Rapid Recruitment of Symbiotic Algae into Developing Scleractinian Coral Tissues
Abstract
1. Introduction
2. Materials and Methods
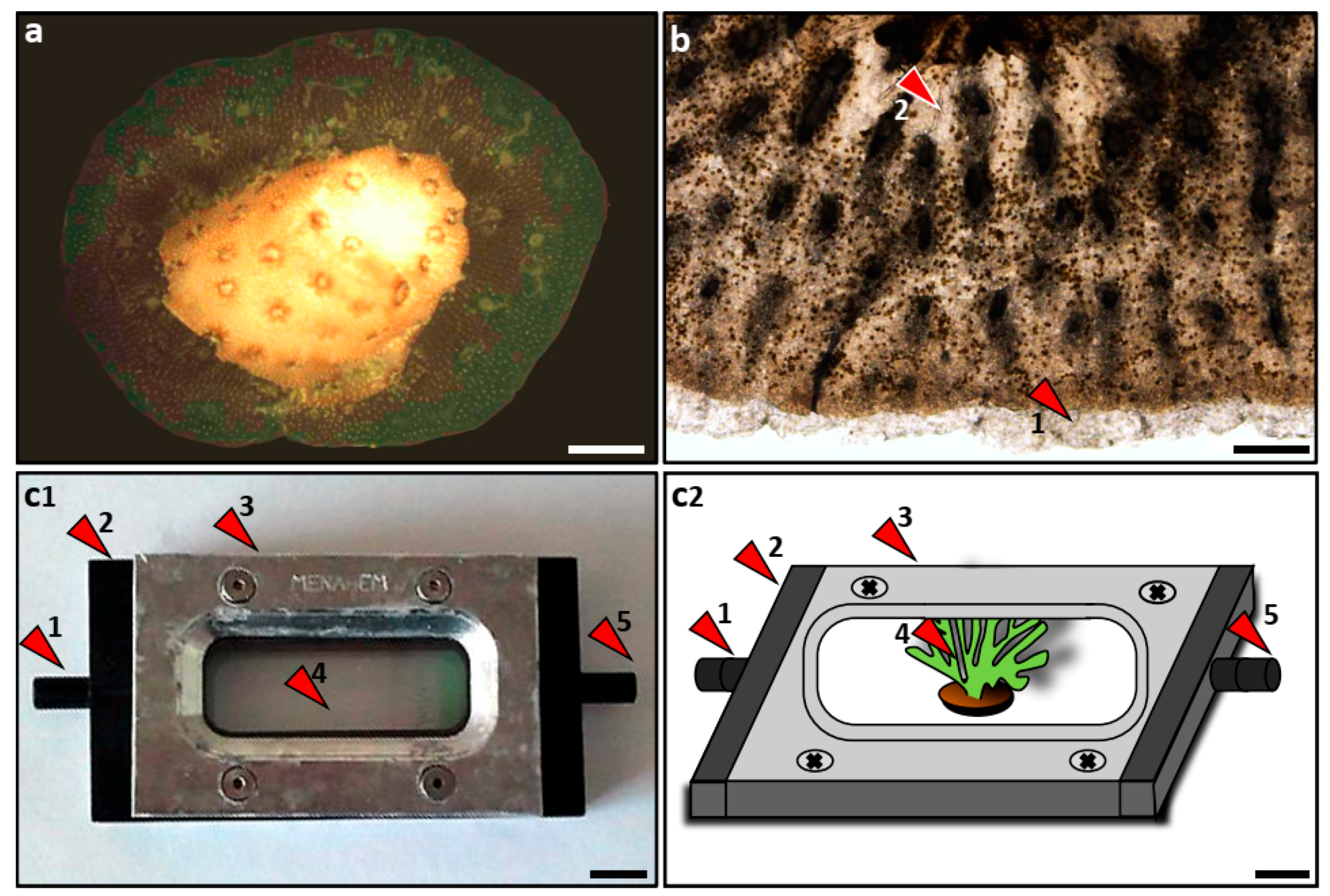
2.1. Nubbin Preparation
2.2. Lateral Tissue Extensions
2.3. Live Tissue Observations
2.4. Zooxanthella Observations
2.5. Endosymbiotic Dinoflagellate Recruitment
2.6. Algal Cell Counting
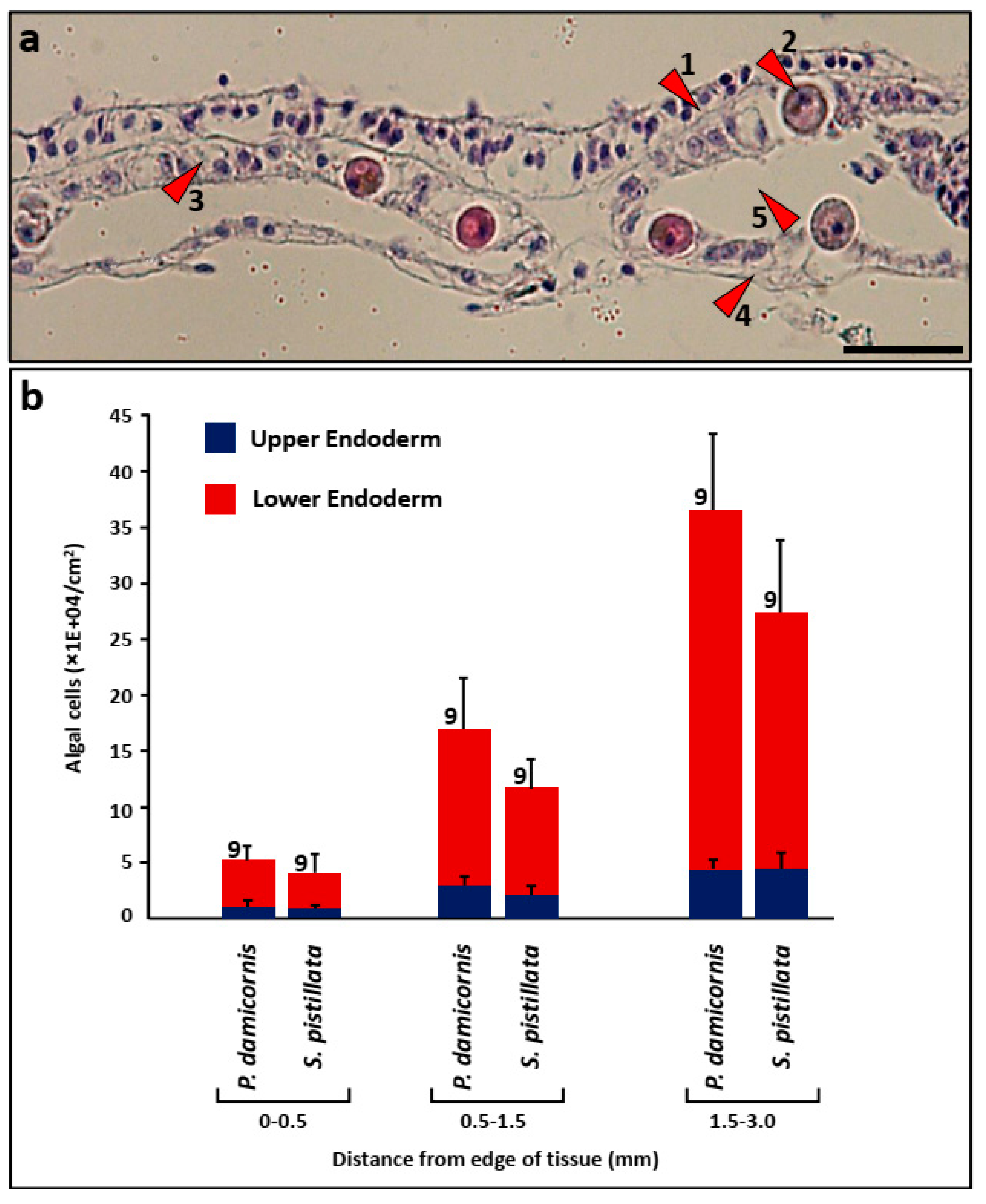
2.7. Histology
3. Results
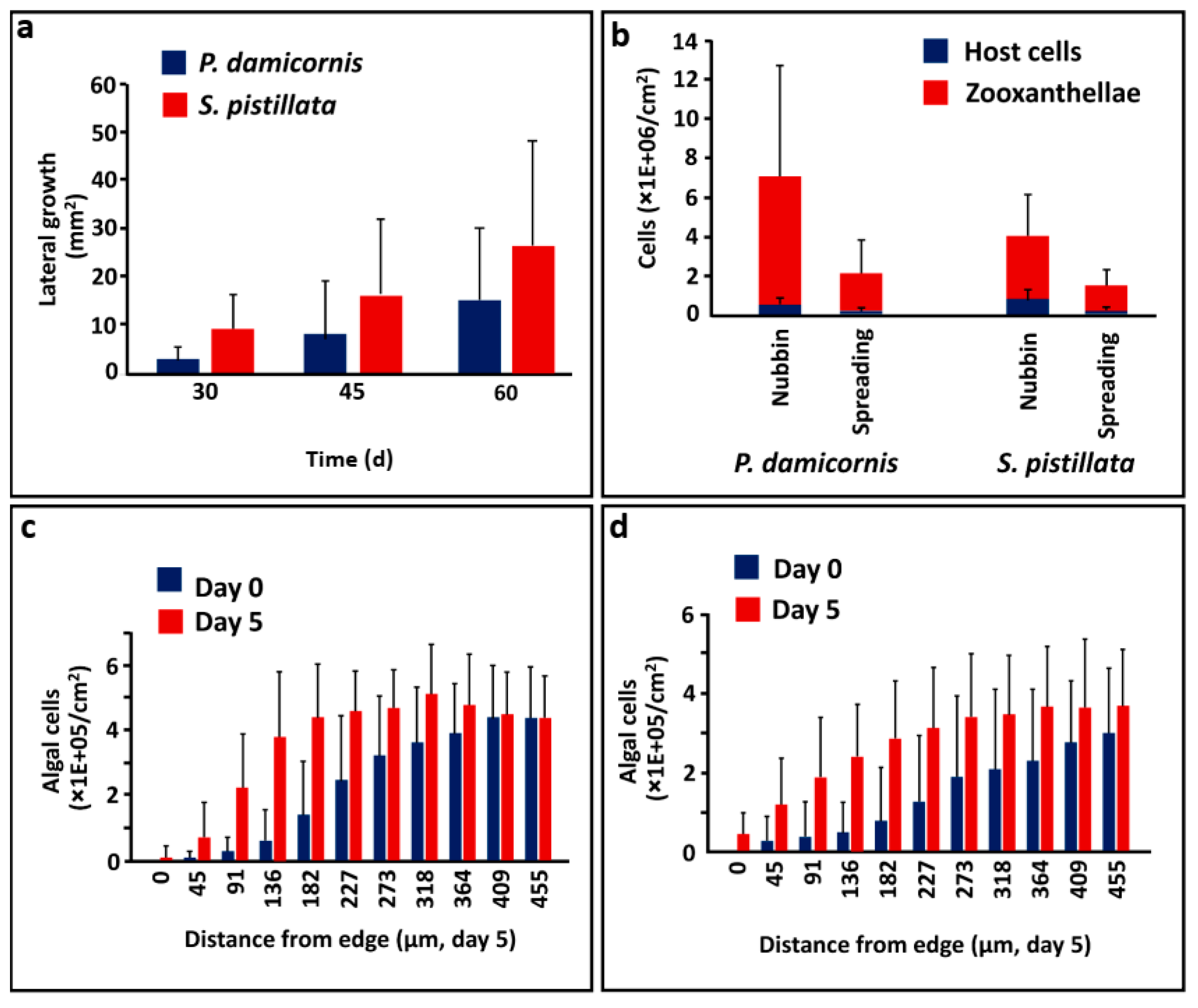
3.1. Forming Lateral Tissue Sheets
3.2. Algal Cells in Coral Tissues
3.3. Algal Cells in Histological Sections
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lajeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Boil. 2018, 28, 2570–2580. [Google Scholar] [CrossRef]
- Fitt, W.K. The role of chemosensory behavior of Symbiodinium microadriaticum, intermediate hosts, and host behavior in the infection of coelenterates and molluscs with zooxanthellae. Mar. Boil. 1984, 81, 9–17. [Google Scholar] [CrossRef]
- Karako-Lampert, S.; Katcoff, D.; Achituv, Y.; Dubinsky, Z.; Stambler, N. Do clades of symbiotic dinoflagellates in scleractinian corals of the Gulf of Eilat (Red Sea) differ from those of other coral reefs? J. Exp. Mar. Boil. Ecol. 2004, 311, 301–314. [Google Scholar] [CrossRef]
- Muller-Parker, G.; D’Elia, C.F.; Cook, C.B. Interactions between Corals and Their Symbiotic Algae. In Coral Reefs in the Anthropocene; Springer Science and Business Media LLC: Berlin, Germany, 2015; pp. 99–116. [Google Scholar]
- Jones, R.J.; Yellowlees, D. Regulation and control of intracellular algae (=zooxanthellae) in hard corals. Philos. Trans. R. Soc. B Boil. Sci. 1997, 352, 457–468. [Google Scholar] [CrossRef]
