The Role of Bacillus amyloliquefaciens on Litopenaeus vannamei During the Maturation of a Biofloc System
Abstract
:1. Introduction
2. Materials and Methods
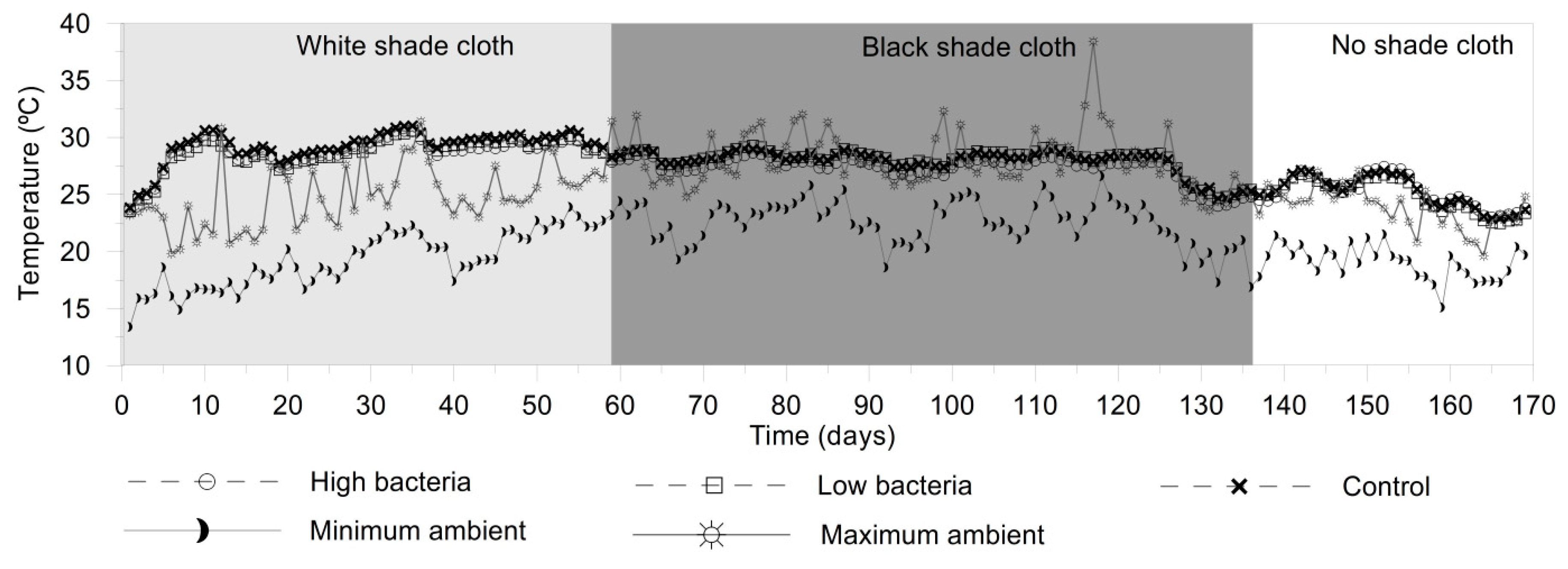
2.1. Location and Shrimp Culture System
2.2. Probiotic Treatments
2.3. Chlorophyll a and Microbial Activity
2.4. Growth Parameters
2.5. Immune Parameters
2.6. Statistical Analysis
3. Results
3.1. Water Quality
3.2. Microbial Activity
3.3. Growth Parameters
3.4. Immune System Parameters
4. Discussion
4.1. Effects on the Biofloc System
4.2. Effects on Shrimps
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Emerenciano, M.; Gaxiola, G.; Cuzon, G. Biofloc technology (BFT): A review for aquaculture application and animal food industry. In Biomass Now-Cultivation and Utilization; Darko, M., Ed.; InTech, Queen’s University: Kingston, ON, Canada, 2013; pp. 301–328. [Google Scholar]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book, 1st ed.; World Aquaculture Society: Baton Rouge, LA, USA, 2009. [Google Scholar]
- Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture 2012, 356, 351–356. [Google Scholar] [CrossRef]
- Wasielesky, W.; Atwood, H.; Stokes, A.; Browdy, C.L. Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture 2006, 258, 396–403. [Google Scholar] [CrossRef]
- Jatobá, A.; Silva, B.C.; Silva, J.S.; Vieira, F.D.; Mouriño, J.L.; Seiffert, W.Q.; Toledo, T.M. Protein levels for Litopenaeus vannamei in semi-intensive and biofloc systems. Aquaculture 2014, 432, 365–371. [Google Scholar] [CrossRef]
- Xu, W.; Morris, T.C.; Samocha, T.M. Effects of C/N ratio on biofloc development, water quality, and performance of Litopenaeus vannamei juveniles in a biofloc-based, high-density, zero-exchange, outdoor tank system. Aquaculture 2016, 453, 169–175. [Google Scholar] [CrossRef]
- Sanders, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 46, 58–61. [Google Scholar] [CrossRef]
- Van Hai, N.; Fotedar, R.A. Review of probiotics in shrimp aquaculture. J. Appl. Aquac. 2010, 22, 251–266. [Google Scholar] [CrossRef]
- Souza, D.M.; Suita, S.M.; Leite, F.P.; Romano, L.A.; Wasielesky, W.; Ballester, E.L. The use of probiotics during the nursery rearing of the pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817) in a zero exchange system. Aquac. Res. 2012, 43, 1828–1837. [Google Scholar] [CrossRef]
- Krummenauer, D.; Poersch, L.; Romano, L.A.; Lara, G.R.; Encarnação, P.; Wasielesky, W. The effect of probiotics in a Litopenaeus vannamei biofloc culture system infected with Vibrio parahaemolyticus. J. Appl. Aquac. 2014, 26, 370–379. [Google Scholar] [CrossRef]
- Farzanfar, A. The use of probiotics in shrimp aquaculture. Pathog. Dis. 2006, 48, 149–158. [Google Scholar]
- Zhou, X.; Wang, Y.; Li, W. Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture 2009, 287, 349–353. [Google Scholar] [CrossRef]
