1. Introduction
The first samplings of marine seaweeds in Hainan Island were performed by Chinese algologists from 1933 to the 1980s and documented 310 species of red, brown and green marine algae [
1,
2]. The next major algal samplings were conducted in 1990 and 1992 during the German-Chinese expeditions to Hainan Island and recorded 203 species [
2]. The third sampling was performed during 2008–2012 by authors of this study. There were 253 species of macroalgae, 30 species of Cyanobacteria and three species of seagrasses found [
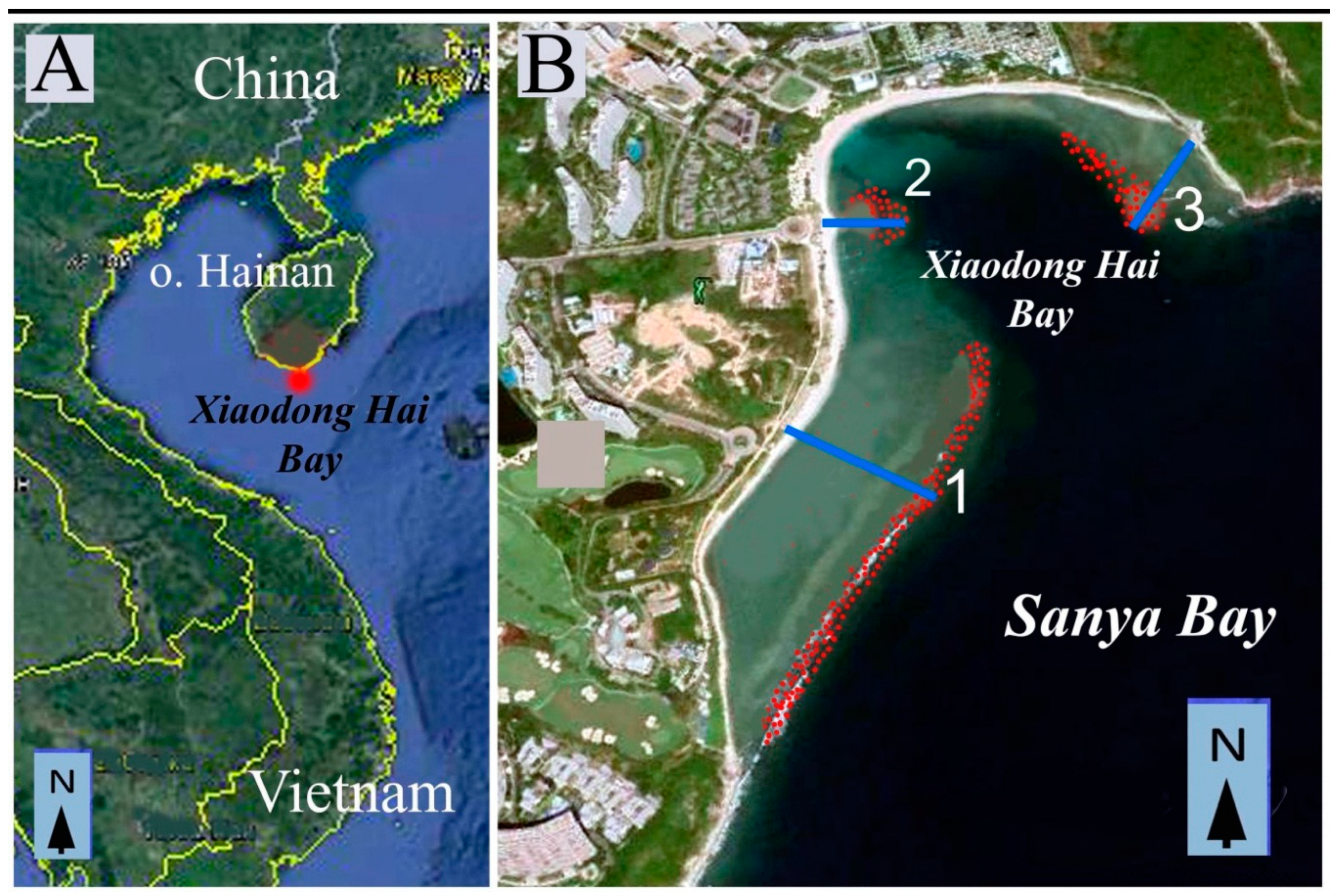
3]. In all three samplings, algae were collected from sites located in the southern, southeastern, and northern parts of the island. Eight of the studied sites were common to all three samplings including Xiaodong Hai (
Figure 1A). Collections of 1990, 1992 and 2008–2012 showed that Xiaodong Hai is the richest locality of the southern part of Hainan in seaweeds. About 200 species of marine macrophytes were found during these years.
The main coastal ecosystems of shallow water of Hainan Island are coral reefs among the most prominent fringing reefs of China. However, almost 80% of the fringing reefs along the coastline of Hainan Island have been damaged due to intensive human activities during the 1970s–1990s. Recently, the eutrophication of Hainan coastal waters, particularly in the shallow gulfs, has increased owing to greater tourist numbers, hotel building along the coast and mariculture in coastal ponds and pools with wastes draining into the sea [
4,
5,
6]. As was shown by our earlier studies of the benthic flora of Hainan Island (2008–2012) [
2,
6], the degradation of coral reefs is evident not only in a decrease in the coverage of the bottom by colonies of hermatypic corals and in change of their species composition, but also in the occupation of substrates free of live corals by macroalgae and cyanobacteria, sometimes leading to catastrophic changes in the diversity and species composition of the benthic flora. These changes have a long-term character, but can manifest in interannual, as well as seasonal changes in the benthic flora.
Here, we report in the results from a sampling campaign conducted to identify the benthic flora in Xiaodong Hai locality between the spring of 2016 and the spring of 2019. The aim of the work was to inventory the recent benthic flora of the locality, identifying interannual and seasonal changes in the diversity, species composition, taxonomic composition and the structure of algal communities.
2. Materials and Methods
2.1. Study Site, Time and Conditions
Hainan Island (
Figure 1A) is located in the South China Sea (19°12ʹN, 109°45ʹE). The island has an area of 33.920 km
2 and a coastline of more than 1610 km. The annual sea-surface temperature (SST) maximum (30.8 °C) is in July and the minimum (18.7 °C) is in January. The mean tidal range is generally less than 1.5 m [
4,
7]. The rainy season in the southern part of Hainan occurs from May to October and accounts for 95% of the yearly rainfall; the dry season occurs from November to April [
8]. Xiaodong Hai locality is situated in the southern part of Hainan Island (18°12’39”N and 109°29’51”E).
