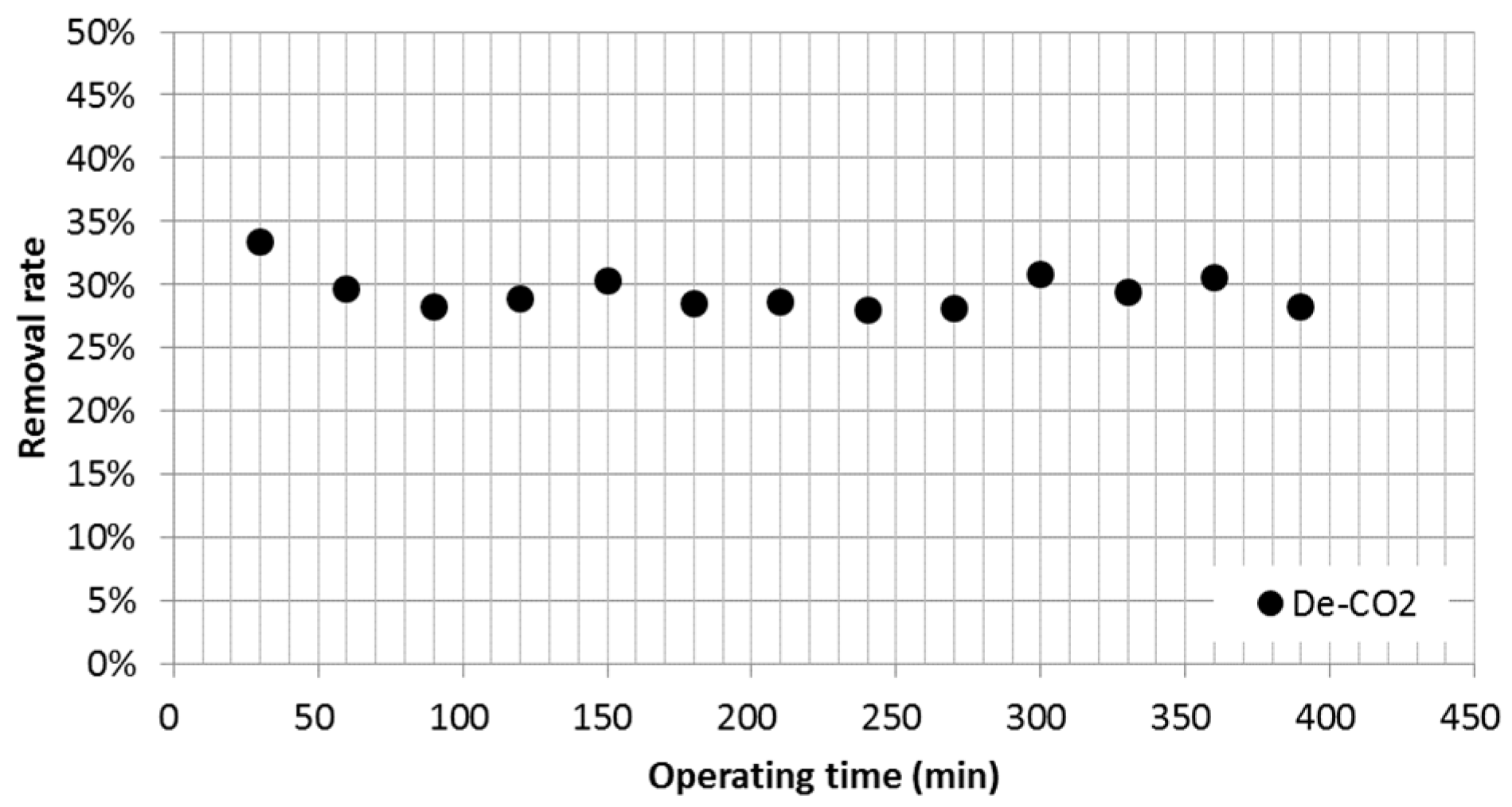
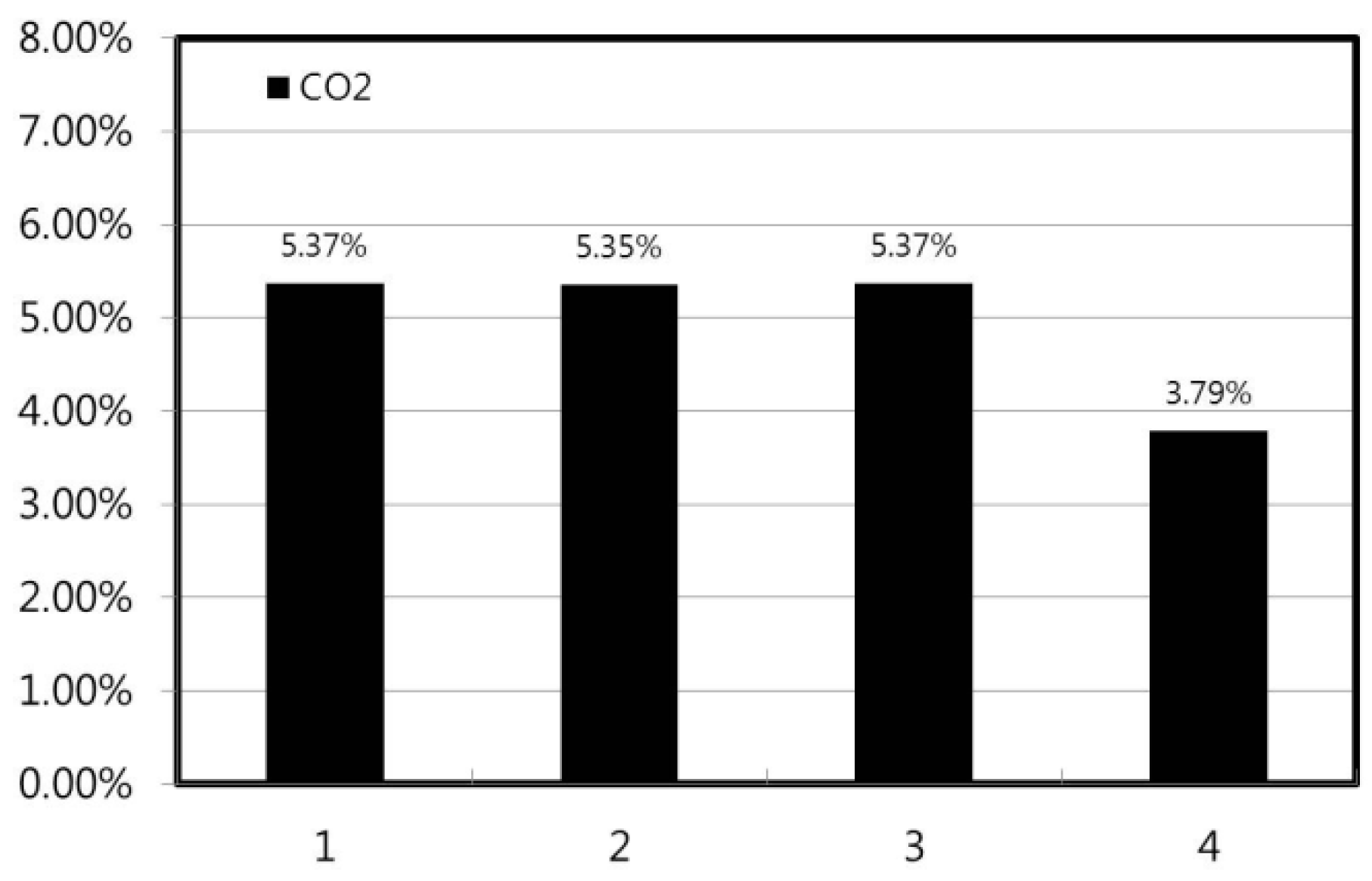
3.3. Carbon Dioxide (CO2) Emissions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DieselNet. Available online: https://www.dieselnet.com (accessed on 21 September 2020).