- Nuez-Ortín, W.G.; Casillas-Hernández, R.; Nolasco-Soria, H. Efecto de Ecobiol Aqua en la Actividad Enzimática del Camarón. Aquafeed, 2013. Available online: www.aquafeed.co/efecto-de-ecobiol-aqua-en-la-actividad-enzimatica-del-camaron/ (accessed on 17 April 2018).
- Pandiyan, P.; Balaraman, D.; Thirunavukkarasu, R.; George, E.G.; Subaramaniyan, K.; Manikkam, S.; Sadayappan, B. Probiotics in aquaculture. Drug Invent. Today 2013, 5, 5–59. [Google Scholar] [CrossRef]
- Rengpipat, S.; Rukpratanporn, S.; Piyatiratitivorakul, S.; Menasaveta, P. Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 2000, 191, 271–288. [Google Scholar] [CrossRef]
- Tseng, D.Y.; Ho, P.L.; Huang, S.Y.; Cheng, S.C.; Shiu, Y.L.; Chiu, C.S.; Liu, C.H. Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish Shellfish Immunol. 2009, 26, 339–344. [Google Scholar] [CrossRef]
- Paiva-Maia, E.; Alves-Modesto, G.; Otavio-Brito, L.; Olivera, A.; Vasconcelos-Gesteira, T.C. Effect of a commercial probiotic on bacterial and phytoplankton concentration in intensive shrimp farming (Litopenaeus vannamei) recirculation systems. Lat. Am. J. Aquat. Res. 2013, 41, 126–137. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; Porchas-Cornejo, M.A.; Martínez-Porchas, M.; Villalpando-Canchola, E.; Gollas-Galván, T.; Martínez-Córdova, L.R. Bacterial biota of shrimp intestine is significantly modified by the use of a probiotic mixture: A high throughput sequencing approach. Helgol. Mar. Res. 2017, 71, 1–10. [Google Scholar] [CrossRef]
- Dalmin, G.; Kathiresan, K.; Purushothaman, A. Effect of probiotics on bacterial population and health status of shrimp in culture pond ecosystem. Indian J. Exp. Biol. 2001, 39, 939–942. [Google Scholar]
- Nimrat, S.; Suksawat, S.; Boonthai, T.; Vuthiphandchai, V. Potential Bacillus probiotics enhance bacterial numbers, water quality and growth during early development of white shrimp (Litopenaeus vannamei). Vet. Microbiol. 2012, 159, 443–450. [Google Scholar] [CrossRef]
- Ramezani-Fard, E.; Zokaeifar, H.; Ebrahimi, M.; Kamarudin, M.S.; Goh, Y.M. Probiotic administration of Litopenaeus vannamei: Is there any negative effect on the fatty acid profile of meat? Iran. J. Fish. Sci. 2014, 13, 550–559. [Google Scholar]
- Jerzsele, A.; Szeker, K.; Csizinszky, R.; Gere, E.; Jakab, C.; Mallo, J.J.; Galfi, P. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult. Sci. 2012, 91, 837–843. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Islam, M.M.; Mun, H.S.; Sim, H.J.; Kim, Y.J.; Yang, C.J. Effects of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult. Sci. 2014, 93, 1963–1971. [Google Scholar] [CrossRef]