Coral reef of the locality has all the characteristic morphological features of the fringing reef (
Figure S1A). The gentle slope occupied by corals, coralline algae and algal turf (
Figure S1B) goes to a depth of 3 m while a poorly marked reef-cross goes into a rather wide (1–2 m) reef-flat occupied by corals,
Sargassum spp. bed (
Figure S1C) and algal turf (
Figure S1D). The bottom of the shallow lagoon (about 50 cm at low tide) is covered with the remains of coral colonies, sometimes with coarse sand, where mainly colonial Cyanobacteria, seagrass and some species of seaweeds settle. Coastline is mostly sandy with protrusions of carbonate base fossil reef, with intertidal pools (depth to 50 cm) and occupied mainly by algal turf. By the time of the study, the coral reef in Xiaodong Hai was significantly damaged and the carbonate reef cover with live corals was no more than 10%. At the same time, a large number of young colonies of branched corals indicates an active coral reef recovery process.
Following 2012, the sewage from Luhuitou region was discharged into the Xiaodong Hai Bay close to transect 2 (
Figure 1B) and the water here was severely polluted. The mean values in April 2019 were 9.36 µM for DIN (sum of NH
4, NO
3 and NO
2), ranging from 1.86 µM to 27.79 µM and were 0.55 µM for PO
4, ranging from 0.26 µM to 0.98 µM (Li et al. unpublished data).
2.2. Collection, Identification and Conservation of Seaweeds
For floristic studies, three transects passing from the shoreline to the slope of the coral reef were visually chosen (
Figure 1B). Sampling of macroalgae was carried out at the end of the dry seasons in 2016 (23 Mar.–01 Apr.), 2017 (2 Mar.–20 Mar.), 2019 (14 Mar.–24 Mar.) and at the end of the rainy seasons in 2016 (20 Nov.–4 Dec.), 2018 (23 Nov.–30 Nov.). At each sampling transect of four different tidal zones algae were collected: the upper intertidal zone + the splash zone, middle and lower intertidal zones, as well as the upper subtidal zone down to 2 m water depths (division into tidal zones followed Perestenko [
9]).
Seaweeds were collected by foot and via snorkeling during low tides. Samples were extracted no less than from five quadrats (10,000 cm2 each) in each sampling from each tidal zone. The abundance was visually determined based on photographs (made perpendicularly to the surface of the substrate occupied by algal community) of analyzed quadrats by estimating the mean substrate surface area occupied by algae. The following indicators of the abundance were used: rare sighting—found only one-two times with less than 10% relative coverage of substrata or found floating or on the beach (washed from the sea); common—found in the most quadrats with 10% to 50% relative coverage; and abundant—found in communities with substrata coverage from 50% to 100%. Dominance in the communities was also visually determined and defined as follows: monodominant if one algal species occupied more than 50% of the surface area; bidominant if two species occupied more than 50% of the surface area; and polydominant if more than two species predominated.
Fresh and dried specimens were identified using monographic publications, floristic studies, and systematic articles cited in previous publications [
3,
6,
10]. The systematics and nomenclature followed Guiry and Guiry [
11].
The collections of macroalgae and their epiphytes were preserved as dried herbarium specimens and were deposited in the herbarium at National Scientific Center of Marine Biology, Far Eastern Branch, Russian Academy of Science, Vladivostok 690041, Russian Federation.
2.3. Statistical Analysis
Data was analyzed with the statistical package Primer 6.1.12 (Primer–E Ltd., Plimut, UK). The similarity in species composition among samples collected at different years or seasons was analyzed by calculating the Bray-Curtis similarity coefficient [
12]. For graphical representation of the data set, cluster analysis (group average method) and non-metric multidimensional scaling (nMDS) ordination were carried out.
3. Results
3.1. Species and Life Forms Diversity
Our results show a total of 198 species and taxonomic forms of macroalgae and 20 species of benthic Cyanobacteria (
Tables S1 and S2).
The collection represents 106 species of the phylum Rhodophyta (54% of all collection) divided into three classes, 16 orders, 29 families and 57 genera; 40 species of Ochrophyta (20% of all) divided into one class (Phaeophyceae), 5 orders, 7 families and 15 genera; 52 species of Chlorophyta (26% of all) divided into two classes, 7 orders, 16 families and 25 genera and 20 species of Cyanobacteria belonging to 6 orders, 9 families and 14 genera.
Among red algae, the largest number of taxa belonged to families such as Rhodomelaceae (23 taxa), Ceramiaceae (14), and to the Order Corallinales (12 taxa); among green algae, Cladophoraceae (13) and Caulerpaceae (6); and among brown algae, Sargassaceae (18) and Dictyotaceae (8 taxa). Among Cyanobacteria, the largest number recorded for the Family Oscillatoriaceae (6 species).
The collection of algae consisted mainly of species (57%) growing on a hard substrate (epilithic algae) and a smaller degree of epiphytic and endophytic algae (26%). Algae growing on hard substrata and epiphytically amounted to 15%.
