Mapping the Landscape of Marine Giant Virus Research: A Scientometric Perspective (1996–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Selection Criteria
2.2. Bibliometric Analysis
3. Results
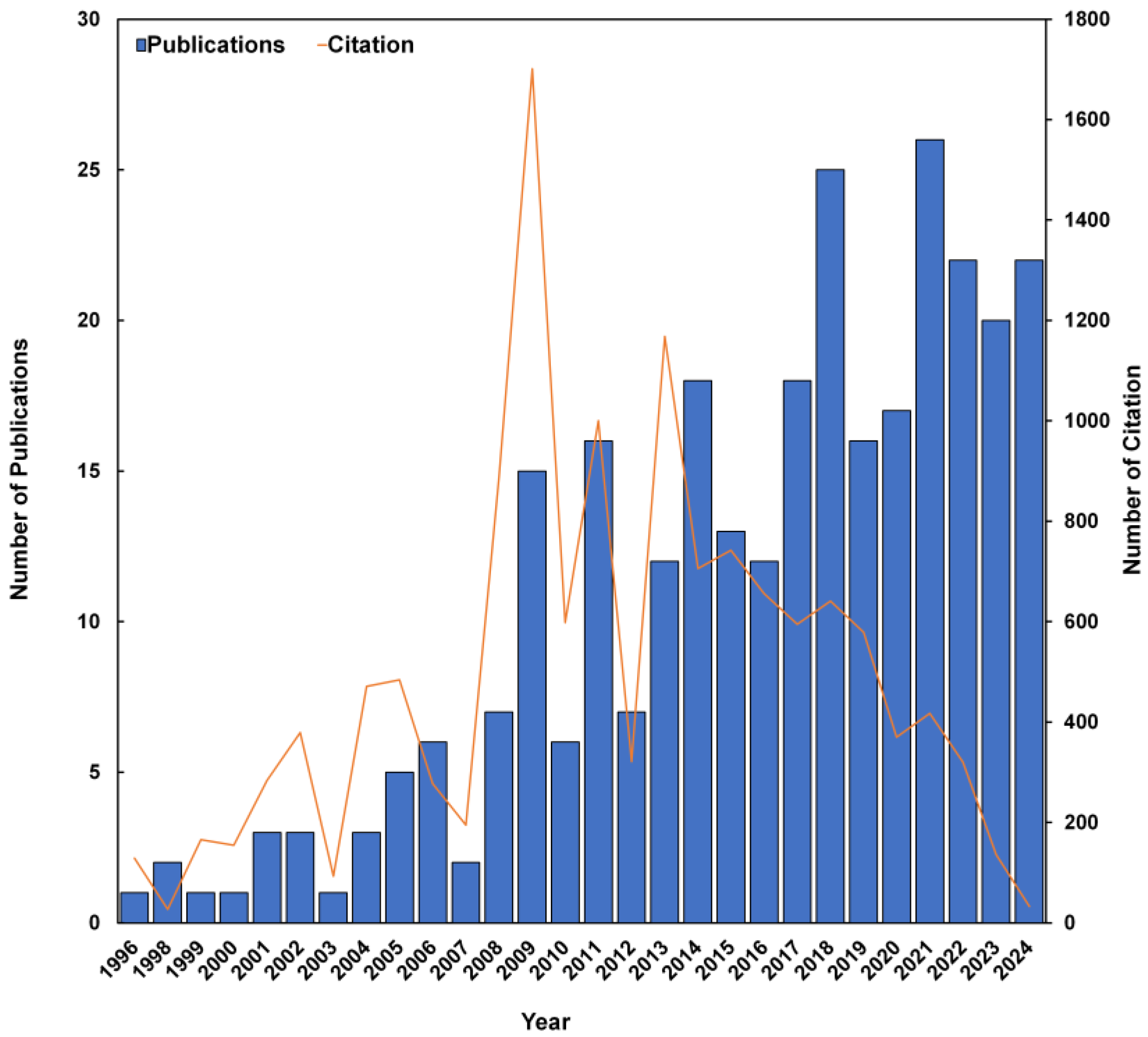
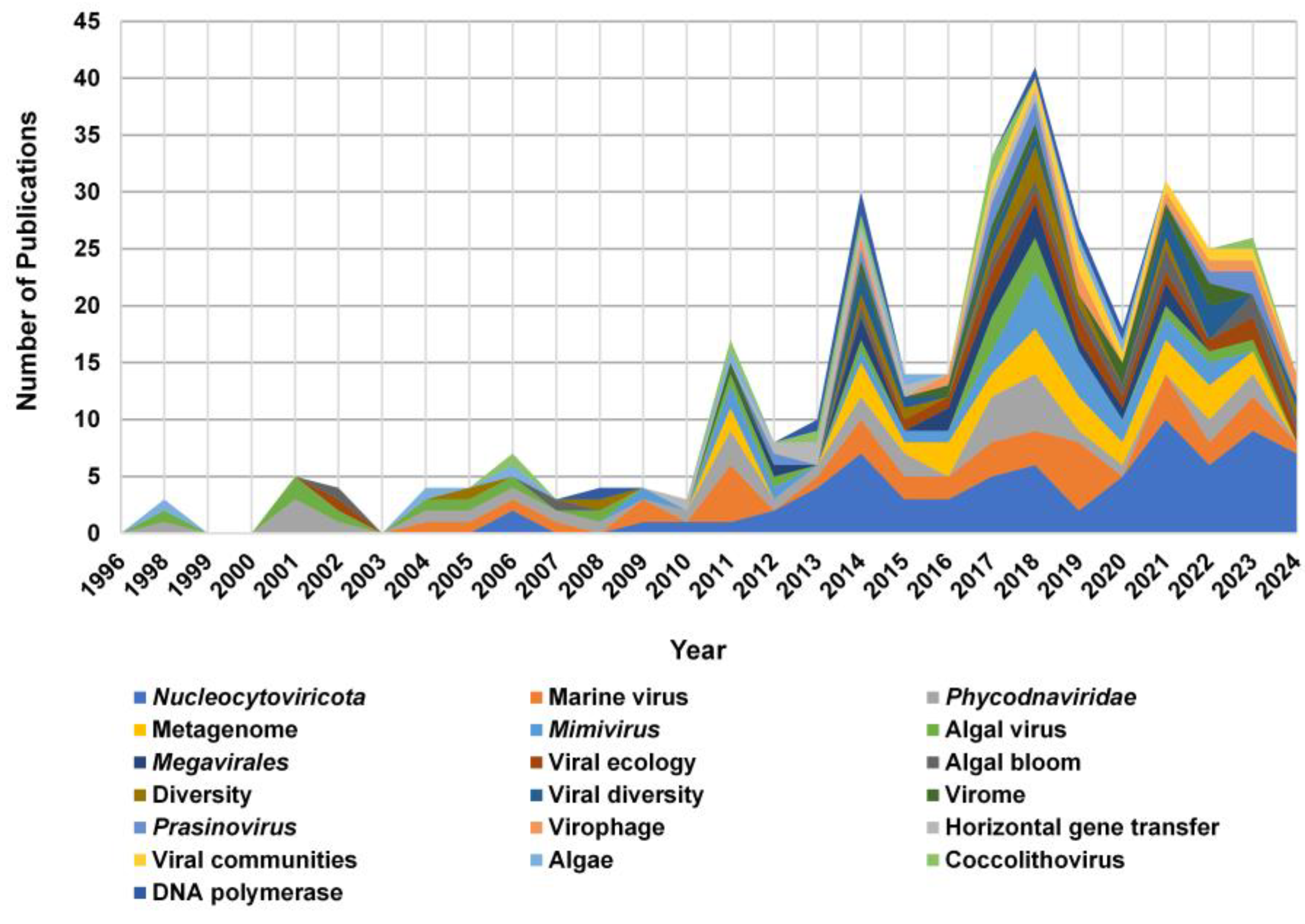
3.1. Annual Trends in Publications and Citations
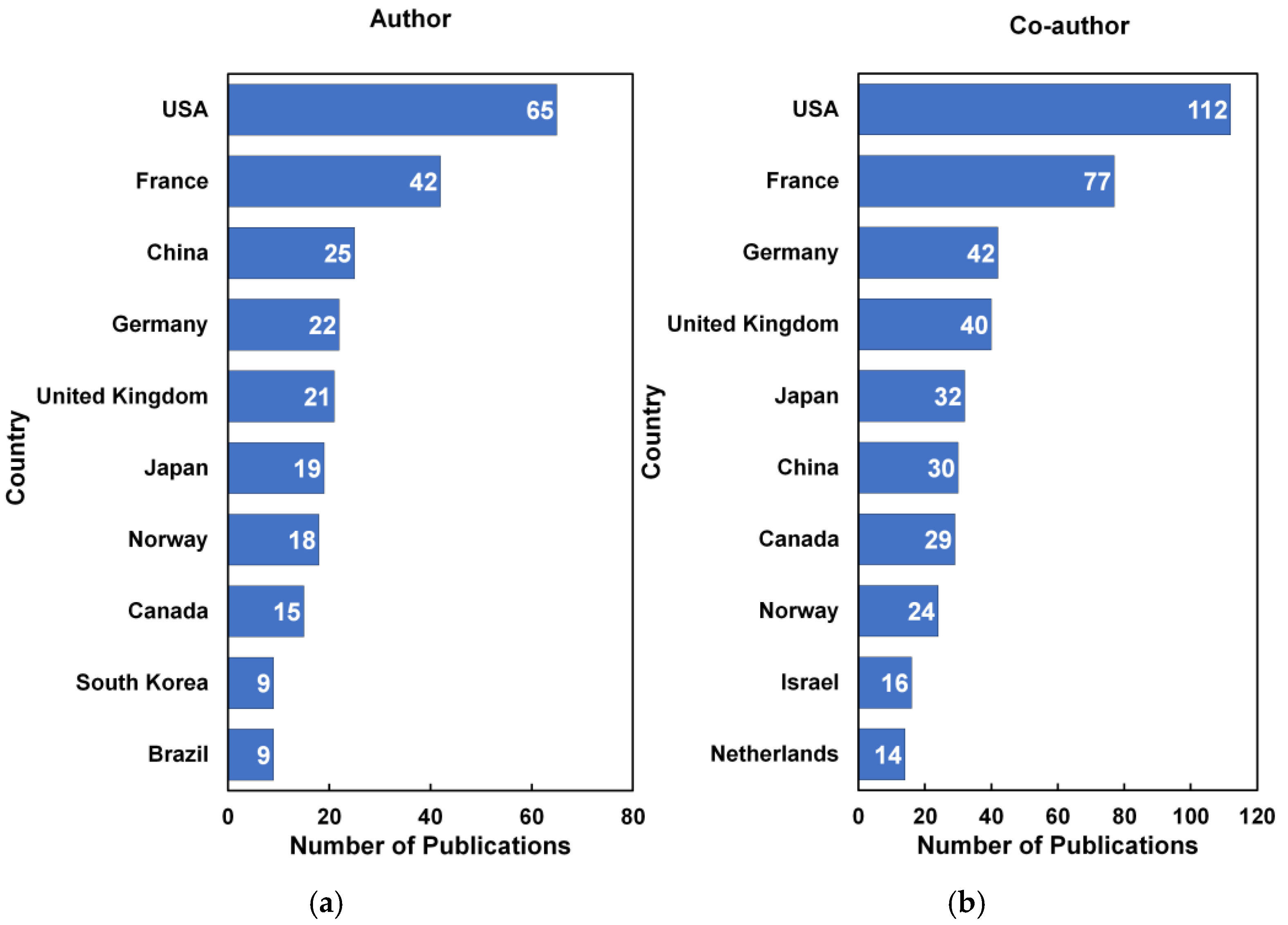
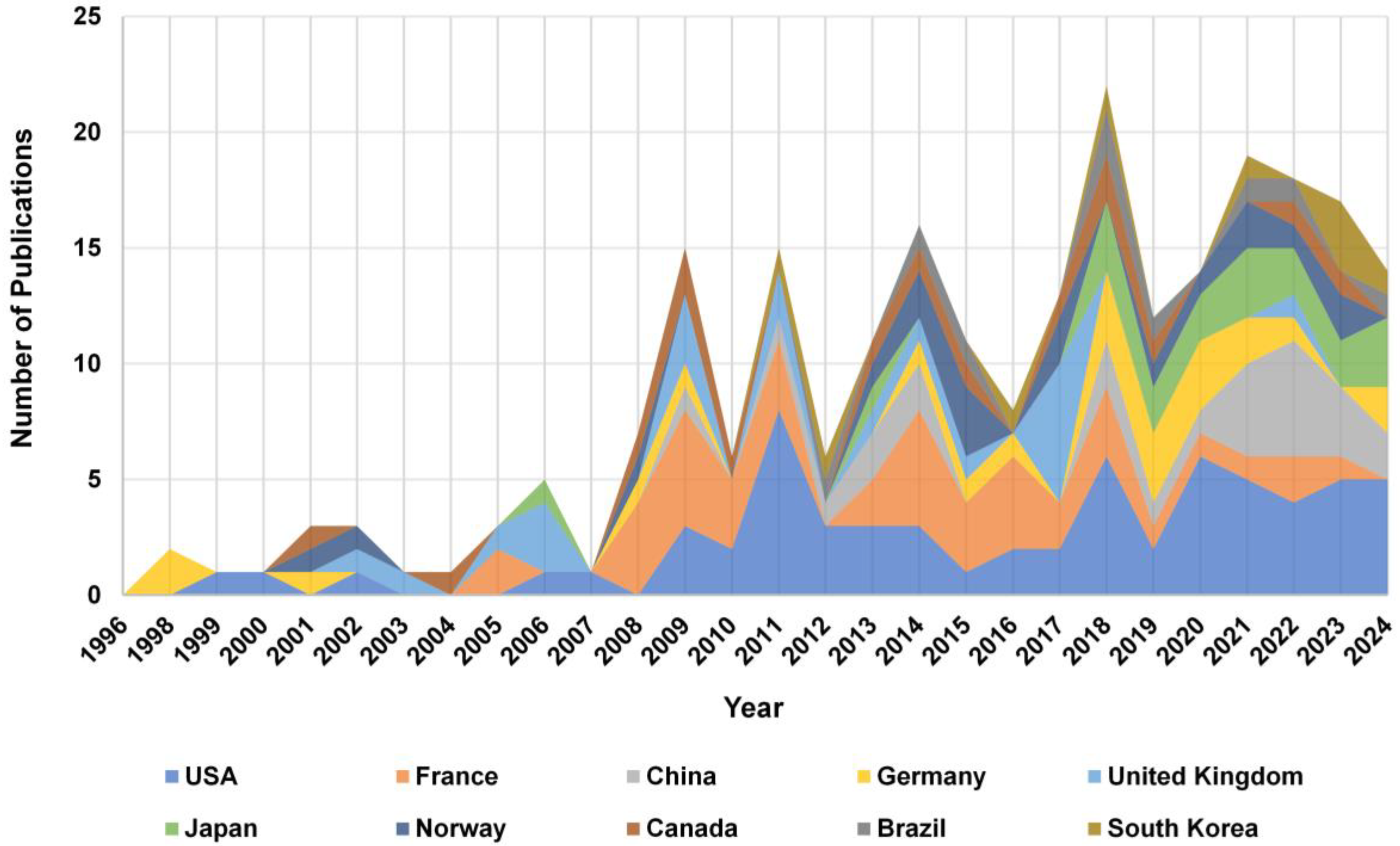
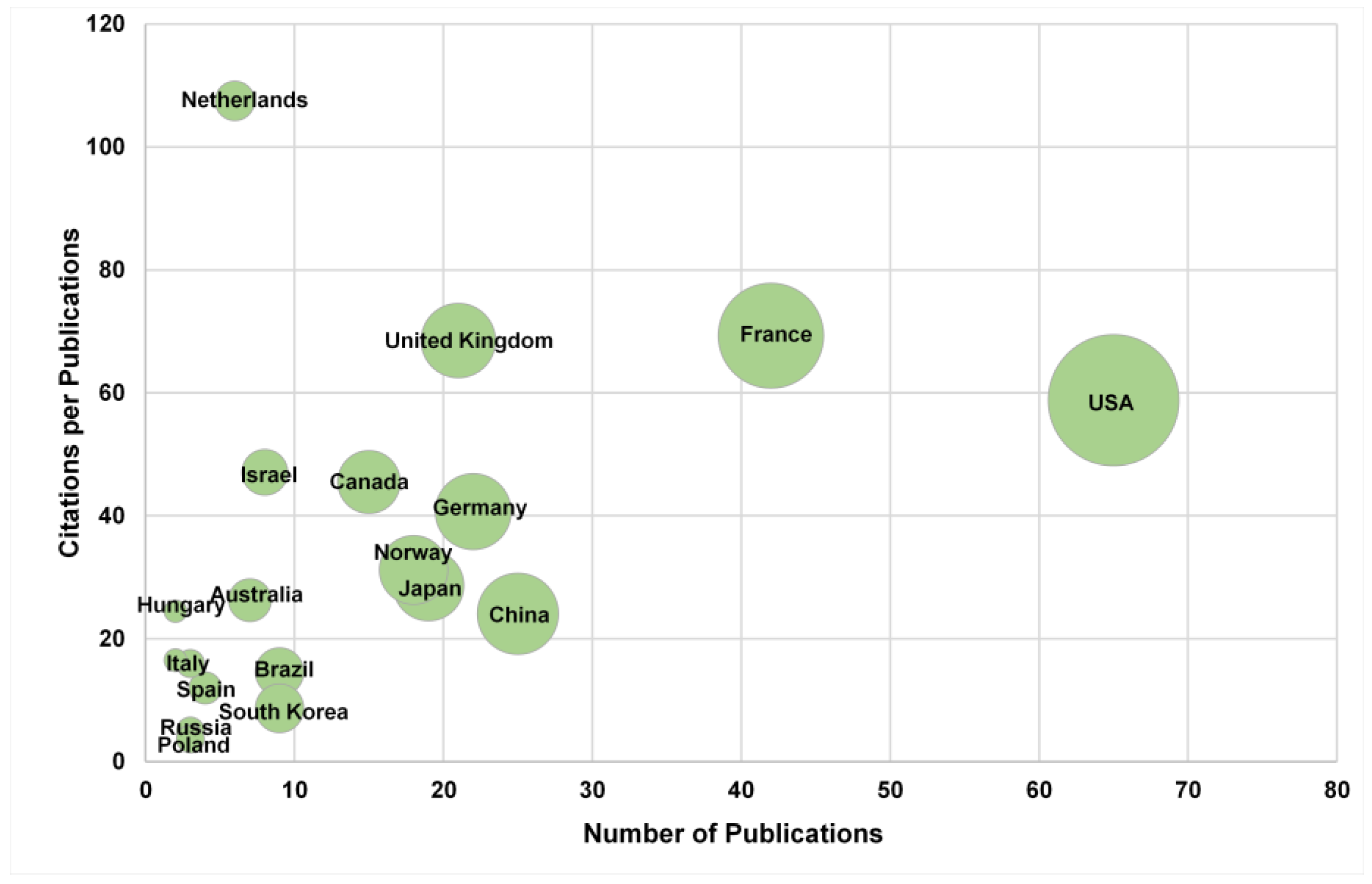
3.2. Citation Analysis of National Contributions to Research
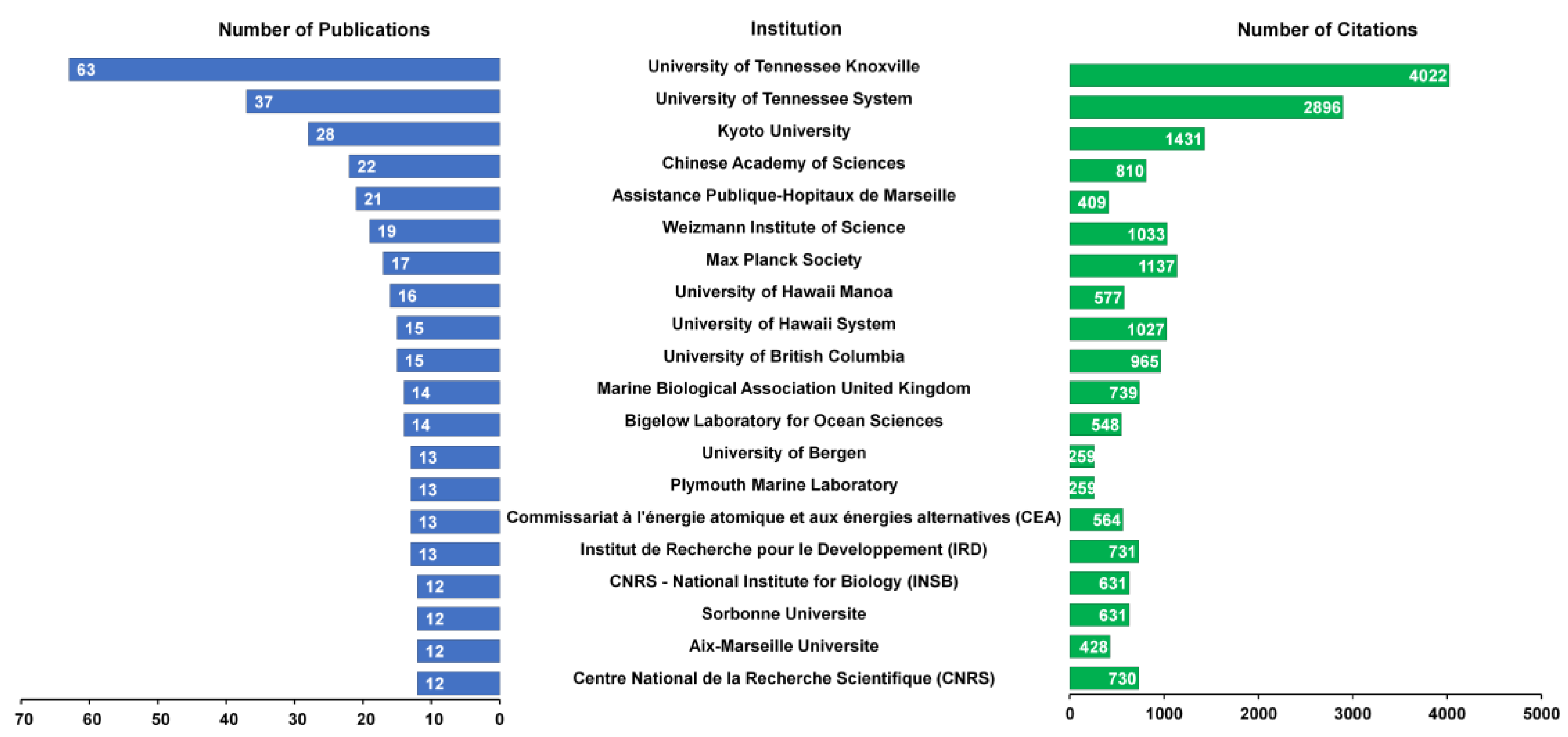
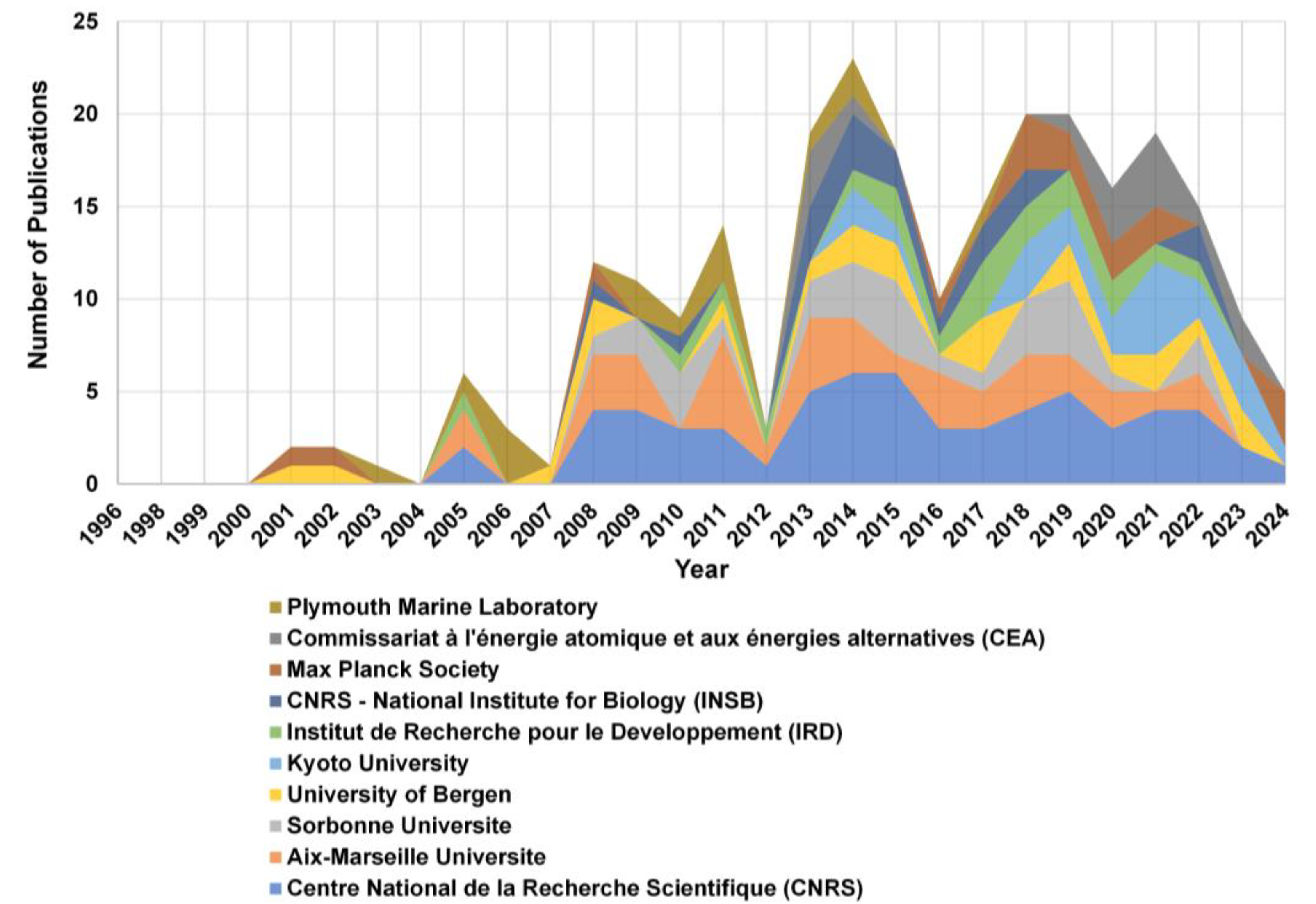
3.3. Institutional Contributions to MGV Research
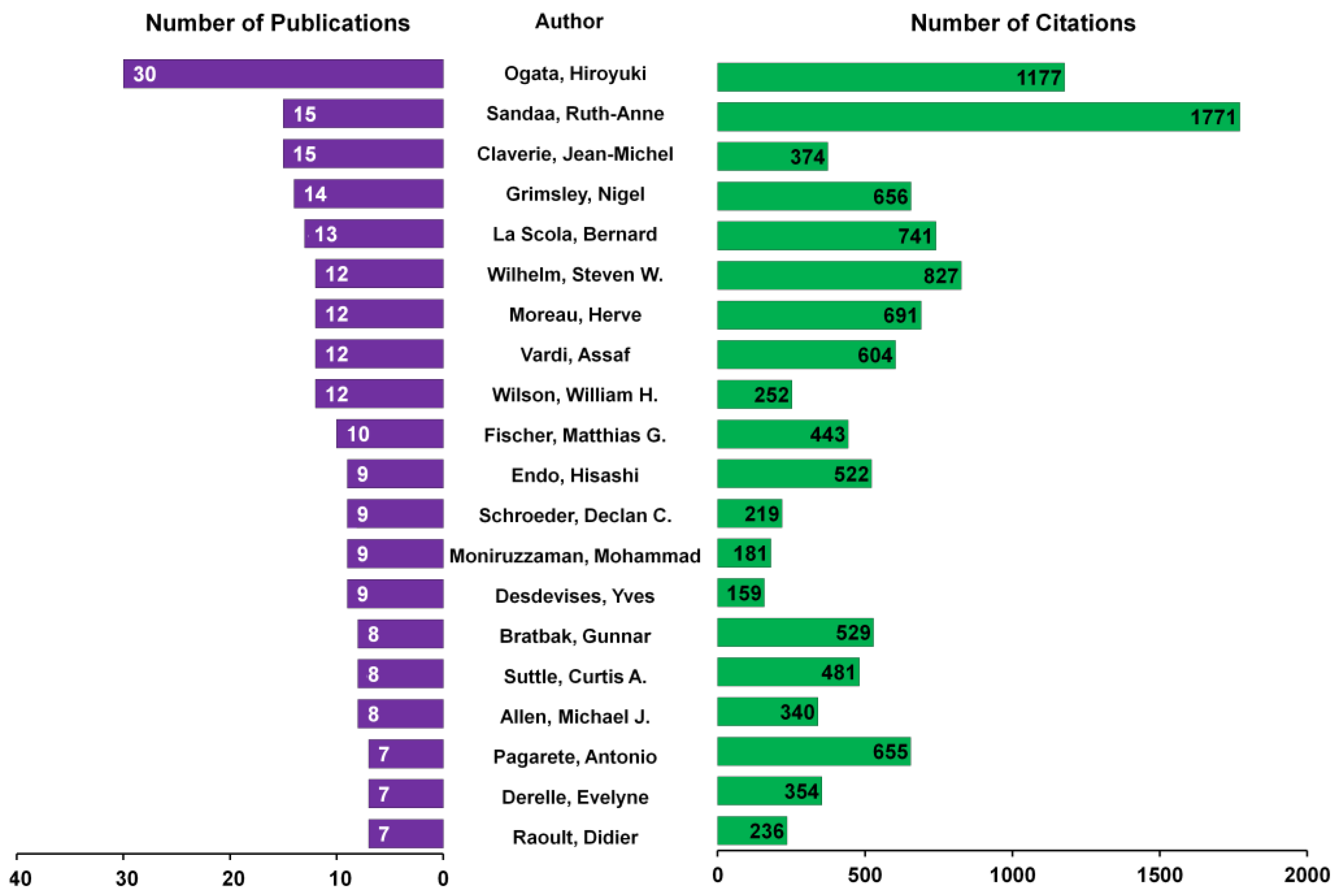
3.4. Analysis of Key Researchers
3.5. Number of MGV Publications by Web of Science Categories
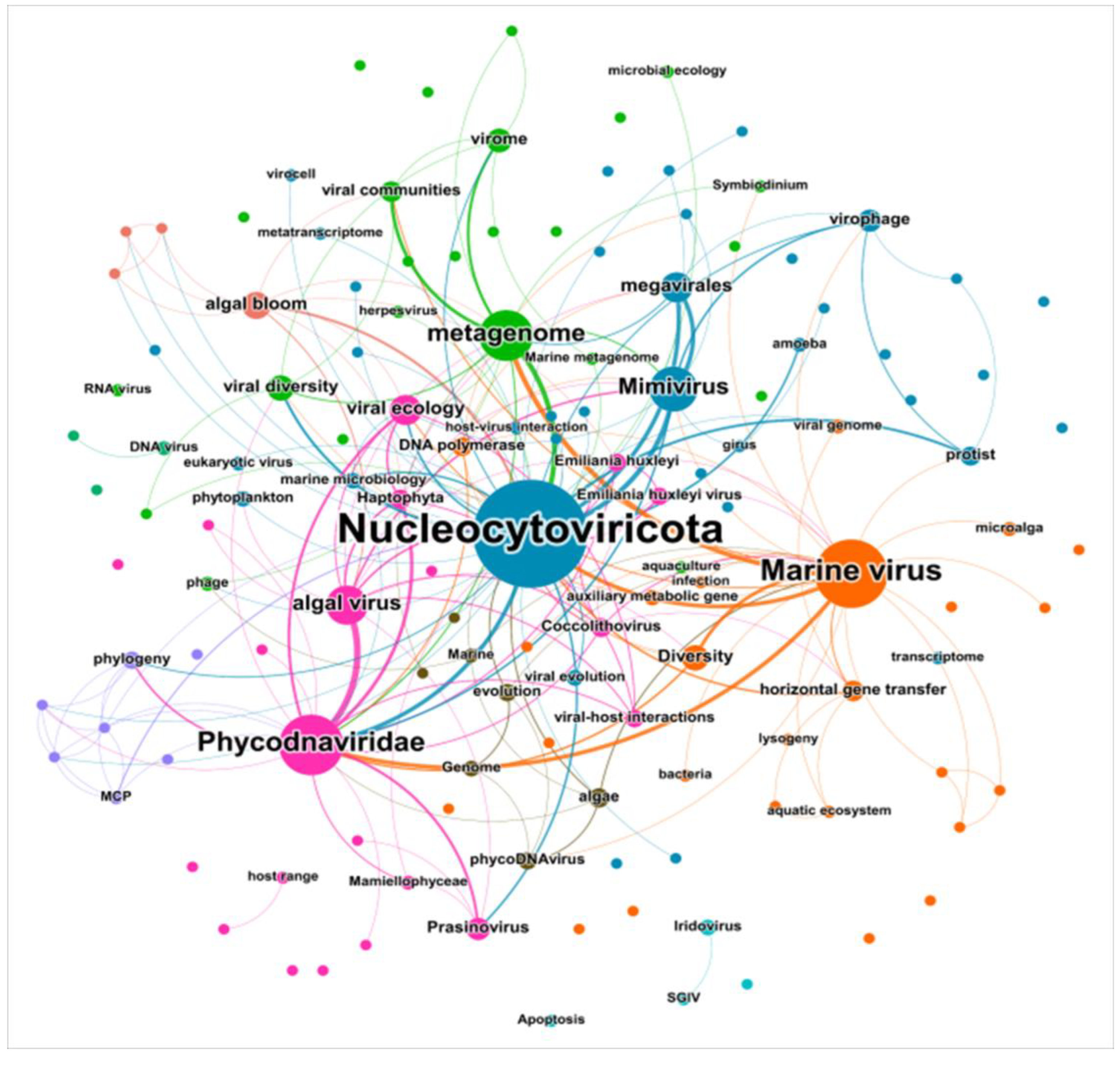
3.6. Analysis of Author Keywords
4. Discussion
4.1. Research Development Stages and Technological Advances by Year
4.2. Citation Analysis and Key Publications
4.3. Research Trends by Country and Institution
4.4. Evolution of Key Themes: Function, Interaction, and Environmental Integration
4.5. Future Perspectives
4.6. Limitations
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMG | Auxiliary Metabolic Gene |
| CNRS | Centre National de la Recherche Scientifique |
| CEA | Commissariat à l’énergie atomique et aux énergies alternatives |
| GOV2.0 | Global Ocean Virome 2.0 |
| IRD | Institut de Recherche pour le Développement |
| CNRS-INSB | Institut des Sciences Biologiques of the French National Centre for Scientific Research |
| MIT | Massachusetts Institute of Technology |
| MGVs | Marine Giant Virus |
| NCLDV | Nucleocytoplasmic Large DNA Virus |
| WoS | Web of Science |
| WoSCC | Web of Science Core Collection |
References
- Costello, M.J.; Chaudhary, C. Marine Biodiversity, Biogeography, Deep-Sea Gradients, and Conservation. Curr. Biol. 2017, 27, R511–R527. [Google Scholar] [CrossRef]