- Larsen, N.; Thorsen, L.; Kpikpi, E.N.; Stuer-Lauridsen, B.; Cantor, M.D.; Nielsen, B.; Brockmann, E.; Derkx, P.M.; Jespersen, L. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl. Microbiol. Biotechnol. 2014, 98, 1105–1118. [Google Scholar] [CrossRef]
- Lei, X.; Piao, X.; Ru, Y.; Zhang, H.; Péron, A.; Zhang, H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler Chickens. Asian Australas. J. Anim. 2015, 28, 239–246. [Google Scholar] [CrossRef]
- Camacho, M.A. Efecto de la Aplicación de Probióticos en Dietas Para Camarón Blanco Litopenaeus vannamei, Mediante Indicadores de Crecimientoy Respuesta Inmune. Master’s Thesis, Instituto Tecnológico de Sonora, Sonora, Mexico, 2012. [Google Scholar]
- Xie, F.; Zhu, T.; Zhang, F.; Zhou, K.; Zhao, Y.; Li, Z. Using Bacillus amyloliquefaciens for remediation of aquaculture water. Springer Plus 2013, 2, 119. [Google Scholar] [CrossRef]
- Huang, L.; Ran, C.; He, S.; Ren, P.; Hu, J.; Zhao, X.; Zhou, Z. Effects of dietary Saccharomyces cerevisiae culture or live cells with Bacillus amyloliquefaciens spores on growth performance, gut mucosal morphology, hsp70 gene expression, and disease resistance of juvenile common carp (Cyprinus carpio). Aquaculture 2015, 438, 33–38. [Google Scholar] [CrossRef]
- Saputra, F.; Shiu, Y.; Chen, Y.; Puspitasari, A.W.; Danata, R.H.; Liu, C.; Hu, S. Dietary supplementation with xylanase-expressing B. amyloliquefaciens R8 improves growth performance and enhances immunity against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2016, 58, 397–405. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Manual of Diagnostic Tests for Aquatic Animals. OiE, 2016. Available online: www.oie.int/en/standard-setting/aquatic-manual/access-online/ (accessed on 19 November 2018).
- Jory, D.E.; Cabrera, T.R.; Dugger, D.M.; Fegan, D.; Lee, P.G.; Lawrence, L.; Jackson, C.; Mcintosh, R.; Castañeda, J.; International, B.; et al. A global Review of shrimp feed management: Status and perspectives. In Aquaculture 2001: Book of Abstracts, 1st ed.; World Aquaculture Society, Ed.; World Aquaculture Society: Baton Rouge, LA, USA, 2001. [Google Scholar]
- Baumgarten, M.G.Z.; Wallner-Kersanach, M.; Niencheski, L.F.H. Manual de Análisis em Oceanografía Química, 1st ed.; Editora da FURG: Rio Grande, Brazil, 2010. [Google Scholar]
- Grasshoff, K. Methods of Seawater Analysis, 1st ed.; WILEY-VCH: Weinheim, Germany, 1976. [Google Scholar]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Van Wyk, P.; Scarpa, J. Water quality requirements and management. In Farming Marine Shrimp in Recirculating Freshwater Systems, 1st ed.; Florida Department of Agriculture and Consumer Services, Ed.; Harbor Branch Oceanographic Institution: Fort Pierce, FL, USA, 1999. [Google Scholar]
- AVAMET. Associació Valenciana de Meteorologia Josep Peinado. AVAMET, 2017. Available online: www.avamet.org (accessed on 21 December 2017).