- Rowan, R. Review—Diversity and ecology of zooxanthellae on coral reefs. J. Phycol. 1998, 34, 407–417. [Google Scholar] [CrossRef]
- Muscatine, L. The role of symbiotic algae in carbon and energy flux in reef corals. In Ecosystems of the World; Coral Reefs, Dubinsky, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 25, pp. 75–87. [Google Scholar]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol. Mol. Boil. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef]
- Chen, W.-N.U.; Hsiao, Y.-J.; Mayfield, A.B.; Young, R.; Hsu, L.-L.; Peng, S.-E. Transmission of a heterologous clade C Symbiodinium in a model anemone infection system via asexual reproduction. PeerJ 2016, 4, 2358. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Lanetty, M.; Scaramuzzi, C.; Quinnell, R.G.; Larkum, A.W.D. Transport of symbiotic zooxanthellae in mesogleal canals of Zoanthus robustus? Coral Reefs 2005, 24, 195–196. [Google Scholar] [CrossRef]
- Rodriguez-Lanetty, M.; Wood-Charlson, E.M.; Hollingsworth, L.L.; Krupp, D.A.; Weis, V.M. Temporal and spatial infection dynamics indicate recognition events in the early hours of a dinoflagellate/coral symbiosis. Mar. Boil. 2006, 149, 713–719. [Google Scholar] [CrossRef]
- Raz-Bahat, M.; Erez, J.; Rinkevich, B. In Vivo light-microscopic documentation for primary calcification processes in the hermatypic coral Stylophora pistillata. Cell Tissue Res. 2006, 325, 361–368. [Google Scholar] [CrossRef]
- Coffroth, M.A.; Poland, D.M.; Petrou, E.L.; Brazeau, D.A.; Holmberg, J.C. Environmental Symbiont Acquisition May Not Be the Solution to Warming Seas for Reef-Building Corals. PLoS ONE 2010, 5, e13258. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, S129–S138. [Google Scholar] [CrossRef]
