Synergistic Effects of Nano-SiO2 on Emulsion Film Stability and Non-Newtonian Rheology of Offshore Oil-Based Drilling Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Emulsion and Offshore Drilling Fluid Preparation
2.2.1. Method for Preparing an Emulsion
2.2.2. Offshore Drilling Fluid Preparation Method
2.3. Testing Methods for Emulsions and Offshore Drilling Fluids
2.3.1. Microstructure
2.3.2. Contact Angle Testing
2.3.3. Degree of Phase Separation
2.3.4. Rheological Properties
2.3.5. Characterization of the Zeta-Potential
2.3.6. High Temperature and High-Pressure Filtration Performance
2.3.7. Microstructure of Mud Cake
2.3.8. Electrical Stability Test
3. Results and Discussion
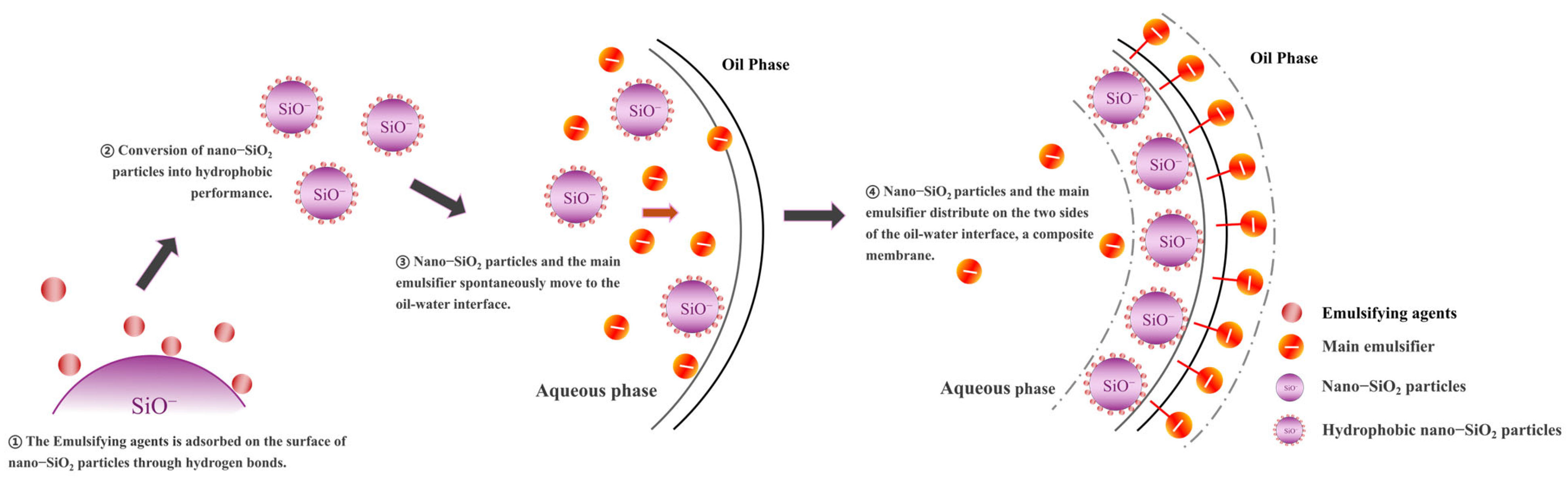
3.1. Mechanism of Synergistic Action Between Nano-SiO2 and Emulsifier
3.1.1. The Mechanism of Emulsion Stability
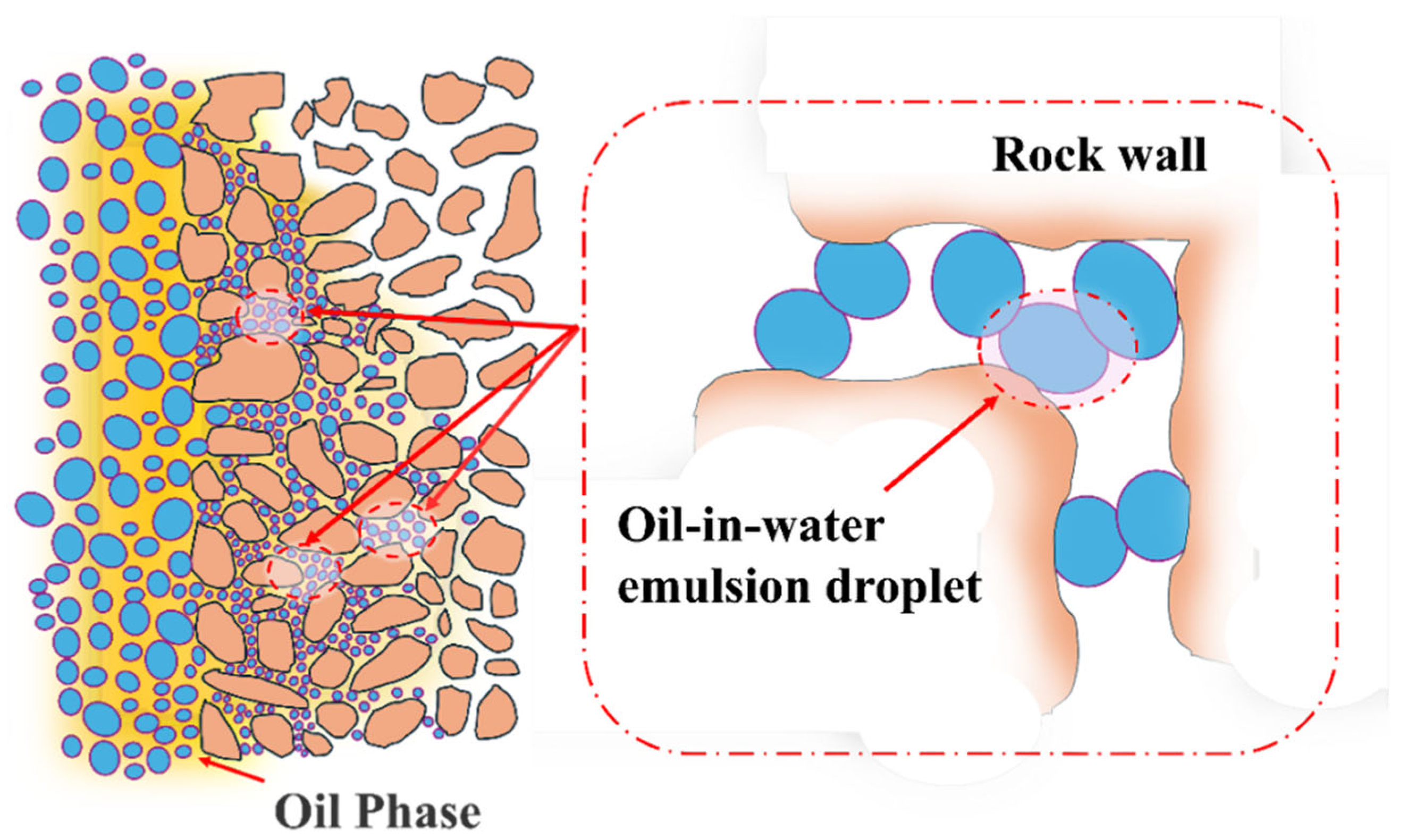