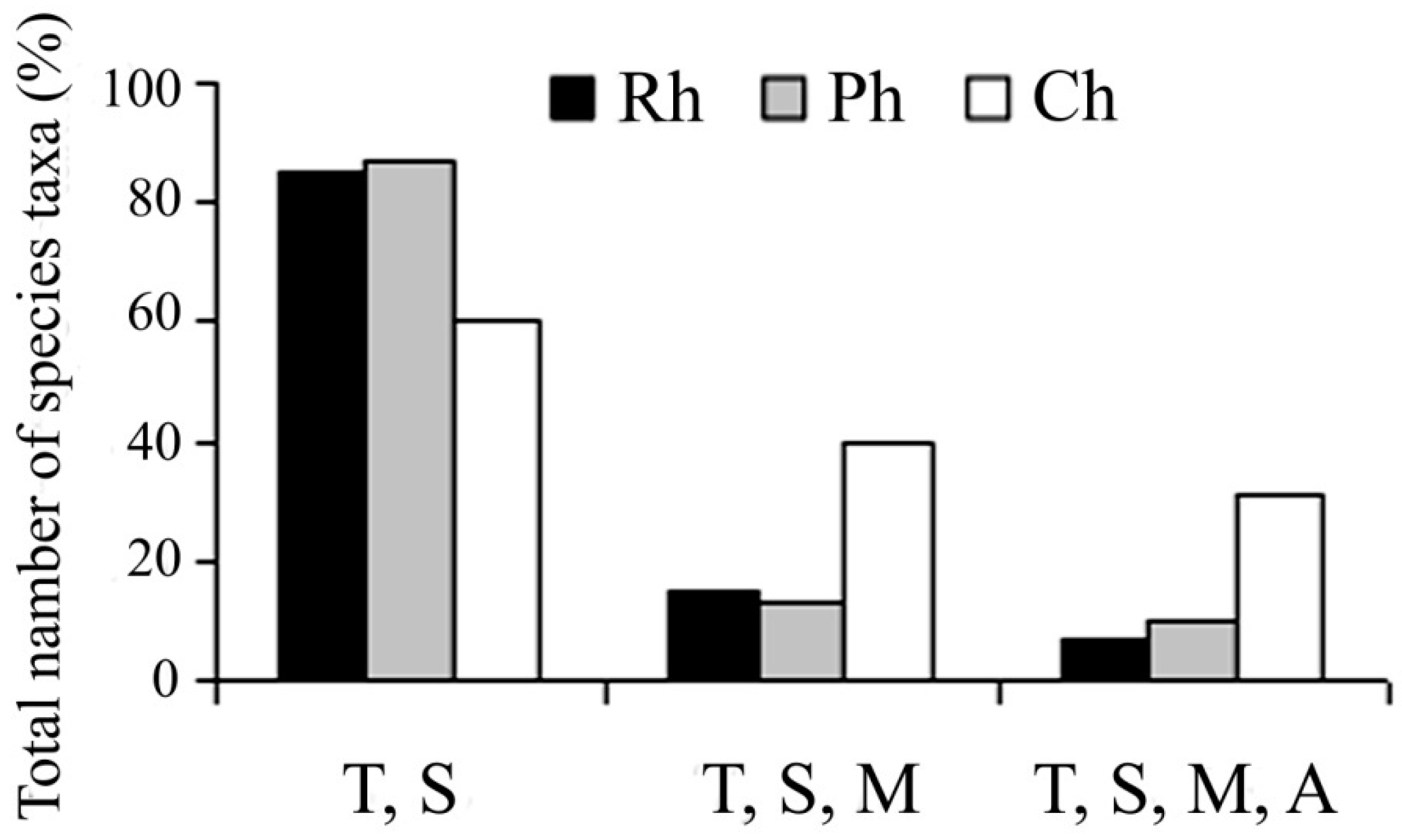
A 79% of the species identified, are typically distributed among tropical or subtropical regions [
11]. Reds and browns significantly prevailed in this group. Species of algae recorded in the tropics or subtropics to temperate zones and sometimes for Arctic and Antarctic accounted for 21% of the collection. This group was the most numerous among green algae (40%). Of the collection, cosmopolitan algae inhabiting tropics to Arctic or Antarctic waters amounted to 14%, and represented the highest numbers of taxa among green algae (31%) (
Figure 2). Algae inhabiting only the Indo-Pacific contained 63 species (32% of all collection) while red algae amounted to 33%, brown to 55% and green to 12%.
3.2. Interannual Differences in Diversity, Taxonomic Composition and Dominance of Species in Algal Communities of the Benthic Flora
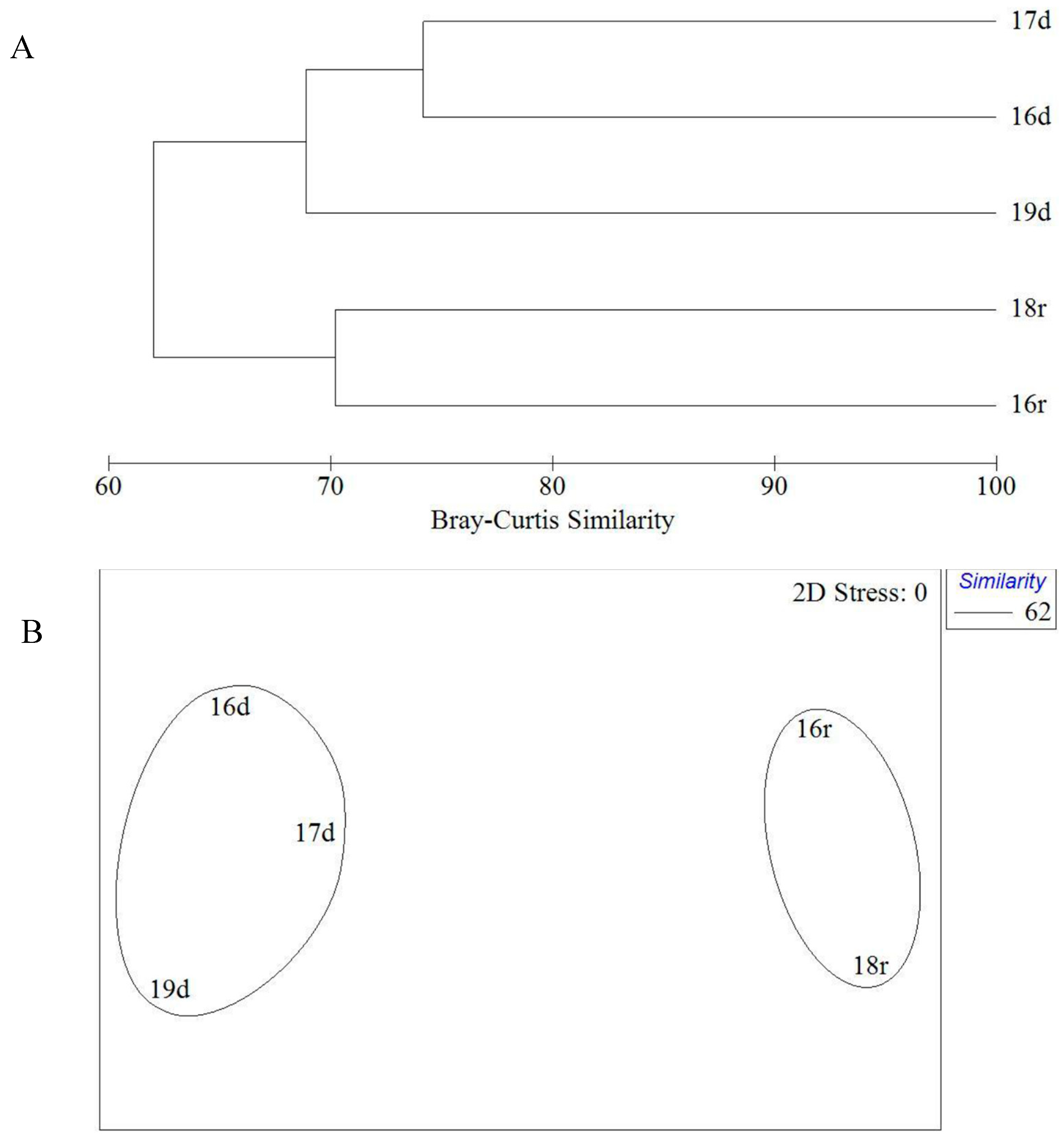
The analysis did not reveal any interannual differences either in dry or rainy seasons. The maximum floristic similarity (70%) was found in the rainy season between 2016 and 2018. The lowest similarity (68%) was found between collections of 2016/2017 and 2019 obtained at the end of the dry season (
Figure 3). Moreover, Xiaodong Hai locality was richer in species number of green algae in 2016 and 2017 than in 2019 (
Table S1).