- Lara, E.; Vaqué, D.; Sà, E.L.; Boras, J.A.; Gomes, A.; Borrull, E.; Díez-Vives, C.; Teira, E.; Pernice, M.C.; Garcia, F.C.; et al. Unveiling the role and life strategies of viruses from the surface to the dark ocean. Sci. Adv. 2017, 3, e1602565. [Google Scholar] [CrossRef]
- Shiah, F.-K.; Lai, C.-C.; Chen, T.-Y.; Ko, C.-Y.; Tai, J.-H.; Chang, C.-W. Viral shunt in tropical oligotrophic ocean. Sci. Adv. 2022, 8, eabo2829. [Google Scholar] [CrossRef]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A.; Schwalbach, M. Viral influence on aquatic bacterial communities. Biol. Bull. 2003, 204, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.G. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef]
- Ku, C. Giant virus-eukaryote interactions as ecological and evolutionary driving forces. mSystems 2021, 6, 10.1128. [Google Scholar]
- Farzad, R.; Ha, A.D.; Aylward, F.O. Diversity and genomics of giant viruses in the North Pacific Subtropical Gyre. Front. Microbiol. 2022, 13, 1021923. [Google Scholar] [CrossRef]
- Fang, Y.; Meng, L.; Xia, J.; Gotoh, Y.; Hayashi, T.; Nagasaki, K.; Endo, H.; Okazaki, Y.; Ogata, H. Genome-resolved year-round dynamics reveal a broad range of giant virus microdiversity. mSystems 2025, 10, e01168-24. [Google Scholar]
- Endo, H.; Blanc-Mathieu, R.; Li, Y.; Salazar, G.; Henry, N.; Labadie, K.; de Vargas, C.; Sullivan, M.B.; Bowler, C.; Wincker, P.; et al. Biogeography of marine giant viruses reveals their interplay with eukaryotes and ecological functions. Nat. Ecol. Evol. 2020, 4, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.C.; Zayed, A.A.; Conceição-Neto, N.; Temperton, B.; Bolduc, B.; Alberti, A.; Ardyna, M.; Arkhipova, K.; Carmichael, M.; Cruaud, C.; et al. Marine DNA Viral Macro- and Microdiversity from Pole to Pole. Cell 2019, 177, 1109–1123.e14. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.A.; Wainaina, J.M.; Dominguez-Huerta, G.; Pelletier, E.; Guo, J.; Mohssen, M.; Tian, F.; Pratama, A.A.; Bolduc, B.; Zablocki, O.; et al. Cryptic and abundant marine viruses at the evolutionary origins of Earth’s RNA virome. Science 2022, 376, 156–162. [Google Scholar] [CrossRef]
- Scola, B.L.; Audic, S.; Robert, C.; Jungang, L.; De Lamballerie, X.; Drancourt, M.; Birtles, R.; Claverie, J.-M.; Raoult, D. A giant virus in amoebae. Science 2003, 299, 2033. [Google Scholar] [CrossRef] [PubMed]
- Philippe, N.; Legendre, M.; Doutre, G.; Couté, Y.; Poirot, O.; Lescot, M.; Arslan, D.; Seltzer, V.; Bertaux, L.; Bruley, C. Pandoraviruses: Amoeba viruses with genomes up to 2.5 Mb reaching that of parasitic eukaryotes. Science 2013, 341, 281–286. [Google Scholar] [CrossRef]
- Legendre, M.; Bartoli, J.; Shmakova, L.; Jeudy, S.; Labadie, K.; Adrait, A.; Lescot, M.; Poirot, O.; Bertaux, L.; Bruley, C. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proc. Natl. Acad. Sci. USA 2014, 111, 4274–4279. [Google Scholar]
- Arslan, D.; Legendre, M.; Seltzer, V.; Abergel, C.; Claverie, J.-M. Distant Mimivirus relative with a larger genome highlights the fundamental features of Megaviridae. Proc. Natl. Acad. Sci. USA 2011, 108, 17486–17491. [Google Scholar]
- Fischer, M.G.; Allen, M.J.; Wilson, W.H.; Suttle, C.A. Giant virus with a remarkable complement of genes infects marine zooplankton. Proc. Natl. Acad. Sci. USA 2010, 107, 19508–19513. [Google Scholar] [CrossRef] [PubMed]
- Van Etten, J.L.; Dunigan, D.D. Chloroviruses: Not your everyday plant virus. Trends Plant Sci. 2012, 17, 1–8. [Google Scholar] [CrossRef]
- Weynberg, K.D.; Allen, M.J.; Wilson, W.H. Marine Prasinoviruses and Their Tiny Plankton Hosts: A Review. Viruses 2017, 9, 43. [Google Scholar] [CrossRef]