3.1.2. Mechanism of Loss Control
3.2. Emulsion Performance Analysis
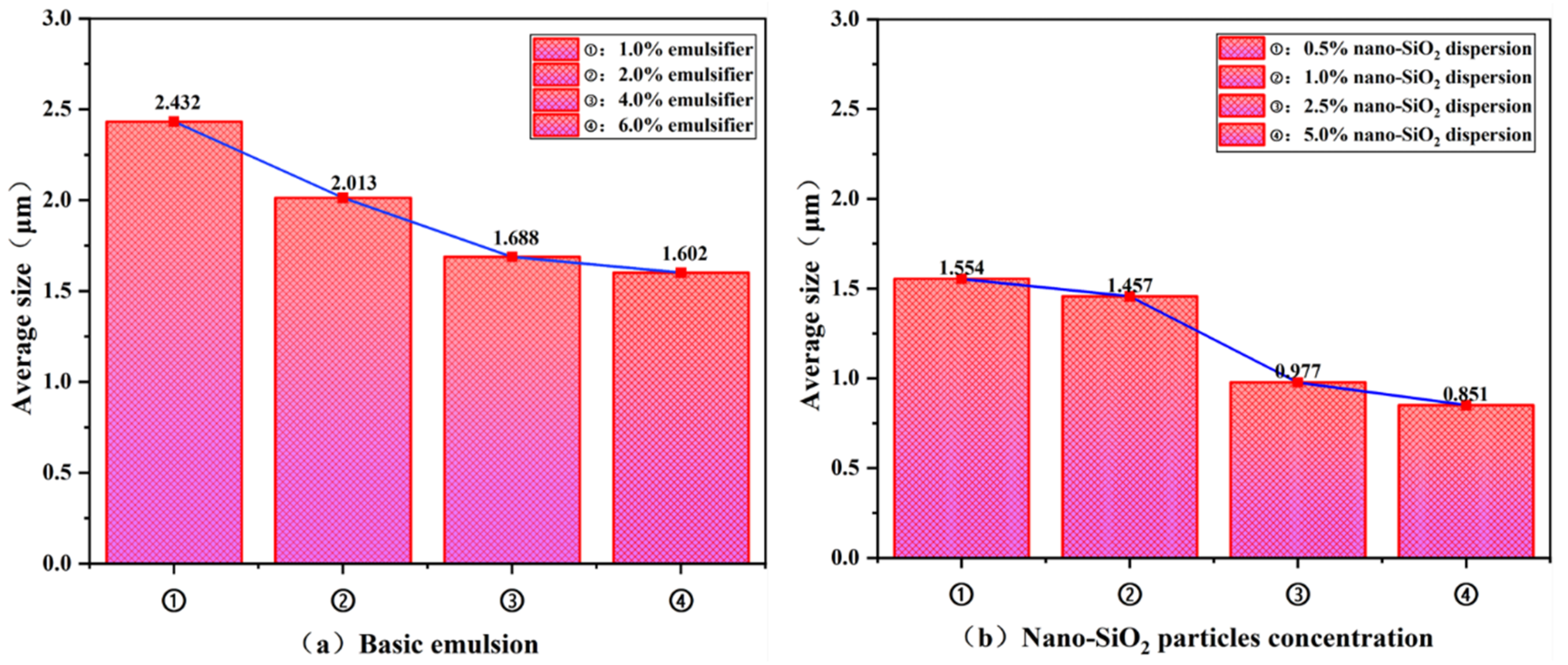
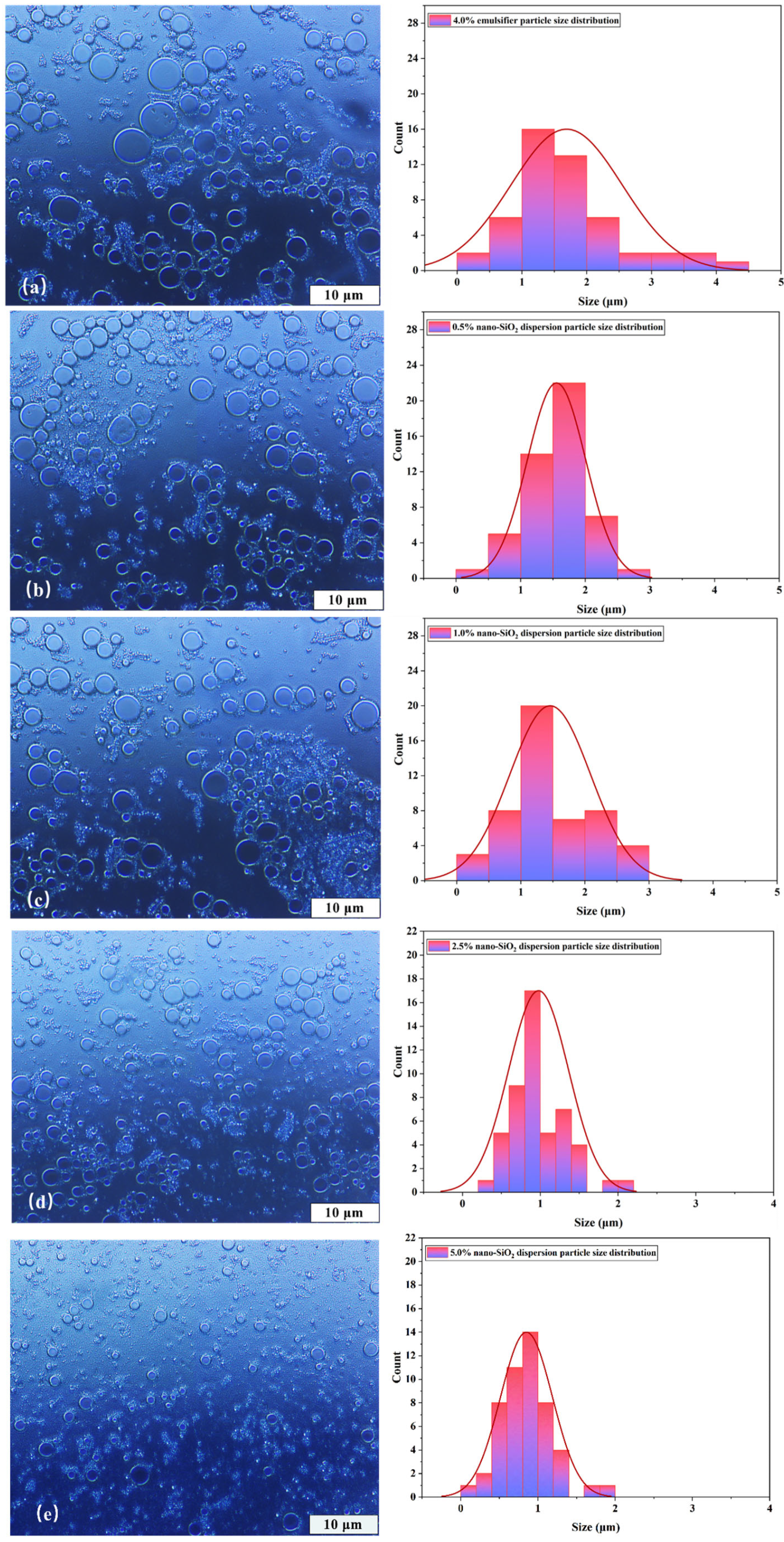
3.2.1. Microscopic Morphology of Emulsion Droplets
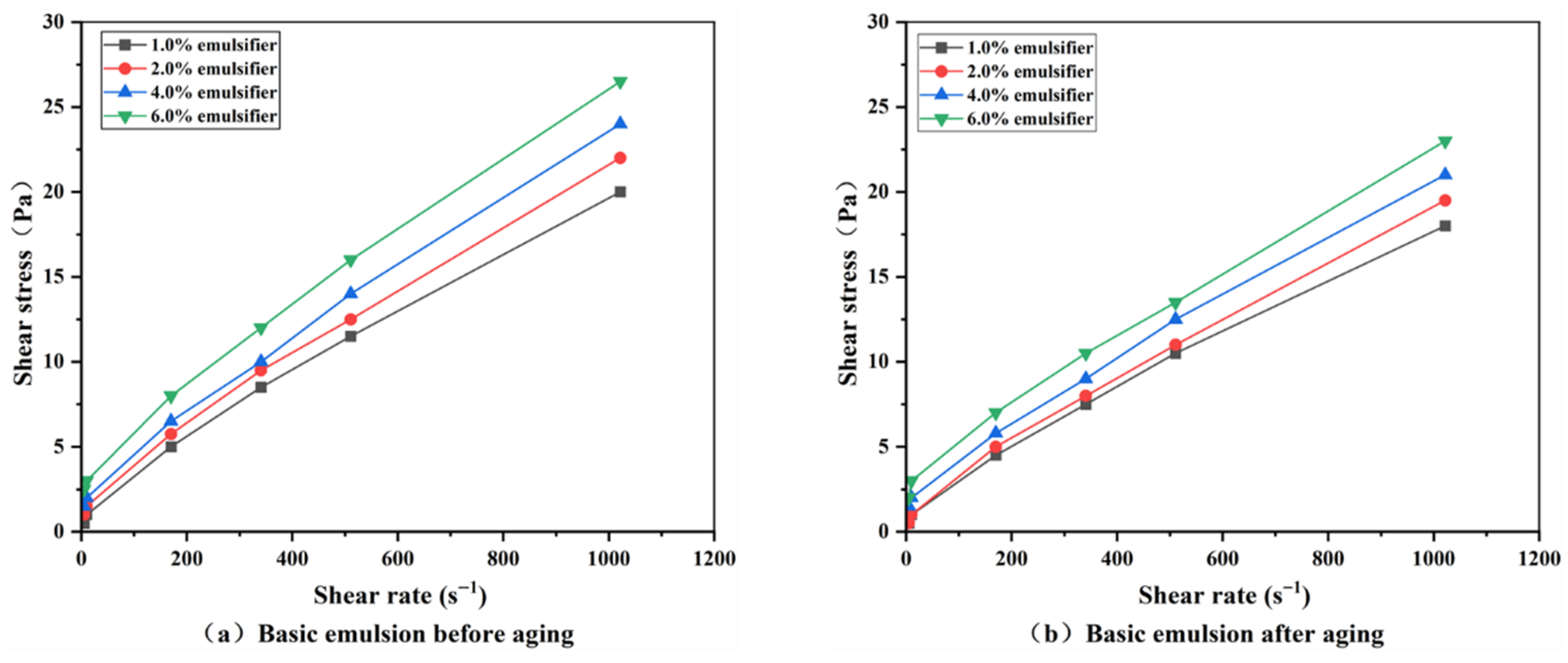