- Gateño, D.; Israel, A.; Barki, Y.; Rinkevich, B. Gastrovascular Circulation in an Octocoral: Evidence of Significant Transport of Coral and Symbiont Cells. Boil. Bull. 1998, 194, 178–186. [Google Scholar] [CrossRef]
- Parrin, A.P.; Goulet, T.L.; Yaeger, M.A.; Bross, L.S.; McFadden, C.S.; Blackstone, N.W. Symbiodinium migration mitigates bleaching in three octocoral species. J. Exp. Mar. Boil. Ecol. 2016, 474, 73–80. [Google Scholar] [CrossRef]
- Marfenin, N.N. Non-radial symmetry of the transport system of Acropora corals. Invertzool 2015, 12, 53–59. [Google Scholar] [CrossRef]
- Gladfelter, E.H. Circulation of fluids in the gastrovascular system of the reef coral acropora cervicornis. Boil. Bull. 1983, 165, 619–636. [Google Scholar] [CrossRef]
- Santos, S.R.; Toyoshima, J.; Iii, R.A.K. Spatial and temporal dynamics of symbiotic dinoflagellates (Symbiodinium: Dinophyta) in the perforate coral Montipora capitata. Galax-J. Coral Reef Stud. 2009, 11, 139–147. [Google Scholar] [CrossRef][Green Version]
- Camaya, A.P.; Sekida, S.; Okuda, K. Changes in the Ultrastructures of the Coral Pocillopora damicornis after Exposure to High Temperature, Ultraviolet and Far-Red Radiation. Cytologia 2016, 81, 465–470. [Google Scholar] [CrossRef]
- Pearse, V.B.; Muscatine, L. Role of symbiotic algae (zooxanthellae) in coral calcification. Boil. Bull. 1971, 141, 350–363. [Google Scholar] [CrossRef]
- Rinkevich, B.; Loya, Y. Does light enhance calcification in hermatypic corals? Mar. Boil. 1984, 80, 1–6. [Google Scholar] [CrossRef]