- IMO (International Maritime Organization). Available online: http://www.imo.org/ (accessed on 21 September 2020).
- MEPC 65/4/3. Air Pollution and Energy Deficiency; Report of the Correspondence Group: London, UK, 2013.
- IMO. Third IMO GHG Study 2014; IMO: London, UK, 2014. [Google Scholar]
- Petroleum Products–Fuels (class F)–Specifications of Marine Fuels. In ISO 8217; International Organization for Standards: Geneva, Switzerland, 2012.
- Ryu, Y.; Dan, T.; Asano, I. Measurement of bunker oil/DME blended fuel viscosity for diesel engine application. J. Jpn. Inst. Mar. Eng. 2012, 47, 95–100. [Google Scholar] [CrossRef][Green Version]
- Ryu, Y.; Dan, T. Combustion and emission characteristics of diesel engine by mixing DME and bunker oil. J. Korean Soc. Mar. Eng. 2012, 36, 117–122. [Google Scholar] [CrossRef]
- Ryu, Y.; Dan, T. Investigation on the effects of dimethyl ether blending to bunker oil for marine diesel engine use. SAE Tech. Pap. 2013. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, Y.; Nam, J. An experimental study of the fuel additive to improve the performance of a 2-stroke large diesel engine. J. Korean Soc. Mar. Eng. 2015, 39, 620–625. (In Korean) [Google Scholar] [CrossRef][Green Version]
- Ryu, Y.; Lee, Y.; Nam, J. Performance and emission characteristics of additives-enhanced heavy fuel oil in large two-stroke marine diesel engine. Fuel 2016, 182, 850–856. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, Y.; Nam, J. Improvement of the performance and emission in a four-stroke diesel engine using fuel additive. J. Korean Soc. Mar. Eng. 2016, 40, 762–767. [Google Scholar] [CrossRef]
- Geng, P.; Mao, H.; Zhang, Y.; Wei, L.; You, K.; Ju, J.; Chen, T. Combustion characteristics and NOx emissions of a waste cooking oil biodiesel blend in a marine auxiliary diesel engine. Appl. Therm. Eng. 2017, 115, 947–954. [Google Scholar] [CrossRef]
- Katayama, N.; Dan, T.; Asano, I.; Kori, Y.; Kanno, K. Combustion analysis of Jatropha oil/marine diesel oil mixed fuel in direct injection diesel engine. In Proceedings of the 6th Pan Asian Association of Maritime Engineering Societies/Advanced Maritime Engineering Conference 2014, PAAMES, Hangzhou, China, 28–30 October 2014; NTMI-01. pp. 1–6. [Google Scholar]
- Hashimoto, M.; Dan, T.; Asano, I.; Otani, T. Combustion characteristics of palm oil in pre-combustion chamber type diesel engine by mixing with gas oil and marine diesel oil. J. Jpn. Inst. Mar. Eng. 2009, 44, 456–462. [Google Scholar] [CrossRef][Green Version]