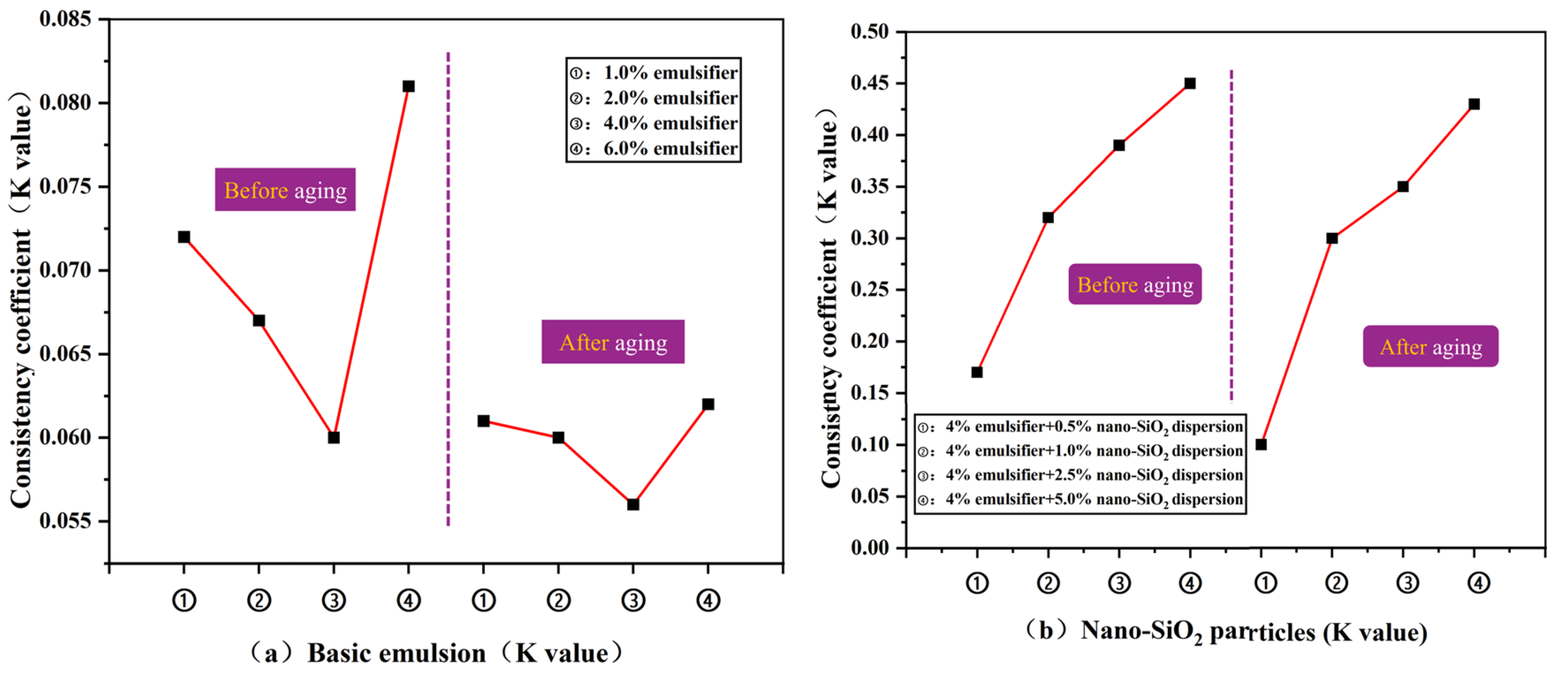
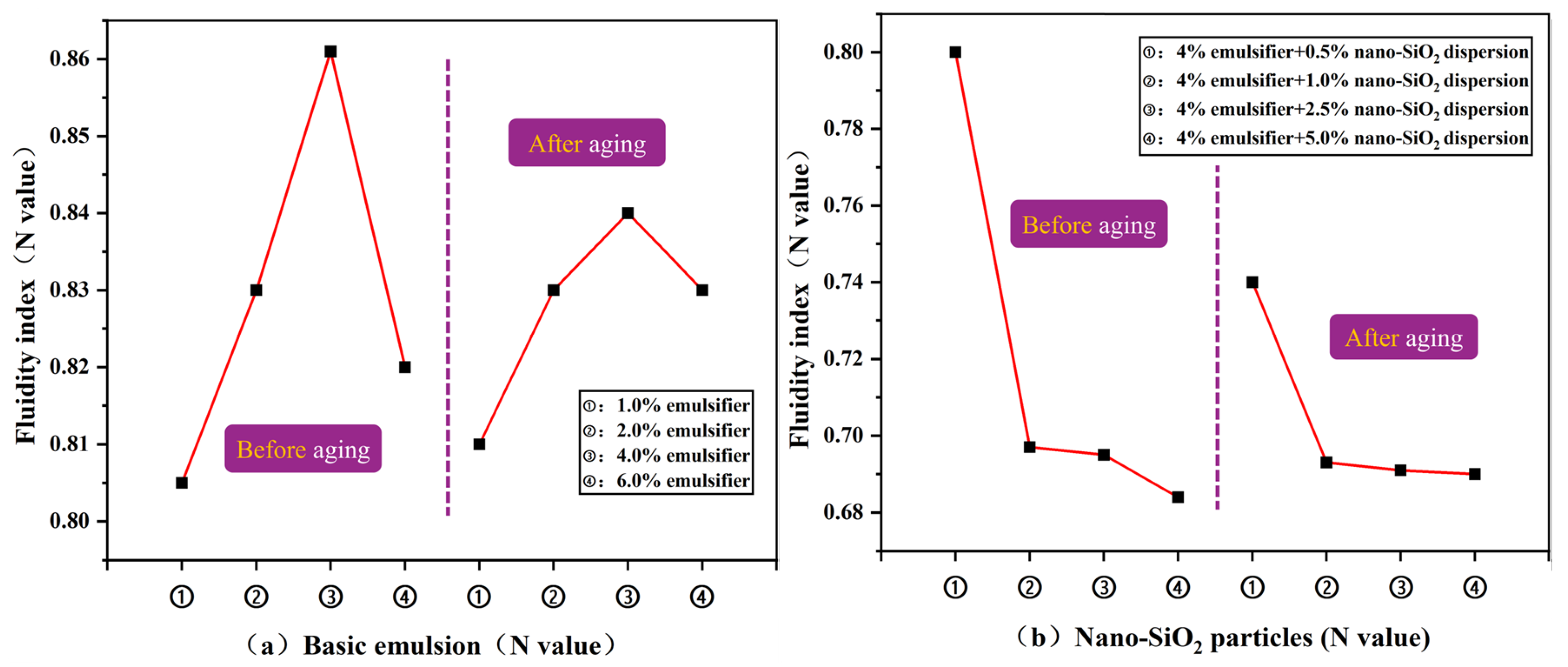
3.2.2. Rheological Properties of Emulsions
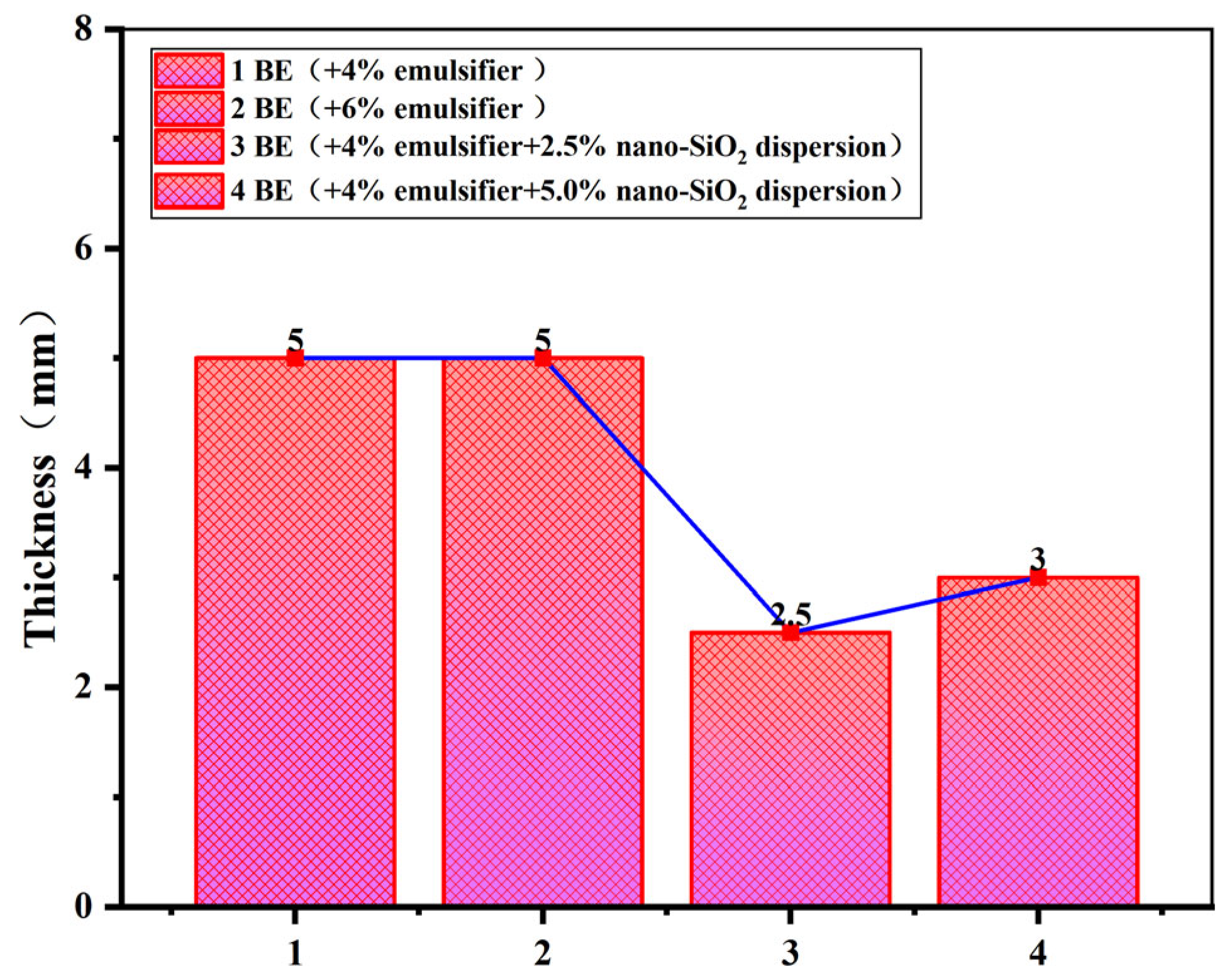
3.2.3. Degree of Separation of Emulsion Phase