- Fang, L.-S.; Chen, Y.-W.J.; Chen, C.-S. Why does the white tip of stony coral grow so fast without zooxanthellae? Mar. Boil. 1989, 103, 359–363. [Google Scholar] [CrossRef]
- Shafir, S.; Van Rijn, J.; Rinkevich, B. Nubbing of Coral Colonies: A Novel Approach for the Development of Inland Broodstocks. Aquar. Sci. Conserv. 2001, 3, 183–190. [Google Scholar] [CrossRef]
- Shafir, S.; Van Rijn, J.; Rinkevich, B. The use of coral nubbins in coral reef ecotoxicology testing. Biomol. Eng. 2003, 20, 401–406. [Google Scholar] [CrossRef]
- Shaish, L.; Abelson, A.; Rinkevich, B. How Plastic Can Phenotypic Plasticity Be? The Branching Coral Stylophora pistillata as a Model System. PLoS ONE 2007, 2, e644. [Google Scholar] [CrossRef]
- Shafir, S.; Rijn, J.V.; Rinkevich, B. Steps in the construction of underwater coral nursery, an essential component in reef restoration acts. Mar. Biol. 2006, 149, 679–687. [Google Scholar] [CrossRef]
- Shafir, S.; Rijn, J.V.; Rinkevich, B. Coral nubbins as source material for coral biological research: A prospectus. Aquaculture 2006, 259, 444–448. [Google Scholar] [CrossRef]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Baruch, R.; Avishai, N.; Rabinowitz, C. UV incites diverse levels of DNA breaks in different cellular compartments of a branching coral species. J. Exp. Boil. 2005, 208, 843–848. [Google Scholar] [CrossRef]
- Holmes, G.; Osborn, J.; Veal, C.J.; Hoegh-Guldberg, O.; Nunez, M. A comparative study of methods for surface area and three-dimensional shape measurement of coral skeletons. Limnol. Oceanogr. Methods 2010, 8, 241–253. [Google Scholar]