Interannual differences in the dominance of species were clear. Communities of algal turf dominated in a mosaic-polydominant arrangement in the lower intertidal and upper subtidal zones (
Table S1). For example, in the spring of 2016,
Centroceras clavulatum,
Gayliella mazoyerae,
Spyridia filamentosa,
Hypnea pannosa (Rhodophyta, Rh);
Padina minor,
Sargassum polycystum,
S. sanyaense (Phaeophyceae);
Caulerpa chemnitzia,
C. racemosa and
C. sertularioides (Chlorophyta, Ch) prevailed in these communities. In the spring of 2019, some of the previously dominant species moved into the category of common or rare species, while others species such as
Amphiroa foliacea,
A. fragilissima,
Taenioma perpusillum,
Acanthophora spicifera,
Chondria repens,
Tolypiocladia glomerulata (Rh);
Hydroclathrus clathratus,
Padina australis,
Sargassum denticarpum,
S. hemiphyllum var.
chinense,
S. oligocystum (Ph) and
Caulerpa nummularia (Ch) have been added to the remaining dominants. During all years of the study,
Laurencia decumbens,
Gelidiella bornetii,
Palisada papillosa (Rh),
Sphacelaria tribuloides (Ph) and
Ulva clathrata (Ch) mainly dominated in mono-, bi- and polydominant communities in the upper and lower intertidal zones.
3.3. Seasonal Differences in Diversity, Taxonomic Composition and the Dominance of Some Species in Algal Communities
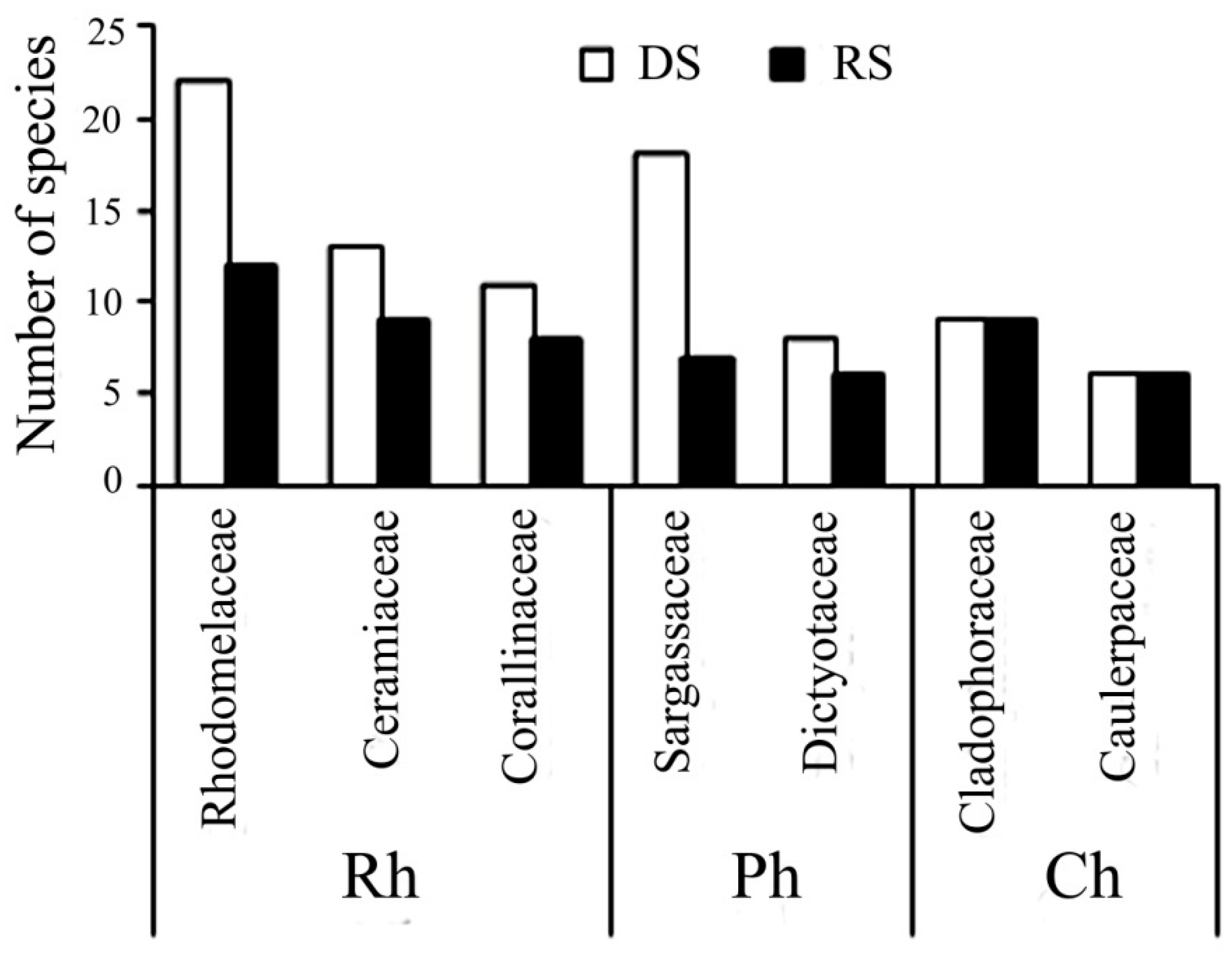
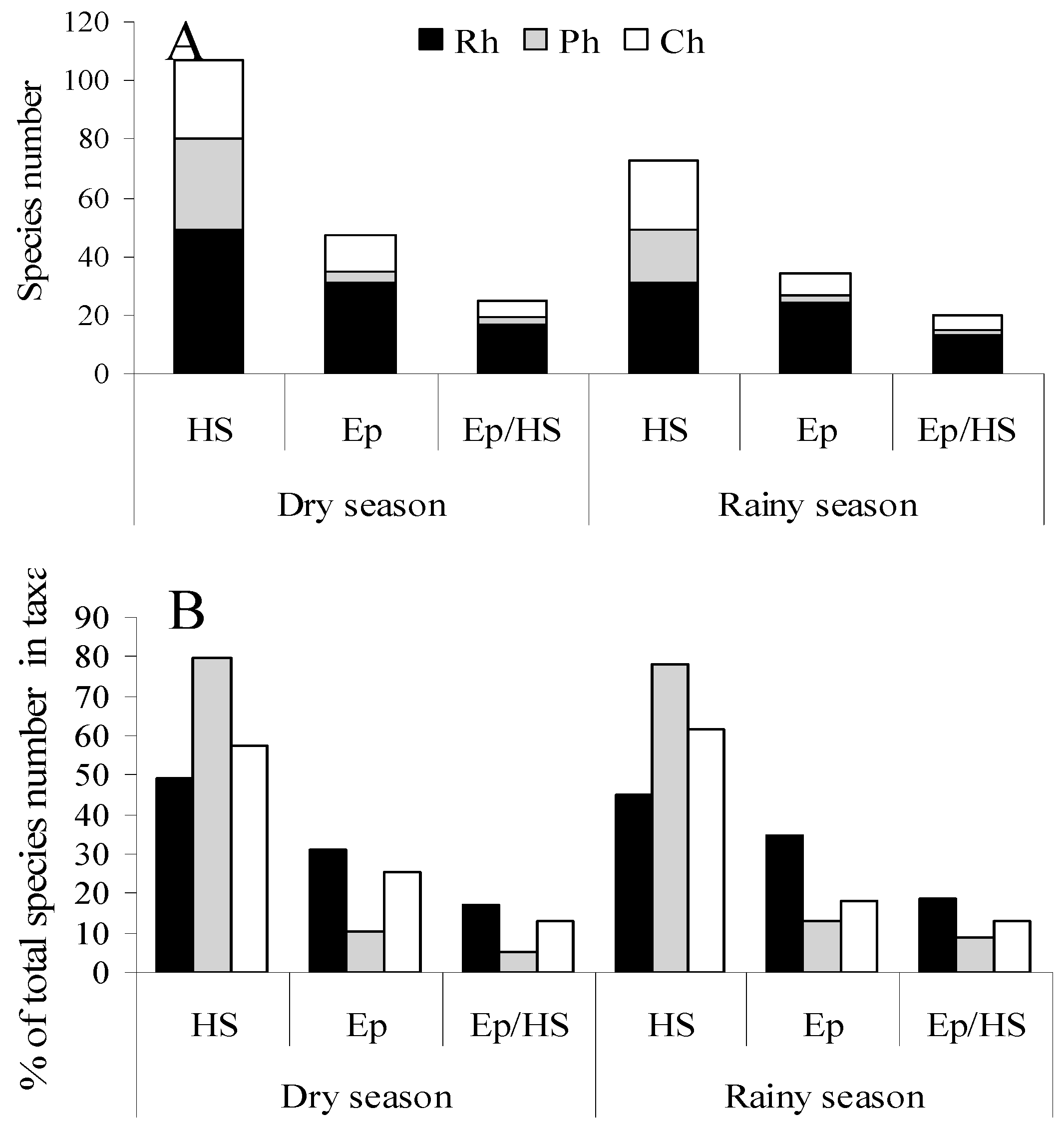