- Allen, M.J.; Schroeder, D.C.; Donkin, A.; Crawfurd, K.J.; Wilson, W.H. Genome comparison of two Coccolithoviruses. Virol. J. 2006, 3, 15. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Martinez-Gutierrez, C.A.; Weinheimer, A.R.; Aylward, F.O. Dynamic genome evolution and complex virocell metabolism of globally-distributed giant viruses. Nat. Commun. 2020, 11, 1710. [Google Scholar] [CrossRef]
- Laber, C.P.; Hunter, J.E.; Carvalho, F.; Collins, J.R.; Hunter, E.J.; Schieler, B.M.; Boss, E.; More, K.; Frada, M.; Thamatrakoln, K.; et al. Coccolithovirus facilitation of carbon export in the North Atlantic. Nat. Microbiol. 2018, 3, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Yoshizawa, S.; Hosaka, T.; Poirier, C.; Choi, C.J.; Hehenberger, E.; Irwin, N.A.T.; Wilken, S.; Yung, C.-M.; Bachy, C.; et al. A distinct lineage of giant viruses brings a rhodopsin photosystem to unicellular marine predators. Proc. Natl. Acad. Sci. USA 2019, 116, 20574–20583. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Erazo Garcia, M.P.; Farzad, R.; Ha, A.D.; Jivaji, A.; Karki, S.; Sheyn, U.; Stanton, J.; Minch, B.; Stephens, D.; et al. Virologs, viral mimicry, and virocell metabolism: The expanding scale of cellular functions encoded in the complex genomes of giant viruses. FEMS Microbiol. Rev. 2023, 47, fuad053. [Google Scholar] [CrossRef] [PubMed]
- La Scola, B.; Desnues, C.; Pagnier, I.; Robert, C.; Barrassi, L.; Fournous, G.; Merchat, M.; Suzan-Monti, M.; Forterre, P.; Koonin, E. The virophage as a unique parasite of the giant mimivirus. Nature 2008, 455, 100–104. [Google Scholar] [CrossRef]
- Hingamp, P.; Grimsley, N.; Acinas, S.G.; Clerissi, C.; Subirana, L.; Poulain, J.; Ferrera, I.; Sarmento, H.; Villar, E.; Lima-Mendez, G.; et al. Exploring nucleo-cytoplasmic large DNA viruses in Tara Oceans microbial metagenomes. Int. Soc. Microb. Ecol. 2013, 7, 1678–1695. [Google Scholar] [CrossRef]