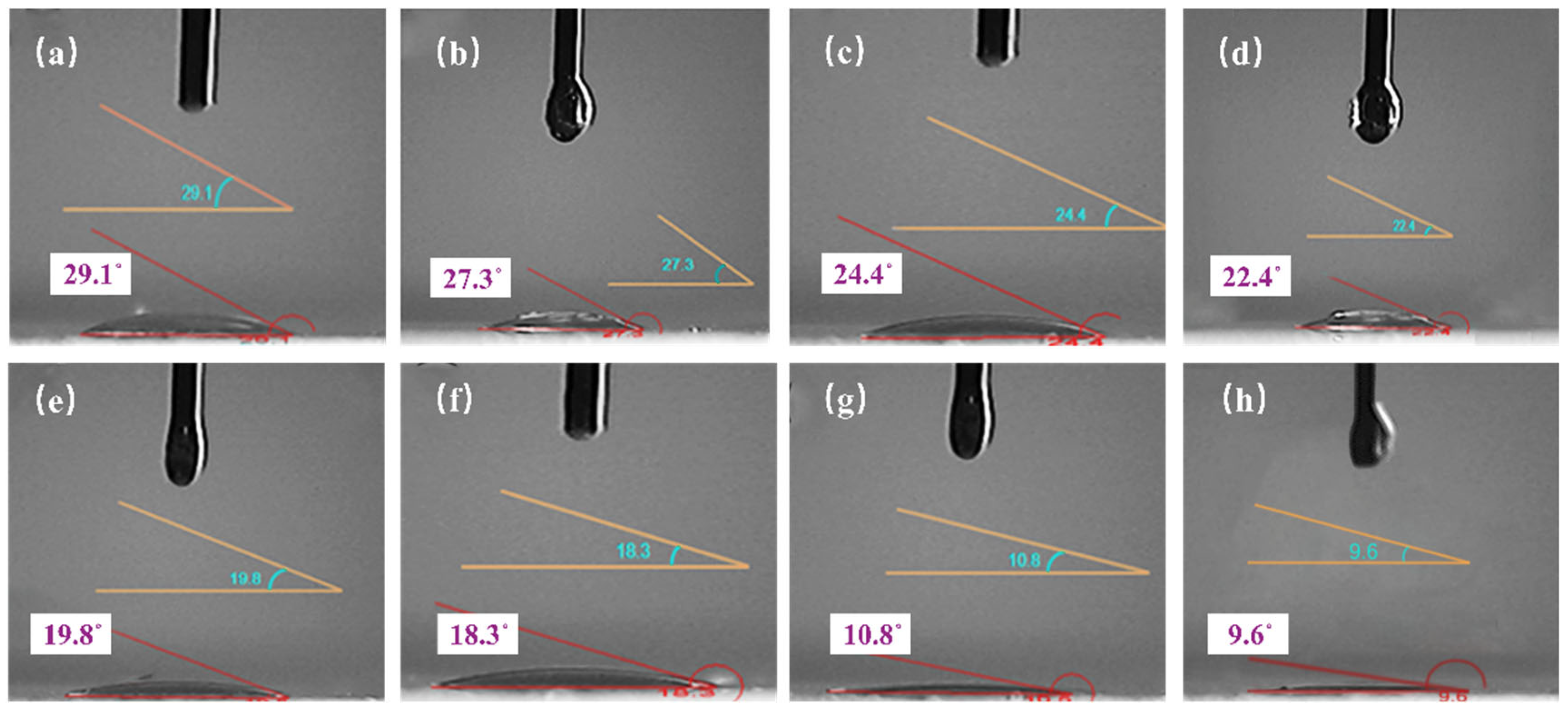
3.2.4. Emulsion Contact Angle Test
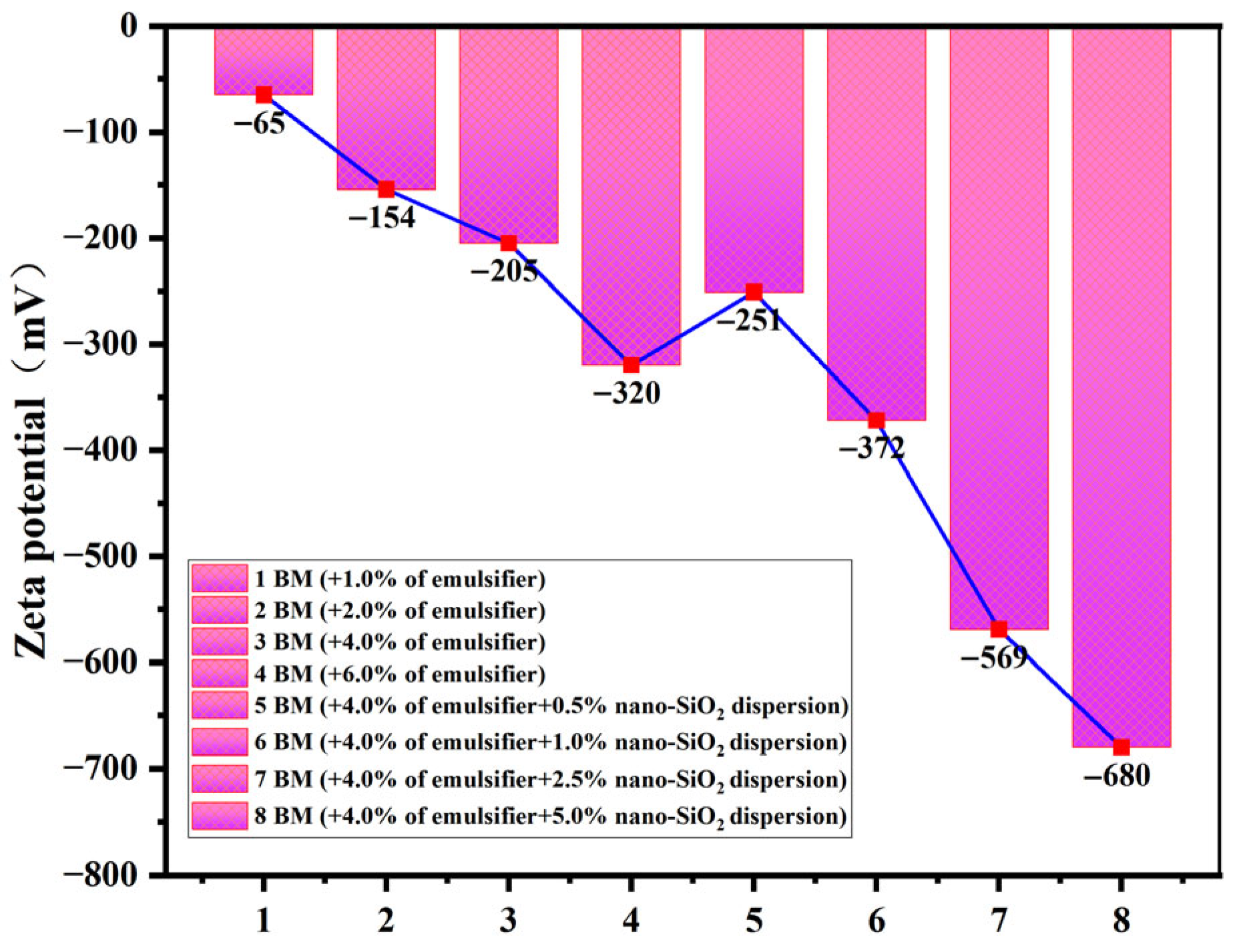
3.2.5. Characterization of the Zeta-Potential of Emulsions
3.3. Offshore Drilling Fluid Analysis