The maximum floristic similarity between the dry and rainy season was significantly less than the interannual similarity, but still accounted for approximately 60% (
Figure 3). In the rainy seasons, species diversity of benthic algae was significantly decreased (more than by 30%), however relative numbers of red, brown and green algae changed slightly. Representatives of the families Rhodomelaceae, Ceramiaceae, and the Order Corallinales (Rh); and Sargassaceae, Dictyotaceae (Ph) common for dry seasons were not found in rainy seasons (
Figure 4).
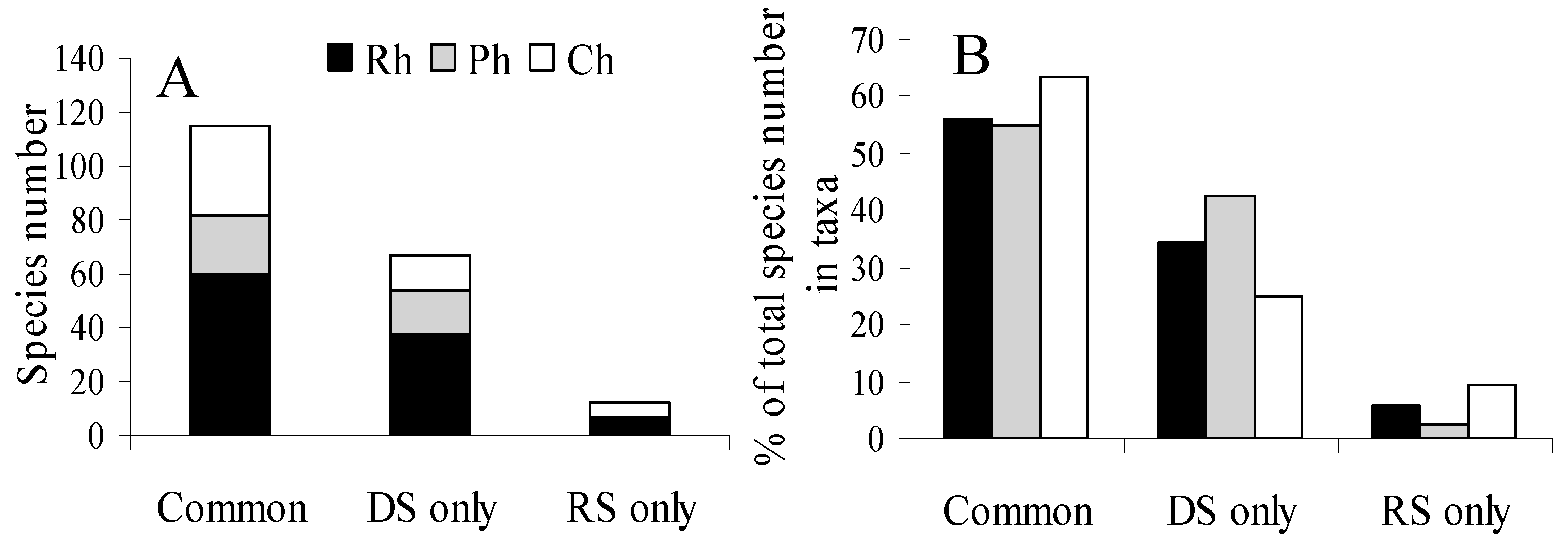
There was a season specificity recorded across algal groups (
Figure 5). Of the 198 species followed during the study, most species (60%) occurred during the both seasons, 35% were specific to only the dry season, and a small amount of rare species (5%) were specific to only the rainy season. Seasonal specificity occurred in all taxonomic groups of macroalgae, especially it strongly manifested in the group of browns, where algae found only at the end of the dry season amounted to 42% of all collected. Aseasonal algae were the most in the group of green algae (63%).
Seasonal specificity in the presence of various life forms was manifested only in an absolute and relative increase in number of species of epiphytic green algae sampled in dry seasons (
Figure 6).
There was no seasonal specificity in the distribution of various taxonomic groups and life forms of algae in tidal zones (
Table S1).