- Roux, S.; Brum, J.R.; Dutilh, B.E.; Sunagawa, S.; Duhaime, M.B.; Loy, A.; Poulos, B.T.; Solonenko, N.; Lara, E.; Poulain, J.; et al. Ecogenomics and potential biogeochemical impacts of globally abundant ocean viruses. Nature 2016, 537, 689–693. [Google Scholar] [CrossRef]
- Minch, B.; Moniruzzaman, M. Expansion of the genomic and functional diversity of global ocean giant viruses. NPJ Viruses 2025, 3, 32. [Google Scholar]
- Gilbert, N.E.; LeCleir, G.R.; Pound, H.L.; Strzepek, R.F.; Ellwood, M.J.; Twining, B.S.; Roux, S.; Boyd, P.W.; Wilhelm, S.W. Giant Virus Infection Signatures Are Modulated by Euphotic Zone Depth Strata and Iron Regimes of the Subantarctic Southern Ocean. mSystems 2023, 8, e01260-22. [Google Scholar] [CrossRef]
- Tokarz-Deptuła, B.; Chrzanowska, S.; Gurgacz, N.; Stosik, M.; Deptuła, W. Virophages—Known and Unknown Facts. Viruses 2023, 15, 1321. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Li, Z. Bibliometric Analysis of Research Hotspots Related to Viruses in The Environmental Field Based on the Web of Science. Pol. J. Environ. Stud. 2024, 33, 4837–4849. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar]
- Zupic, I.; Čater, T. Bibliometric methods in management and organization. Organ. Res. Methods 2015, 18, 429–472. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Hood, W.W.; Wilson, C.S. The Literature of Bibliometrics, Scientometrics, and Informetrics. Scientometrics 2001, 52, 291–314. [Google Scholar] [CrossRef]
- Barabási, A.L.; Jeong, H.; Néda, Z.; Ravasz, E.; Schubert, A.; Vicsek, T. Evolution of the social network of scientific collaborations. Phys. A Stat. Mech. Its Appl. 2002, 311, 590–614. [Google Scholar] [CrossRef]