- Fitt, W.K.; Spero, H.J.; Halas, J.; White, M.W.; Porter, J.W. Recovery of the coral Montastrea annularis in the Florida Keys after the 1987 Caribbean “bleaching event”. Coral Reefs 1993, 12, 57–64. [Google Scholar] [CrossRef]
- Littman, R.A.; Van Oppen, M.J.; Willis, B.L. Methods for sampling free-living Symbiodinium (zooxanthellae) and their distribution and abundance at Lizard Island (Great Barrier Reef). J. Exp. Mar. Boil. Ecol. 2008, 364, 48–53. [Google Scholar] [CrossRef]
- Tang, E.P.Y. Why do dinoflagellates have lower growth rates? J. Phycol. 1996, 32, 80–84. [Google Scholar] [CrossRef]
- Wilkerson, F.P.; Parker, G.M.-; Muscatine, L. Temporal patterns of cell division in natural populations of endosymbiotic algae. Limnol. Oceanogr. 1983, 28, 1009–1014. [Google Scholar] [CrossRef]
- Camaya, A.P. Growth, Cell Division and Dysfunction of Coral Tissues and Symbiotic Zooxanthellae in the Scleractinian Pocillopora Damicornis (Linnaeus) Revealed by Light and Electron Microscopy. Ph.D. Thesis, Kochi University, Kochi, Japan, 2017. [Google Scholar]
- Hirose, M.; Yamamoto, H.; Nonaka, M. Metamorphosis and acquisition of symbiotic algae in planula larvae and primary polyps of Acropora spp. Coral Reefs 2008, 27, 247–254. [Google Scholar] [CrossRef]
- Kass-Simon, G.S.A.A.; Scappaticci, A.A., Jr. The behavioral and developmental physiology of nematocysts. Can. J. Zool. 2002, 80, 1772–1794. [Google Scholar] [CrossRef]
- Hennige, S.J.; Smith, D.J.; Perkins, R.; Consalvey, M.; Paterson, D.M.; Suggett, D.J. Photoacclimation, growth and distribution of massive coral species in clear and turbid waters. Mar. Ecol. Prog. Ser. 2008, 369, 77–88. [Google Scholar] [CrossRef]