Seasonal differences were clearly manifested in the dominance of certain species in algal communities (
Table S1). Amphiroa foliacea, Amphiroa fragilissima, Ganonema farinosum, Hypnea pannosa, Palisada papillosa, Spyridia filamentosa, Tolypiocladia glomerulata (Rh); Colpomenia sinuosa, Hydroclathrus clathratus, Padina australis (Ph); Caulerpa sertularioides (Ch) dominated in algal communities only in dry seasons. Such species as Asparagopsis taxiformis, Ceramium cimbricum, Herposiphonia secunda f. tenella, Jania adhaerens, J. capillacea, Pterocladiella caerulescens (Rh); Lobophora variegata, Sphacelaria rigidula (Ph); Ulva clathrata, Caulerpa racemosa (Ch) prevailed only in rainy seasons.
3.4. Algal Communities and Their Distribution in Tidal Zones
The splash zone on separate remnants of patch-reefs was occupied mainly by colonial Cyanobacteria forming black crusts (
Figure S2A). The upper intertidal zone consisted of fossil base of coral reef, individual large boulders and remains of coral colonies were occupied by monodominant communities (resistant to complete desiccation) of crust algae and algal mat (0.2–0.5 cm high) (
Figure S2C). The middle intertidal zone (consisted of rocky substratum) was also occupied by monodominant communities of crust and turf-forming algae, and carbonate base of coral reef was occupied by bi- and polydominant communities of algal turf (
Figure S2D,E). In the lower intertidal and upper subtidal zones, polydominant (mosaic) communities of algal turf with varying dominant species (
Figure S2F) mainly occupied on reefs, which are now formed by dead coral. Dense or sometimes interrupted
Sargassum spp. belt with the mosaic dominance of such species as
Sargassum aquifolium,
S. denticarpum,
S. hemiphyllum var.
chinense,
S. oligocystum,
S. polycystum occupied reef-flat (
Figure S2G). Another most distributed upright growing community in these zones was presented by monodominant community of the brown algae
Padina spp.
4. Discussion
A total about 200 species of macroalgae and 20 species of Cyanobacteria were found during the study in Xiaodong Hai locality. The group of species inhabiting only the tropics or subtropics dominated among macrophytes. At the same time, algae inhabiting temperate waters, amounted to 20% of all collection. Most found species of the collection widely distributed in warm waters of Atlantic, Indian and Pacific Oceans and others found only in the Indo-Pacific region. Such biogeographic distribution of species in Xiaodong Hai gives grounds to attribute the benthic flora of the southern part of Hainan Island, according to the scheme of Briggs [
13], to the warm temperate region of the Northern hemisphere. In previous works, a similar geographical distribution we found for macroalgae from other localities in Hainan [
3], as well as from some southern islands of Japan [
14,
15] and in the southern Vietnam [
16]. The long-term study of the benthic flora of the coral reef in Xiaodong Hai locality (German-China expeditions in 1990 and 1992, our investigations in 2008, 2009, 2015–2019) has confirmed the richness of species of the recent flora and the similarity of species composition with other localities in Sanya Bay [
17]. At the same time, the coral reef in Xiaodong Hai differs significantly in algal species composition from those of the northern localities of Hainan Island, for example Meixia and Xianhai localities situated to the North from Xiaodong Hai by ~1°50′. In these localities,
Monostroma latissimum Wittrock dominated in the upper intertidal in the springtime, and in the upper subtidal such species, as
Sargassum henslowianum C. Agardh,
S. microcystum C. Agardh,
S. horneri (Turner) C. Agardh were common, however these species were not found in Sanya Bay [
3].
Current investigations in Xiaodong Hai locality confirmed our assumption about decadal changes in the flora of benthic algae and the reasons causing these changes [
3]. The conspicuous changes in algal species diversity and composition occurred from the 1930s to 2019, as exemplified by a decline in species richness and the appearance of new algal findings. The largest numbers of taxa losses were recorded for families Liagoraceae, Gracilariaceae (Rh), Caulerpaceae, Codiaceae (Ch) and Sargassaceae (Ph). The majority of lost species (not found again) were low productive, annual or perennial representatives inhabiting hard substrata (epilithic algae) with volumnar form of thalli. At the same time, the following families contributed to taxa increases: Ceramiaceae, Rhodomelaceae (Rh), Sphacelariaceae, Dictyotaceae (Ph), Cladophoraceae and Ulvaceae (Ch). Representatives of these families are mainly high productive ephemeral and epiphytic algae with fine filamentous forms and high surface area-volume ratios. These serious changes occurred in the benthic flora due probably to over-exploitation of the reef ecosystems, especially in the 1950s–1970s; eutrophication of shallow waters in the 1990s–2010s, most notably sewage disposal (coastal urbanization, agriculture, mariculture, and tourism industry, etc.).
4.1. Interannual Differences in the Marine Flora
In the course of monitoring of the marine flora in Xiaodong Hai, there were no significant interannual differences in diversity, and taxonomic of macrophytes. It probably can be explained by the absence of significant differences in the levels of major climatic factors between the years in the period 2016–2019, affecting the composition of the flora and the structure of algal communities. However, in a few pieces of the literature on interannual changes in the diversity of the benthic flora in subtropical/tropical areas, we often found serious interannual variations in the number and composition of species. For example, in the work of Tribolett and Vroom [
18], devoted to the study of the underwater ecosystems of the Mariana Archipelago, showed that the total number of macroalgal genera on the islands shelf was increased 1.4 times from 2003 to 2005. In another work of Vroom and Timmers [
19], describing interannual changes in flora and fauna at Gardner Pinnacles, Northwestern Hawaiian Islands, found that species diversity of eukaryotic algae from 2003 to 2004 was decreased by 2.5 times. In the first case, the enrichment of the coral reef ecosystem of the Mariana Archipelago with macrophytes, according to the authors, could be associated with differing oceanographic conditions such as sea surface temperature, human impacts such as fishing, as well as typhoons, and volcanic activity that occurred on the archipelago during the study years (2003–2005). In the second case, the authors explain the decrease in algal diversity on Gardner Pinnacles in 2004 by the early seasonal changes that caused losses in biomass and algal diversity. Previously, Vroom et al. [
20], Sergeeva et al. [
21], Harley et al. [
22] showed a significant impact of natural disasters (increase in water temperature, causing coral bleaching and death, as well as tsunamis and strong typhoons physically freeing substrata from plants) on the composition and diversity of the benthic flora. It was also shown that the next year/years after the catastrophe, species composition of the flora might change dramatically, as due to the disappearance of large perennial and annual algal macrophytes, and at the expense of occupation of free substrate (dead coral colonies, etc.) with ephemeric and opportunistic algae [
21].