- Cobo, M.J.; López-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. Science mapping software tools: Review, analysis, and cooperative study among tools. J. Am. Soc. Inf. Sci. Technol. 2011, 62, 1382–1402. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Brussaard, C.P.; Short, S.M.; Frederickson, C.M.; Suttle, C.A. Isolation and phylogenetic analysis of novel viruses infecting the phytoplankton Phaeocystis globosa (Prymnesiophyceae). Appl. Environ. Microbiol. 2004, 70, 3700–3705. [Google Scholar] [CrossRef]
- Raoult, D.; Audic, S.; Robert, C.; Abergel, C.; Renesto, P.; Ogata, H.; La Scola, B.; Suzan, M.; Claverie, J.-M. The 1.2-megabase genome sequence of Mimivirus. Science 2004, 306, 1344–1350. [Google Scholar] [CrossRef]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef]
- Guglielmini, J.; Woo, A.C.; Krupovic, M.; Forterre, P.; Gaia, M. Diversification of giant and large eukaryotic dsDNA viruses predated the origin of modern eukaryotes. Proc. Natl. Acad. Sci. USA 2019, 116, 19585–19592. [Google Scholar]
- Aylward, F.O.; Moniruzzaman, M.; Ha, A.D.; Koonin, E.V. A phylogenomic framework for charting the diversity and evolution of giant viruses. PLoS Biol. 2021, 19, e3001430. [Google Scholar]
- Coutinho, F.H.; Silveira, C.B.; Gregoracci, G.B.; Thompson, C.C.; Edwards, R.A.; Brussaard, C.P.D.; Dutilh, B.E.; Thompson, F.L. Marine viruses discovered via metagenomics shed light on viral strategies throughout the oceans. Nat. Commun. 2017, 8, 15955. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, F.; Thurber, R.V. Viruses manipulate the marine environment. Nature 2009, 459, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Arslan, D.; Abergel, C.; Claverie, J.-M. Genomics of Megavirus and the elusive fourth domain of Life. Commun. Integr. Biol. 2012, 5, 102–106. [Google Scholar] [CrossRef]
- Abergel, C.; Legendre, M.; Claverie, J.-M. The rapidly expanding universe of giant viruses: Mimivirus, Pandoravirus, Pithovirus and Mollivirus. FEMS Microbiol. Rev. 2015, 39, 779–796. [Google Scholar] [CrossRef]