- Furtado, P.S.; Gaona, C.A.; Poersch, L.H.; Wasielesky, W. Application of different doses of calcium hydroxide in the farming shrimp Litopenaeus vannamei with the biofloc technology (BFT). Aquac. Int. 2014, 22, 1009–1023. [Google Scholar] [CrossRef]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W. Effect of calcium hydroxide, carbonate and sodium bicarbonate on water quality and zootechnical performance of shrimp Litopenaeus vannamei reared in bio-flocs technology (BFT) systems. Aquaculture 2011, 321, 130–135. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J. Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture 2003, 224, 193–201. [Google Scholar] [CrossRef]
- Wright, S.; Jeffrey, S.; Mantoura, R.; Llewellyn, C.; Bjornland, T.; Repeta, D.; Welschmeyer, N. Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar. Ecol. Prog. Ser. 1991, 77, 183–196. [Google Scholar] [CrossRef]
- Hooker, S.; Firestone, E.; Claustre, H.; Ras, J. The First SeaWiFS HPLC Analysis Round-Robin Experiment (SeaHARRE-1). NASA Technical Reports Server, 2001. Available online: https://ntrs.nasa.gov/search.jsp?R=20010072242 (accessed on 18 December 2017).
- Schveitzer, R.; Arantes, R.; Costódio, P.F.; Santo, C.M.; Arana, L.V.; Seiffert, W.Q.; Andreatta, E.R. Effect of different biofloc levels on microbial activity, water quality and performance of Litopenaeus vannamei in a tank system operated with no water exchange. Aquac. Eng. 2013, 56, 59–70. [Google Scholar] [CrossRef]
- Dodds, W.K.; Cole, J.J. Expanding the concept of trophic state in aquatic ecosystems: It’s not just the autotrophs. Aquat. Sci. 2007, 69, 427–439. [Google Scholar] [CrossRef]
- Maggioni, D.S.; Andreatta, E.R.; Hermes, E.M.; Barracco, M.A. Evaluation of some hemato-immunological parameters in female shrimp Litopenaeus vannamei submitted to unilateral eyestalk ablation in association with a diet supplemented with superdoses of ascorbic acid as a form of immunostimulation. Aquaculture 2004, 241, 501–515. [Google Scholar] [CrossRef]
- Li, K.; Zheng, T.; Tian, Y.; Xi, F.; Yuan, J.; Zhang, G.; Hong, H. Beneficial effects of Bacillus licheniformis on the intestinal microflora and immunity of the white shrimp, Litopenaeus vannamei. Biotechnol. Lett. 2007, 29, 525–530. [Google Scholar] [CrossRef]
- Macias-Sancho, J.; Poersch, L.H.; Bauer, W.; Romano, L.A.; Wasielesky, W.; Tesser, M.B. Fishmeal substitution with Arthrospira (Spirulina platensis) in a practical diet for Litopenaeus vannamei: Effects on growth and immunological parameters. Aquaculture 2014, 426, 120–125. [Google Scholar] [CrossRef]
- Souza, D.M.; Borges, V.D.; Furtado, P.; Romano, L.A.; Wasielesky, W.; Monserrat, J.M.; Garcia, L.D. Antioxidant enzyme activities and immunological system analysis of Litopenaeus vannamei reared in biofloc technology (BFT) at different water temperatures. Aquaculture 2016, 451, 436–443. [Google Scholar] [CrossRef]
- Ekasari, J.; Azhar, M.H.; Surawidjaja, E.H.; Nuryati, S.; Schryver, P.D.; Bossier, P. Immune response and disease resistance of shrimp fed biofloc grown on different carbon sources. Fish Shellfish Immunol. 2014, 41, 332–339. [Google Scholar] [CrossRef]