- Ogunkoya, D.; Li, S.; Rojas, O.; Fang, T. Performance, combustion, and emissions in a diesel engine operated with fuel-in-water emulsions based on lignin. J. Appl. Energy 2015, 154, 851–861. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Rujiravanit, R.; Ueno, T.; Saito, N. Non-thermal plasma technology for abatement of pollutant emission from marine diesel engine. J. Korean Soc. Mar. Eng. 2016, 40, 929–934. [Google Scholar] [CrossRef]
- Raptotasios, S.I.; Sakellaridis, N.F.; Papagiannakis, R.G.; Hountalas, D.T. Application of a multi-zone combustion model to investigate the NOx reduction potential of two-stroke marine diesel engines using EGR. Appl. Energy 2015, 157, 814–823. [Google Scholar] [CrossRef]
- Verschaeren, R.; Schaepdryver, W.; Serruys, T.; Bastiaen, M.; Vervaeke, L.; Verhelst, S. Experimental study of NOx reduction on a medium speed heavy duty diesel engine by the application of EGR (exhaust gas recirculation) and Miller timing. Energy 2014, 76, 614–621. [Google Scholar] [CrossRef]
- Camarillo, M.K.; Stringfellow, W.T.; Hanlon, J.S.; Watson, K.A. Investigation of selective catalytic reduction for control of nitrogen oxides in full-scale dairy energy production. Appl. Energy 2013, 106, 328–336. [Google Scholar] [CrossRef]
- Ryu, Y.; Kim, H.; Cho, G.; Kim, H.; Nam, J. A study on the installation of SCR system for generator diesel engine of existing ship. J. Korean Soc. Mar. Eng. 2015, 39, 412–417. (In Korean) [Google Scholar] [CrossRef]
- Ntziachristos, L.; Saukko, E.; Lehtoranta, K.; Ronkko, T.; Timonen, H.; Simone, P.; Keskinen, J. Particle emissions characterization from a medium-speed marine diesel engine with two fuels at different sampling conditions. Fuel 2016, 186, 456–465. [Google Scholar] [CrossRef]
- Ryu, Y.; Kim, T.; Kim, J.; Nam, J. Experimental study on the De-NOx by using wet-type exhaust gas cleaning system for marine diesel engines. J. Korean Soc. Mar. Eng. 2017, 41, 412–417. (In Korean) [Google Scholar] [CrossRef]
- Kim, T.; Choi, S.; Kim, J.; Song, J. A study on the NOx reduction of flue gas using un-divided electrolysis of seawater. Korean Chem. Eng. Res. 2012, 50, 825–829. (In Korean) [Google Scholar] [CrossRef][Green Version]
- Kim, T.W.; Kim, J.H.; Song, J.Y. A study on the NOx reduction of flue gas using seawater electrolysis. J. Korean Oil Chem. Soc. 2012, 29, 570–576. [Google Scholar] [CrossRef]