- Sullivan, M.B.; Weitz, J.S.; Wilhelm, S. Viral ecology comes of age. Environ. Microbiol. Rep. 2017, 9, 33–35. [Google Scholar] [CrossRef]
- Mizuno, C.M.; Rodriguez-Valera, F.; Kimes, N.E.; Ghai, R. Expanding the Marine Virosphere Using Metagenomics. PLoS Genet. 2013, 9, e1003987. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [PubMed]
- Weitz, J.S.; Wilhelm, S.W. Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol. Rep. 2012, 4, 17. [Google Scholar] [CrossRef]
- Koslová, A.; Hackl, T.; Bade, F.; Sanchez Kasikovic, A.; Barenhoff, K.; Schimm, F.; Mersdorf, U.; Fischer, M.G. Endogenous virophages are active and mitigate giant virus infection in the marine protist Cafeteria burkhardae. Proc. Natl. Acad. Sci. USA 2024, 121, e2314606121. [Google Scholar] [PubMed]
- Mallapaty, S. AI scans RNA ‘dark matter’ and uncovers 70,000 new viruses. Nature 2024, 634, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gu, Z.; Luo, X.; Guo, Q.; Lai, L.; Deng, M. Learning a generalized graph transformer for protein function prediction in dissimilar sequences. GigaScience 2024, 13, giae093. [Google Scholar] [CrossRef]
- Colson, P.; de Lamballerie, X.; Fournous, G.; Raoult, D. Reclassification of giant viruses composing a fourth domain of life in the new order Megavirales. Intervirology 2012, 55, 321–332. [Google Scholar] [CrossRef]
- Schulz, F.; Roux, S.; Paez-Espino, D.; Jungbluth, S.; Walsh, D.A.; Denef, V.J.; McMahon, K.D.; Konstantinidis, K.T.; Eloe-Fadrosh, E.A.; Kyrpides, N.C. Giant virus diversity and host interactions through global metagenomics. Nature 2020, 578, 432–436. [Google Scholar] [CrossRef]
- Brussaard, C.P.D. Viral Control of Phytoplankton Populations—A Review. J. Eukaryot. Microbiol. 2004, 51, 125–138. [Google Scholar]
- Pesant, S.; Not, F.; Picheral, M.; Kandels-Lewis, S.; Le Bescot, N.; Gorsky, G.; Iudicone, D.; Karsenti, E.; Speich, S.; Troublé, R.; et al. Open science resources for the discovery and analysis of Tara Oceans data. Sci. Data 2015, 2, 150023. [Google Scholar] [CrossRef]