- Xu, W.J.; Pan, L.Q. Evaluation of dietary protein level on selected parameters of immune and antioxidant systems, and growth performance of juvenile Litopenaeus vannamei reared in zero-water exchange biofloc-based culture tanks. Aquaculture 2014, 426, 181–188. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1955, 193, 265–275. [Google Scholar]
- Gaona, C.A.P.; Poersch, L.H.; Krummenauer, D.; Foes, G.K.; Wasielesky, W.J.; Wasielesky, W. The effect of solids removal on water quality, growth and survival of Litopenaeus vannamei in a biofloc technology culture system. Int. J. Recirc. Aquac. 2011, 12, 1–12. [Google Scholar] [CrossRef]
- Ray, A.J. Study shows lower biofloc concentration may improve shrimp production. Glob. Aquac. Advocate 2012, 15, 28–31. [Google Scholar]
- Toledo, T.M.; Silva, B.C.; Vieira, F.N.; Mouriño, J.L.P.; Seiffert, W.Q. Effects of different dietary lipid levels and fatty acids profile in the culture of white shrimp Litopenaeus vannamei (Boone) in biofloc technology: Water quality, biofloc composition, growth and health. Aquac. Res. 2016, 47, 1841–1851. [Google Scholar] [CrossRef]
- Montero, J.I. Tendencias tecnológicas en los invernaderos mediterráneos. In Manejo Del Clima en el Invernadero Mediterráneo, 1st ed.; Sánchez-Guerrero, M.C., Alonso, F.J., Lorenzo, P., Medrano, E., Eds.; Intituto de Invetigación y Formación Agraria y Pesquera: Seville, Spain, 2009; pp. 115–127. [Google Scholar]
- Cheng, W.; Liu, C.; Kuo, C. Effects of dissolved oxygen on hemolymph parameters of freshwater giant prawn, Macrobrachium rosenbergii (de Man). Aquaculture 2003, 220, 843–856. [Google Scholar] [CrossRef]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W. The effects of different alkalinity levels on Litopenaeus vannamei reared with biofloc Technology (BFT). Aquac. Int. 2015, 23, 345–358. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J. Acute toxicity of ammonia on Litopenaeus vannamei (Boone) juveniles at different salinity levels. J. Exp. Mar. Biol. Ecol. 2001, 259, 109–119. [Google Scholar] [CrossRef]
- Ray, A.J.; Lewis, B.L.; Browdy, C.L.; Leffler, J.W. Suspended solids removal to improve shrimp (Litopenaeus vannamei) production and an evaluation of a plant-based feed in minimal-exchange, superintensive culture systems. Aquaculture 2010, 299, 89–98. [Google Scholar] [CrossRef]
- Correia, E.S.; Wilkenfeld, J.S.; Morris, T.C.; Wei, L.; Prangnell, D.I.; Samocha, T.M. Intensive nursery production of the Pacific white shrimp Litopenaeus vannamei using two commercial feeds with high and low protein content in a biofloc-dominated system. Aquac. Eng. 2014, 59, 48–54. [Google Scholar] [CrossRef]
- Samocha, T.M.; Patnaik, S.; Speed, M.; Ali, A.; Burger, J.M.; Almeida, R.V.; Ayub, Z.; Harisanto, M.; Horowitz, A.; Brock, D.L. Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquac. Eng. 2007, 36, 184–191. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Z.; Dai, X.; Avnimelech, Y. Effects of addition of maize starch on the yield, water quality and formation of bioflocs in an integrated shrimp culture system. Aquaculture 2014, 418, 79–86. [Google Scholar] [CrossRef]
- Martins, T.G.; Odebrecht, C.; Jensen, L.V.; D’Oca, M.G.; Wasielesky, W. The contribution of diatoms to bioflocs lipid content and the performance of juvenile Litopenaeus vannamei (Boone, 1931) in a BFT culture system. Aquac. Res. 2016, 47, 1315–1326. [Google Scholar] [CrossRef]