- An, S. Air pollution protection onboard by seawater and electrolyte. J. Korean Soc. Mar. Eng. 2006, 30, 93–101. [Google Scholar]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Haynes, W.M. Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 134–135. [Google Scholar]
- Andreasen, A.; Mayer, S. Use of seawater scrubbing for SO2 removal from marine engine exhaust gas. Energy Fuels 2007, 21, 3274–3279. [Google Scholar] [CrossRef]
- Henze, M.; Mark, C.M.; Loosdrecht, V.; Ekama, G.A.; Brdjanovic, D. Biological Wastement Treatment. IWA Publishing Books, 2008; p. 236. [Google Scholar]
- Wang, L.; Bassiri, M.; Najafi, R.; Robson, M. Hypochlorous acis as a potential wound care agent. J. Burns Wounds 2007, 6, 65–79. [Google Scholar]
- Kuropka, J. Removal of nitrogen oxides from flue gases in a packed column. Environ. Prot. Eng. 2011, 37, 13–22. [Google Scholar]
- Guo, R.; Pan, W.; Zhang, X.; Xu, H.; Jin, Q.; Ding, C.; Guo, S. The absorption kinetics of NO into weakly acidic NaClO Solution. Sep. Sci. Technol. 2013, 48, 2871–2875. [Google Scholar] [CrossRef]
- Mondal, M.K.; Chelluboyana, V.R. New experimental results of combined SO2 and NO removal from simulated gas stream by NaClO as low-cost absorbent. Chem. Eng. J. 2013, 217, 48–53. [Google Scholar] [CrossRef]
- Chang, M.; Lee, H.; Wu, F.; Lai, C.R. Simultaneous removal of nitrogen oxide/nitrogen dioxide/sulfur dioxide from gas streams by combined plasma scrubbing technology. J. Air Waste MGMT Assoc. 2004, 54, 941–949. [Google Scholar] [CrossRef]
- Mok, Y. Absorption-reduction technique assisted by ozone injection and sodium sulfide for NOx removal from exhaust gas. Chem. Engi. J. 2006, 118, 63–67. [Google Scholar] [CrossRef]
- Shen, C.H.; Rochelle, G.T. Nitrogen dioxide absorption and sulfide oxidation in aqueous sulfide. J. Air Waste MGMT Assoc. 2011, 49, 332–338. [Google Scholar] [CrossRef]
- Schulz, K.; Riebesell, U.; Rost, B.; Thomas, S.; Zeebe, R.E. Determination of the rate constants for the carbon dioxide to bicarbonate inter-conversion in pH-buffered seawater systems. Mar. Chem. 2006, 100, 53–65. [Google Scholar] [CrossRef]
- Stolaroff, J.K.; Keith, D.W.; Lowry, G.V. Carbon dioxide capture from atmospheric air using sodium hydroxide spray. Environ. Sci. Tech. 2008, 42, 2728–2735. [Google Scholar] [CrossRef]

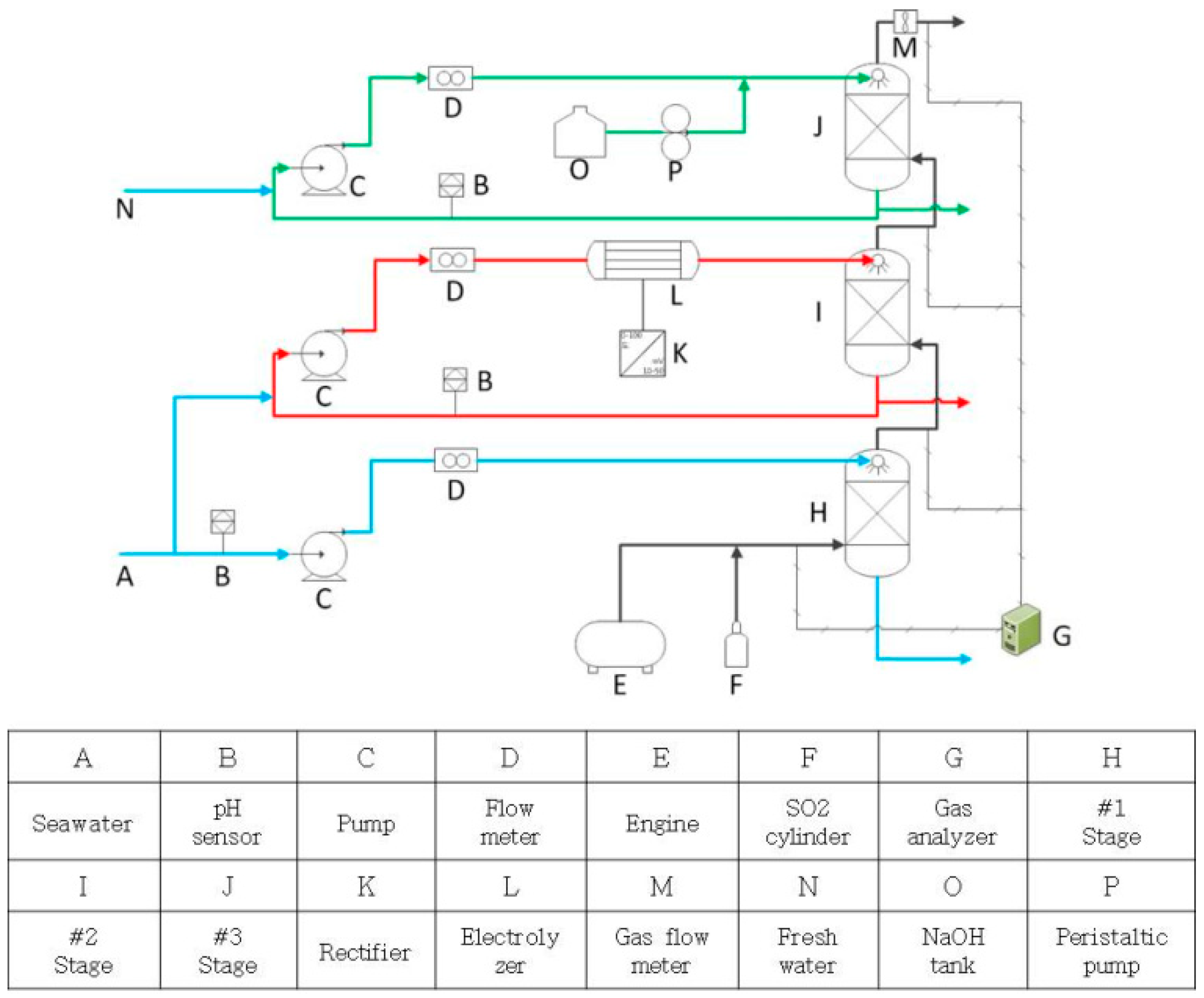



| Outside ECA (Global Requirement) | Inside ECA |
|---|---|
| 4.5% m/m prior to 1 January 2012 | 1.5% m/m prior to 1 July 2010 |