- Wilson, W.H.; Schroeder, D.C.; Allen, M.J.; Holden, M.T.G.; Parkhill, J.; Barrell, B.G.; Churcher, C.; Hamlin, N.; Mungall, K.; Norbertczak, H.; et al. Complete Genome Sequence and Lytic Phase Transcription Profile of a Coccolithovirus. Science 2005, 309, 1090–1092. [Google Scholar] [CrossRef]
- Kristensen, D.M.; Mushegian, A.R.; Dolja, V.V.; Koonin, E.V. New dimensions of the virus world discovered through metagenomics. Trends Microbiol. 2010, 18, 11–19. [Google Scholar] [CrossRef]
- Yoon, H.S.; Price, D.C.; Stepanauskas, R.; Rajah, V.D.; Sieracki, M.E.; Wilson, W.H.; Yang, E.C.; Duffy, S.; Bhattacharya, D. Single-Cell Genomics Reveals Organismal Interactions in Uncultivated Marine Protists. Science 2011, 332, 714–717. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.E.; Seo, M.D.; Lee, S.; Lee, T.-K. Mapping the Landscape of Marine Giant Virus Research: A Scientometric Perspective (1996–2024). J. Mar. Sci. Eng. 2025, 13, 1797. https://doi.org/10.3390/jmse13091797
Kim KE, Seo MD, Lee S, Lee T-K. Mapping the Landscape of Marine Giant Virus Research: A Scientometric Perspective (1996–2024). Journal of Marine Science and Engineering. 2025; 13(9):1797. https://doi.org/10.3390/jmse13091797
Chicago/Turabian StyleKim, Kang Eun, Man Deok Seo, Sukchan Lee, and Taek-Kyun Lee. 2025. "Mapping the Landscape of Marine Giant Virus Research: A Scientometric Perspective (1996–2024)" Journal of Marine Science and Engineering 13, no. 9: 1797. https://doi.org/10.3390/jmse13091797
APA StyleKim, K. E., Seo, M. D., Lee, S., & Lee, T.-K. (2025). Mapping the Landscape of Marine Giant Virus Research: A Scientometric Perspective (1996–2024). Journal of Marine Science and Engineering, 13(9), 1797. https://doi.org/10.3390/jmse13091797