| Species | Genotype | Nubbins (n) | Mortality | Developing LPS | LPS Lateral Sizes | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % of Initial | n | % of Alive | < 40 mm2 | 40–100 mm2 | > 100 mm2 | |||
| P. damicornis | 1 | 38 | 0 | 0 | 14 | 37 | 10 | 3 | 1 |
| 2 | 42 | 2 | 5 | 10 | 25 | 9 | 1 | 0 | |
| 3 | 41 | 5 | 12 | 19 | 53 | 19 | 0 | 0 | |
| 4 | 44 | 12 | 27 | 7 | 22 | 7 | 0 | 0 | |
| 5 | 44 | 4 | 9 | 12 | 30 | 12 | 0 | 0 | |
| Total/% | 209 | 23 | 11 | 62 | 33 | 57 | 4 | 1 | |
| S. pistillata | 1 | 45 | 0 | 0 | 28 | 62 | 24 | 4 | 0 |
| 2 | 44 | 0 | 0 | 33 | 75 | 26 | 6 | 1 | |
| 3 | 43 | 1 | 2 | 27 | 64 | 23 | 3 | 1 | |
| 4 | 43 | 0 | 0 | 29 | 67 | 24 | 4 | 1 | |
| 5 | 45 | 0 | 0 | 27 | 60 | 20 | 6 | 1 | |
| Total/% | 220 | 1 | 0.5 | 144 | 66 | 117 | 23 | 4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bockel, T.; Rinkevich, B. Rapid Recruitment of Symbiotic Algae into Developing Scleractinian Coral Tissues. J. Mar. Sci. Eng. 2019, 7, 306. https://doi.org/10.3390/jmse7090306
Bockel T, Rinkevich B. Rapid Recruitment of Symbiotic Algae into Developing Scleractinian Coral Tissues. Journal of Marine Science and Engineering. 2019; 7(9):306. https://doi.org/10.3390/jmse7090306
Chicago/Turabian StyleBockel, Thomas, and Baruch Rinkevich. 2019. "Rapid Recruitment of Symbiotic Algae into Developing Scleractinian Coral Tissues" Journal of Marine Science and Engineering 7, no. 9: 306. https://doi.org/10.3390/jmse7090306
APA StyleBockel, T., & Rinkevich, B. (2019). Rapid Recruitment of Symbiotic Algae into Developing Scleractinian Coral Tissues. Journal of Marine Science and Engineering, 7(9), 306. https://doi.org/10.3390/jmse7090306