- Emerenciano, M.; Cuzon, G.; Arévalo, M.; Gaxiola, G. Biofloc technology in intensive broodstock farming of the pink shrimp Farfantepenaeus duorarum: Spawning performance, biochemical composition and fatty acid profile of eggs. Aquac. Res. 2014, 45, 1713–1726. [Google Scholar] [CrossRef]
- Schrader, K.K.; Green, B.W.; Perschbacher, P.W. Development of phytoplankton communities and common off-flavors in a biofloc technology system used for the culture of channel catfish (Ictalurus punctatus). Aquac. Eng. 2011, 45, 118–126. [Google Scholar] [CrossRef]
- Llario, F.; Rodilla, M.; Escrivá, J.; Falco, S.; Sebastiá-Frasquet, M.T. Phytoplankton evolution during the creation of a biofloc system for shrimp culture. Int. J. Environ. Sci. Technol. 2019, 16, 211–222. [Google Scholar] [CrossRef]
- Vinatea, L.; Gálvez, A.O.; Browdy, C.L.; Stokes, A.; Venero, J.; Haveman, J.; Lewis, B.L.; Lawson, A.; Shuler, A.; Leffler, J.W. Photosynthesis, water respiration and growth performance of Litopenaeus vannamei in a super-intensive raceway culture with zero water exchange: Interaction of water quality variables. Aquac. Eng. 2010, 42, 17–24. [Google Scholar] [CrossRef]
- Vilani, F.G.; Schveitzer, R.; Fonseca-Arantes, R.; Vieira, F.; Espírito Santo, C.M.; Seiffert, W.Q. Strategies for water preparation in a biofloc system: Effects of carbon source and fertilization dose on water quality and shrimp performance. Aquac. Eng. 2016, 74, 70–75. [Google Scholar] [CrossRef]
- Krummenauer, D.; Peixoto, S.; Cavalli, R.O.; Poersch, L.H.; Wasielesky, W. Superintensive culture of white shrimp, Litopenaeus vannamei, in a biofloc technology system in southern Brazil at different stocking densities. J. World Aquac. Soc. 2011, 42, 726–733. [Google Scholar] [CrossRef]
- Ray, A.J.; Dillon, K.S.; Lotz, J.M. Water quality dynamics and shrimp (Litopenaeus vannamei) production in intensive, mesohaline culture systems with two levels of biofloc management. Aquac. Eng. 2011, 45, 127–136. [Google Scholar] [CrossRef]
- Baloi, M.; Arantes, R.; Schveitzer, R.; Magnotti, C.; Vinatea, L. Performance of Pacific white shrimp Litopenaeus vannamei raised in biofloc systems with varying levels of light exposure. Aquac. Eng. 2013, 52, 39–44. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M. Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morphology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquac. Int. 2015, 23, 203–217. [Google Scholar] [CrossRef]
- Johansson, M.W.; Keyser, P.; Sritunyalucksana, K.; Söderhäll, K. Crustacean haemocytes and haematopoiesis. Aquaculture 2000, 191, 45–52. [Google Scholar] [CrossRef]
- Cuéllar-Anjel, J. Métodos de diagnósticos de enfermedades en camarones marinos de cultivo. In Guía Técnica: Patología e Inmunología de Camarones Peneidos, 1st ed.; Morales, V., Cuéllar-Anjel, J., Eds.; Programa CYTED Red II-D Vannamei: City of Panama, Panama, 2008; pp. 1–54. [Google Scholar]
- Guo, H.; Xian, J.A.; Li, B.; Ye, C.X.; Wang, A.L.; Miao, Y.T.; Liao, S.A. Gene expression of apoptosis-related genes, stress protein and antioxidant enzymes in hemocytes of white shrimp Litopenaeus vannamei under nitrite stress. Comp. Biochem. Phys. C 2013, 157, 366–371. [Google Scholar] [CrossRef]




| High Bacteria Treatment | Low Bacteria Treatment | Control Treatment | |