In the present work, the main characteristic difference in the marine flora of different years was the unequal composition of the dominant species in algal turf communities. As we supposed earlier [
23], the interannual changes in the composition of dominant and accompanying species in algal turf communities in the upper subtidal zone may be determined by the periodic character of annual changes in vegetation in the course of succession. The formation of new algal turf communities in Sanya Bay begins immediately after elimination of old communities at the end of the dry season (from February to May) [
8,
10,
23]. The species composition of the algal turf communities and their structure during the formation depends on many factors: the number of plants remaining on the substrate after the disappearance of the old communities, on the diversity and amount of spores (propagules) in the environment, on the intensity of abiotic factors such as light, temperature, salinity, nutrient content, wave action, etc. and also biotic factors such as the presence of herbivorous animals, interspecific competition for the substrate, resources and others. The formation of ephemeric communities in the intertidal zone may begin at any time of the year and the progress of their succession depends mainly on changes in the levels of external factors such as air and water temperatures, drying duration, the amount of rainfall and salinity.
4.2. Seasonal Changes
During our study, the largest number of species was found during the dry seasons as compared to the rainy seasons (DS:RS = 186:131) (
Table S1). In our opinion, the richness of the marine flora in dry seasons was caused by optimal conditions for the occurrence and development of algae-ephemerals (especially blue–green algae) and the mass occurrence and vegetation of annual plants.
The decrease in species richness in the upper and middle intertidal zones occurring during rainy seasons seems to be connected with the decrease in salinity that did not contribute to the appearance of algae ephemerals. Algae that completed vegetation remained in the community on the substrates in the form of patches; basal stem, “sleeping” or germinating spores or propagules were difficult for collectors during sampling and could not always be identified to the species level. The latter took place in the rainy period during collecting and determining Sargassum spp., when most species have either completed their vegetation or just started to grow.
Our new data on seasonal changes in the benthic flora of Xiaodong Hai (Sanya Bay) confirm previously obtained results for Luhuitou locality of the same bay [
8,
21]. Investigations of factors influencing seasonal changes in algal species composition in other tropical and subtropical regions do not contradict our results and conclusions. In Nanwan Bay in southern Taiwan (the nearest to Hainan Island), species diversity on the lower reef flat and the reef slope (the low intertidal and upper subtidal zones) showed insignificant seasonal variations [
24]. However, essential changes were observed in the intertidal zone of other subtropical and tropical regions. On coral reefs in Brazil, most of the taxa were present during the summer rainy season (Feb.–Mar.). In the rocky intertidal of the Colombian Caribbean, the macroalgal community was most diverse in Oct., which historically has been the rainiest and calmest month of the year [
25]. On the Great Barrier Reef in Australia, extensive ephemeral blooms of small, fleshy brown macroalgae, such as
Chnoospora and
Hydroclathrus, have been observed on shallow reef flats predominantly during winter and early spring [
26]. The authors of these studies assume that replacement in the vegetation at shallow sites is induced by sharp changes in climate that occur during seasonal changes. On the north-west coast of India (Port Okha), Thakur with coauthors [
27] found that seasonal variation in species composition of stranded seaweeds was correlated with natural species succession. The vegetation season on the Okha coast is spread over seven months, running from Nov. to May. During this period, extensive growth and succession of different types of seaweed associations have been observed. On coral reefs in Okinawa, natural changes in vegetation are connected with a succession of algal communities that occurs in late spring (May–June) when extensive bands of drift algae were found on the shore [
28].
The decrease in species richness in the intertidal zone that occurred during rainy seasons seems to be connected with decrease in salinity, especially in the upper and middle intertidal zones (that did not promote the appearance of ephemeral algae), as well as with the end of the growing season of many annual and perennial species, especially living in the lower intertidal and upper subtidal zone. Every year the community of algal turf in Sanya Bay begin to grow (renew) at the end of the dry season [
10,
23]. During the succession that may continue by one-two months less or more of one year, species composition and the structure of community change under the influence of external (abiotic and biotic) and inner (duration and character of vegetation of some species) factors. We assume those communities at different seasons under different stages of the succession have varying species composition of both dominant and accompanying species. The results of this work confirmed this assumption.
5. Conclusions
Monitoring of the benthic flora of the Xiaodong Hai coral reef carried out in 2016–2019 showed a high level of diversity of macrophytes (198 species and their taxonomic forms) and colonial Cyanobacteria (20 species). Among macrophytes, red algae (54%) prevailed, the most numerous were the species from families such as Rhodomelaceae, Ceramiaceae and the order Corallinales; among green algae (26% of all collection) taxa from Cladophoraceae and Caulerpaceae dominated; in the group of brown algae (20% species) Sargassaceae and Dictyotaceae showed the highest numbers of algal taxa. Analysis of the collection give grounds to attribute the benthic flora of Xiaodonghai locality to the warm temperate region of the Northern hemisphere.
Algae were unevenly distributed over tidal zones: the predominant number of species was found in the lower intertidal and upper subtidal zones, and only 30% of the species dwelled in the upper and middle intertidal zones. Polydominant communities of algal turf and communities of upright growing Sargassum spp. predominated in the lower intertidal and upper subtidal zones. The upper and middle intertidal were occupied by mono- and bidominant communities of algal mat/turf and monodominant communities of crust algae. The splash zone was occupied by colonial Cyanobacteria.
Interannual differences in diversity, species composition and the structure of algal communities were insignificant. Differences were observed mainly in composition of dominant species in polydominant communities of algal turf. At the same time, seasonal differences in the floras were evident: the flora in dry seasons was richer in macrophytic species especially in fine filamentous epiphytic algae from the families Rhodomelaceae and Ceramiaceae (Rh) as well as in Cyanobacteria.
Among algae collected during rainy seasons, about 30% of taxa (common for dry seasons) were not found. It should be mentioned that many large annual and perennial macrophytes during the rainy season finished their vegetation, but were remained on the substrate or epiphytic in the form of small patches, “influxes”, “stems”, propagules, seedlings, becoming invisible to the collectors. Seasonal differences in the floras were observed in composition of dominant species of algal turf. We assume that interseasonal differences found in Xiaodong Hai were caused by objective reasons (impact of external and internal factors on the formation process of algal communities and subjective factors, such as fullness of algal sampling and the possibility of their identification).
Supplementary Materials
The following are available online at
https://www.mdpi.com/2077-1312/7/8/243/s1, Figure S1: Coral reef in Xiaodong Hai locality, Figure S2: Algal communities in Xiaodong Hai at different seasons of 2016–2019, Table S1: List of the seaweeds of Xiaodong Hai in 2016–2019, Table S2: List of benthic Cyanobacteria in Xiaodong Hai (2016–2019).