| 3.5% m/m on and after 1 January 2012 | 1.0% m/m on and after 1 July 2010 |
| 0.5% m/m on and after 1 January 2020 | 0.1% m/m on and after 1 January 2015 |
| Region | Fuel Type | Year | ||
|---|---|---|---|---|
| 2012 | 2030 | 2050 | ||
| Global | HFO | 3114 | 3114 | 3114 |
| LSFO | 3114 | 3114 | 3114 | |
| MGO | 3206 | 3206 | 3206 | |
| LNG | 2750 | 2750 | 2750 | |
| Item | Description |
|---|---|
| Engine type | D4BB-G3, four-stroke diesel engine |
| Bore × stroke | 91.1 × 100 mm |
| Combustion type | Indirect injection |
| No. of cylinders | 4 inline |
| Displacement volume | 2607 cm3 |
| MCR output | 45 PS @ 1800 rpm |
| Compression ratio | 22:1 |
| Fuel | Diesel oil |
| Item | Description |
|---|---|
| Spraying order | 1st stage: seawater 2nd stage: electrolyzed seawater 3rd stage: NaOH (+Na2S) |
| Absorber type | Packed bed |
| Packing | 1” Tripack (polypropylene) |
| Exhaust gas flow | 130 ± 10 Nm3/h |
| Retention time | 6.3–6.9 s |
| Liquid–gas ratio | 43–50 L/m3 |
| CNOx,i | 887–942 ppmv |
| CSO2,i | 430–470 ppmv |
| CCO2,i | 5.30–5.45 vol.% |
| Cl2 concentration | 4.8–5.3 g Cl2/L |
| NaOH concentration | 6.1–7.7 g NaOH/L |
| Gas | Solubility in Water [mol/L] |
|---|---|
| NO | 1.51 × 10−6 |
| NO2 | 2.44 × 10−5 |
| SO2 | 1.25 × 10−3 |
| CO2 | 3.48 × 10−5 |
| Cl2 | 8.20 × 10−5 scientific notion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, Y.; Kim, T.; Kim, J.; Nam, J. Investigation on the Emission Characteristics with a Wet-Type Exhaust Gas Cleaning System for Marine Diesel Engine Application. J. Mar. Sci. Eng. 2020, 8, 850. https://doi.org/10.3390/jmse8110850
Ryu Y, Kim T, Kim J, Nam J. Investigation on the Emission Characteristics with a Wet-Type Exhaust Gas Cleaning System for Marine Diesel Engine Application. Journal of Marine Science and Engineering. 2020; 8(11):850. https://doi.org/10.3390/jmse8110850
Chicago/Turabian StyleRyu, Younghyun, Taewoo Kim, Jungsik Kim, and Jeonggil Nam. 2020. "Investigation on the Emission Characteristics with a Wet-Type Exhaust Gas Cleaning System for Marine Diesel Engine Application" Journal of Marine Science and Engineering 8, no. 11: 850. https://doi.org/10.3390/jmse8110850
APA StyleRyu, Y., Kim, T., Kim, J., & Nam, J. (2020). Investigation on the Emission Characteristics with a Wet-Type Exhaust Gas Cleaning System for Marine Diesel Engine Application. Journal of Marine Science and Engineering, 8(11), 850. https://doi.org/10.3390/jmse8110850