|---|---|---|---|
| pH | 7.77 ± 0.39 | 7.74 ± 0.43 | 7.74 ± 0.40 |
| (n = 450) | (n = 450) | (n = 450) | |
| Alkalinity (mg CaCO3/L) | 108.89 ± 30.05 | 96.70 ± 27.29 | 101.56 ± 27.92 |
| (n = 42) | (n = 42) | (n = 42) | |
| Dissolved oxygen (mg/L) | 5.88 ± 0.48 | 5.97 ± 0.37 | 5.95 ± 0.38 |
| (n = 507) | (n = 507) | (n = 507) | |
| Dissolved oxygen (%) | 91.4 ± 8.38 | 93.0 ± 6.66 | 93.7 ± 7.73 |
| (n = 507) | (n = 507) | (n = 507) | |
| Salinity (g/L) | 22.5 ± 0.08 | 22.5 ± 0.08 | 22.5 ± 0.07 |
| (n = 498) | (n = 498) | (n = 498) |
| High Bacteria Treatment | Low Bacteria Treatment | Control Treatment | p | |
|---|---|---|---|---|
| Weight gain (g) | 16.59 ± 1.15 | 17.41 ± 1.42 | 17.76 ± 0.65 | 0.33 |
| (n = 3) | (n = 3) | (n = 3) | ||
| Weekly weight gain (g/week) | 0.69 ± 0.01 | 0.72 ± 0.06 | 0.73 ± 0.03 | 0.33 |
| (n = 3) | (n = 3) | (n = 3) | ||
| Biomass production (ton/m2) | 21.74 ± 1.83 | 23.00 ± 2.67 | 21.07 ± 2.03 | 0.58 |
| (n = 3) | (n = 3) | (n = 3) | ||
| FCR | 1.91 ± 0.11 | 1.81 ± 0.15 | 1.96 ± 0.21 | 0.53 |
| (n = 3) | (n = 3) | (n = 3) | ||
| Survival (%) | 65.77 ± 4.97 | 66.19 ± 3.48 | 59.53 ± 4.92 | 0.21 |
| (n = 3) | (n = 3) | (n = 3) |
| Immature Biofloc System (day 86) | |||
| High Bacteria Treatment | Low Bacteria Treatment | Control Treatment | |
| TPC (mg/mL) | 93.18 ± 3.99 a | 89.49 ± 21.16 a | 82.58 ± 10.10 a |
| (n = 3) | (n = 3) | (n = 3) | |
| GH (%) | 30.00 ± 1.40 a | 31.33 ± 3.58 a | 25.13 ± 0.99 b |
| (n = 3) | (n = 3) | (n = 3) | |
| HH (%) | 70.00 ± 1.40 a | 68.67 ± 3.58 a | 74.87 ± 0.99 b |
| (n = 3) | (n = 3) | (n = 3) | |
| Mature Biofloc System (day 169) | |||
| High Bacteria Treatment | Low Bacteria Treatment | Control Treatment | |
| TPC (mg/mL) | 123.14 ± 8.82 a | 120.62 ± 12.26 a | 117.75 ± 25.60 a |
| (n = 3) | (n = 3) | (n = 3) | |
| GH (%) | 46.66 ± 2.80 a | 46.28 ± 2.89 a | 39.03 ± 3.89 b |
| (n = 3) | (n = 3) | (n = 3) | |
| HH (%) | 53.34 ± 2.80 a | 53.72 ± 2.89 a | 60.97 ± 2.89 b |
| (n = 3) | (n = 3) | (n = 3) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llario, F.; Falco, S.; Sebastiá-Frasquet, M.-T.; Escrivá, J.; Rodilla, M.; Poersch, L.H. The Role of Bacillus amyloliquefaciens on Litopenaeus vannamei During the Maturation of a Biofloc System. J. Mar. Sci. Eng. 2019, 7, 228. https://doi.org/10.3390/jmse7070228
Llario F, Falco S, Sebastiá-Frasquet M-T, Escrivá J, Rodilla M, Poersch LH. The Role of Bacillus amyloliquefaciens on Litopenaeus vannamei During the Maturation of a Biofloc System. Journal of Marine Science and Engineering. 2019; 7(7):228. https://doi.org/10.3390/jmse7070228
Chicago/Turabian StyleLlario, Ferran, Silvia Falco, María-Teresa Sebastiá-Frasquet, Julia Escrivá, Miguel Rodilla, and Luís Henrique Poersch. 2019. "The Role of Bacillus amyloliquefaciens on Litopenaeus vannamei During the Maturation of a Biofloc System" Journal of Marine Science and Engineering 7, no. 7: 228. https://doi.org/10.3390/jmse7070228
APA StyleLlario, F., Falco, S., Sebastiá-Frasquet, M.-T., Escrivá, J., Rodilla, M., & Poersch, L. H. (2019). The Role of Bacillus amyloliquefaciens on Litopenaeus vannamei During the Maturation of a Biofloc System. Journal of Marine Science and Engineering, 7(7), 228. https://doi.org/10.3390/jmse7070228