Author Contributions
E.A.T. and X.L. conceived and designed the experiments; E.A.T., T.V.T., A.V.S., Y.R. and X.L. performed the experiments, E.A.T., T.V.T., and X.L. performed the data analysis, and drafted the manuscript; H.H. and X.L. supervised the project. All the authors reviewed and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (41476134), the Open Project of State Key Laboratory of Marine Resource Utilization in South China Sea (DX2017003), the Foundation of Hainan University (KYQD(ZR)1805) and the grant ‘China–Russia special funds 2019′ from the Chinese Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tseng, C.K. The past, present and future of phycology in China. Hydrobiologia 2004, 512, 11–20. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Xia, B.M.; Bartsch, I. Retrospective Analysis of Diversity and Species Composition of Marine Macroalgae of Hainan Island (China). Ocean Sci. J. 2016, 51, 1–22. [Google Scholar] [CrossRef]
- Titlyanova, T.V.; Titlyanov, E.A.; Kalita, T.L. Marine algal flora of Hainan Island: A comprehensive synthesis. Coast. Ecosyst. 2014, 1, 28–53. [Google Scholar]
- Zhang, Q.; Xu, X.Z.; Long, X.M. A numerical study on internal tides in the northeast of the South China Sea. J. Trop. Oceanol. 1996, 14, 15–23. [Google Scholar]
- Zhang, G.; Que, H.; Liu, X.; Xu, H. Abalone mariculture in China. J. Shellfish Res. 2004, 23, 947–950. [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V.; Xia, B.M.; Bartsch, I. Checklist of marine benthic green algae (Chlorophyta) on Hainan, a subtropical island off the coast of China: Comparisons between the 1930s and 1990–2009 reveal environmental changes. Bot. Mar. 2011, 54, 523–535. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Q.; Chen, G.; Fong, T.C.; Wong, D.C.; Huang, H.; Wang, H.; Zhao, M. Status monitoring and health assessment of Luhuitou fringing reef of Sanya, Hainan, China. Chin. Sci. Bull. 2006, 51, 81–88. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Huang, H.; Li, X.B. Seasonal changes in benthic algal communities of the upper subtidal zone in Sanya Bay (Hainan Island, China). J. Mar. Biol. Assoc. UK 2014, 91, 51–64. [Google Scholar] [CrossRef]
- Perestenko, L.P. Algae of Peter the Great Bay; Nauka: Leningrad, Russian, 1980. (In Russian) [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V.; Li, X.B.; Hansen, G.I.; Huang, H. Seasonal changes in the intertidal algal communities of Sanya Bay (Hainan Island, China). J. Mar. Biol. Assoc. UK 2014, 94, 879–893. [Google Scholar] [CrossRef] [Green Version]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. Galeay: National University of Ireland, 2019. Available online: http://www.algaebase.org (accessed on 20 June 2019).
- Clarke, K.R.; Green, R.H. Statistical design and analysis for a ‘biological effects’ study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Briggs, J.C. Marine Zoogeography; McGraw-Hill: New York, NY, USA, 1974; 475p. [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V.; Kalita, T.L.; Tokeshi, M. Decadal changes in the algal assemblages of tropical-subtropical Yonaguni Island in the western Pacific. Coast. Ecosyst. 2016, 3, 16–37. [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V.; Tokeshi, M. Recent (2012–2017) seaweed flora of Tomioka Peninsula, Shimoshima Island (the East China Sea, Japan). Coast. Ecosyst. 2019, 6, 1–21. [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V.; Belous, O.S. Checklist of the marine flora of Nha Trang Bay (Vietnam, South China Sea) and decadal changes in the species diversity composition between 1953 and 2010. Bot. Mar. 2015, 58, 367–377. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Belous, O.S.; Kalita, T.L. Inventory change (1990s–2010s) in the marine flora of Sanya Bay (Hainan Island, China). J. Mar. Biol. Assoc. UK 2015, 95, 461–470. [Google Scholar] [CrossRef]
- Tribolett, A.D.; Vroom, P.S. Temporal and spatial comparison of the relative abundance of macroalgae Across the Mariana Archipelago between 2003 and 2005. Phycologia 2007, 46, 187–197. [Google Scholar] [CrossRef]
- Vroom, P.S.; Timmers, M.A.V. Spatial and temporal comparison of algal biodiversity and benthic cover at Gardner Pinnacles, Northwestern Hawaiian Islands. J. Phycol. 2009, 45, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Vroom, P.; Walters, L.; Beach, K.; Coyer, J.; Smith, J.; Abgrall, M.-J.; Byron, D.; Kathryn, D.; Konar, B.; Liss, J.; et al. Hurricane-induced propagation and rapid regrowth of the weedy brown alga Dictyota in the Florida Keys. Fla. Sci. 2005, 68, 161–174. [Google Scholar]
- Sergeeva, O.S.; Titlyanova, T.V.; Titlyanov, E.A. Species composition and distribution of seaweeds of fringing coral reef of Sesoko Island (Ryukyu Archipelago) before and after the natural catastrophe in 1998. Rus. J. Mar. Biol. 2007, 33, 37–48. [Google Scholar] [CrossRef]
- Harley, C.D.G.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A. Effects of climate change on global seaweed communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V. Changes in the species composition of benthic macroalgal communities of the upper subtidal zone on a coral reef in Sanya Bay (Hainan Island, China) during 2009–2012. Rus. J. Mar. Biol. 2013, 39, 413–419. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wong, S.L.; Chang, J.S.; Hwang, R.L.; Dai, C.F.; Yu, Y.C.; Shyu, Y.T.; Sheu, F.; Lee, T.M. Macroalgal assemblage structure on a coral reef in Nanwan Bay in southern Taiwan. Bot. Mar. 2004, 47, 439–453. [Google Scholar] [CrossRef]
- García, C.B.; Díaz-Pulido, G. Dynamics of a macroalgal rocky intertidal community in the Colombian Caribbean. Bol. Investig. Mar. Costeras 2006, 35, 7–18. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; McCook, L.J.; Larkum, A.W.D.; Lotze, H.K.; Raven, J.A.; Schaffelke, B.; Smith, J.; Steneck, R.S. Vulnerability of macroalgae of the Great Barrier Reef to climate change. In Climate Change and the Great Barrier Reef: A Vulnerability Assessment; Johnson, J.E., Marshall, P.A., Eds.; Great Barrier Reef Marine Park Authority and the Australian Greenhouse Office: Townsville, QLD, Australia, 2007; pp. 153–192. [Google Scholar]
- Thakur, M.C.; Reddy, C.R.K.; Jha, B. Seasonal variation in biomass and species composition of seaweeds stranded along Port Okha, northwest coast of India. J. Earth Syst. Sci. 2008, 117, 211–218. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Yakovleva, I.M.; Belous, O.S. Influence of winter and spring/summer algal communities on the growth and physiology of adjacent scleractinian corals. Bot. Mar. 2006, 49, 200–207. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).