3.3.1. Offshore Drilling Fluid Formulation
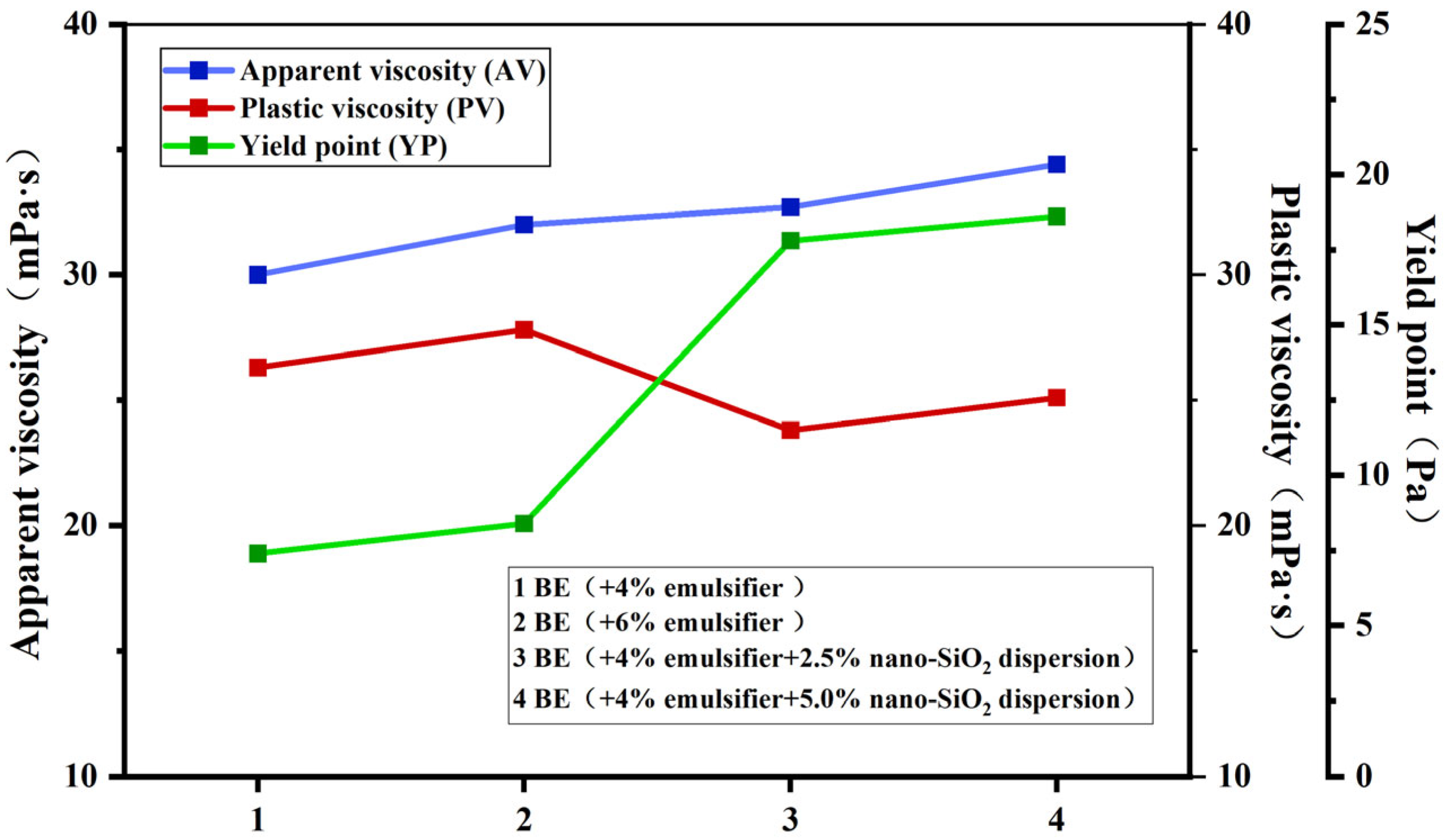
3.3.2. Offshore Drilling Rheological Properties
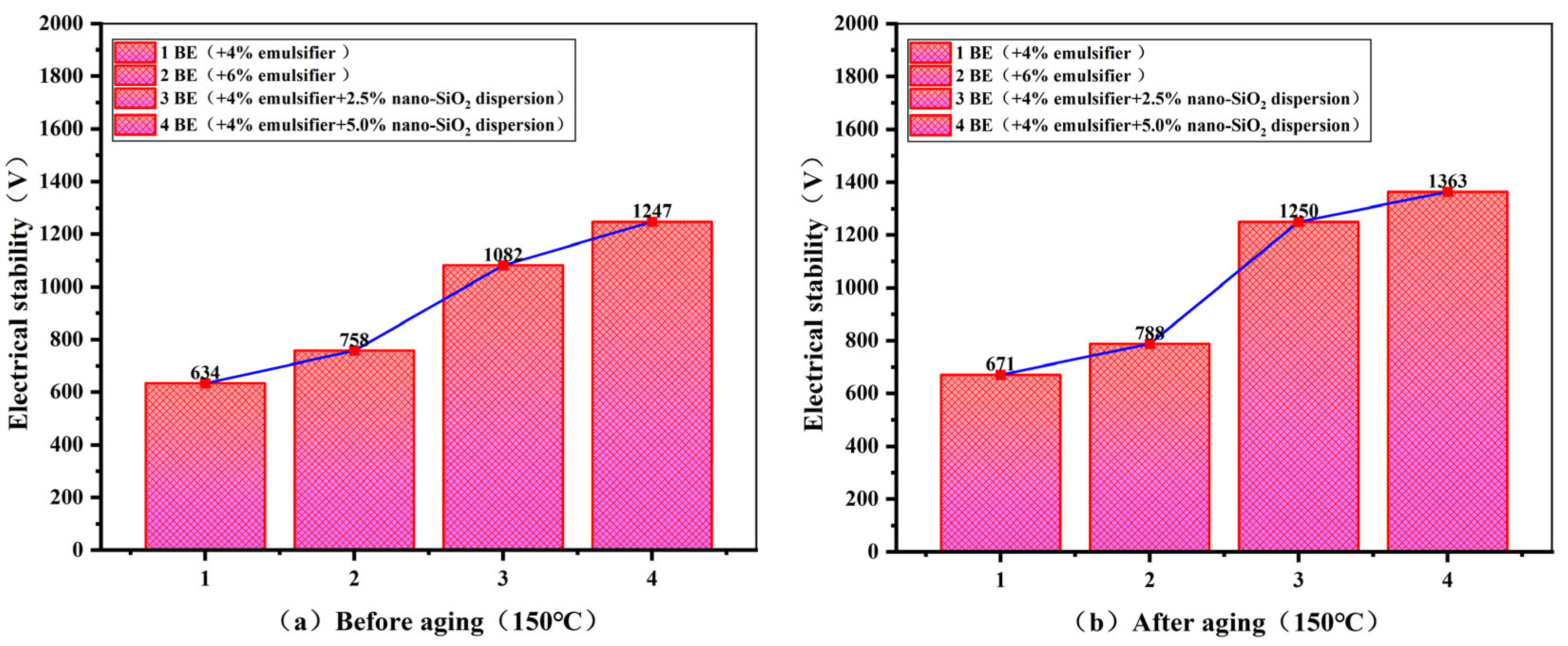
3.3.3. Offshore Drilling Fluid Electrical Stability Performance
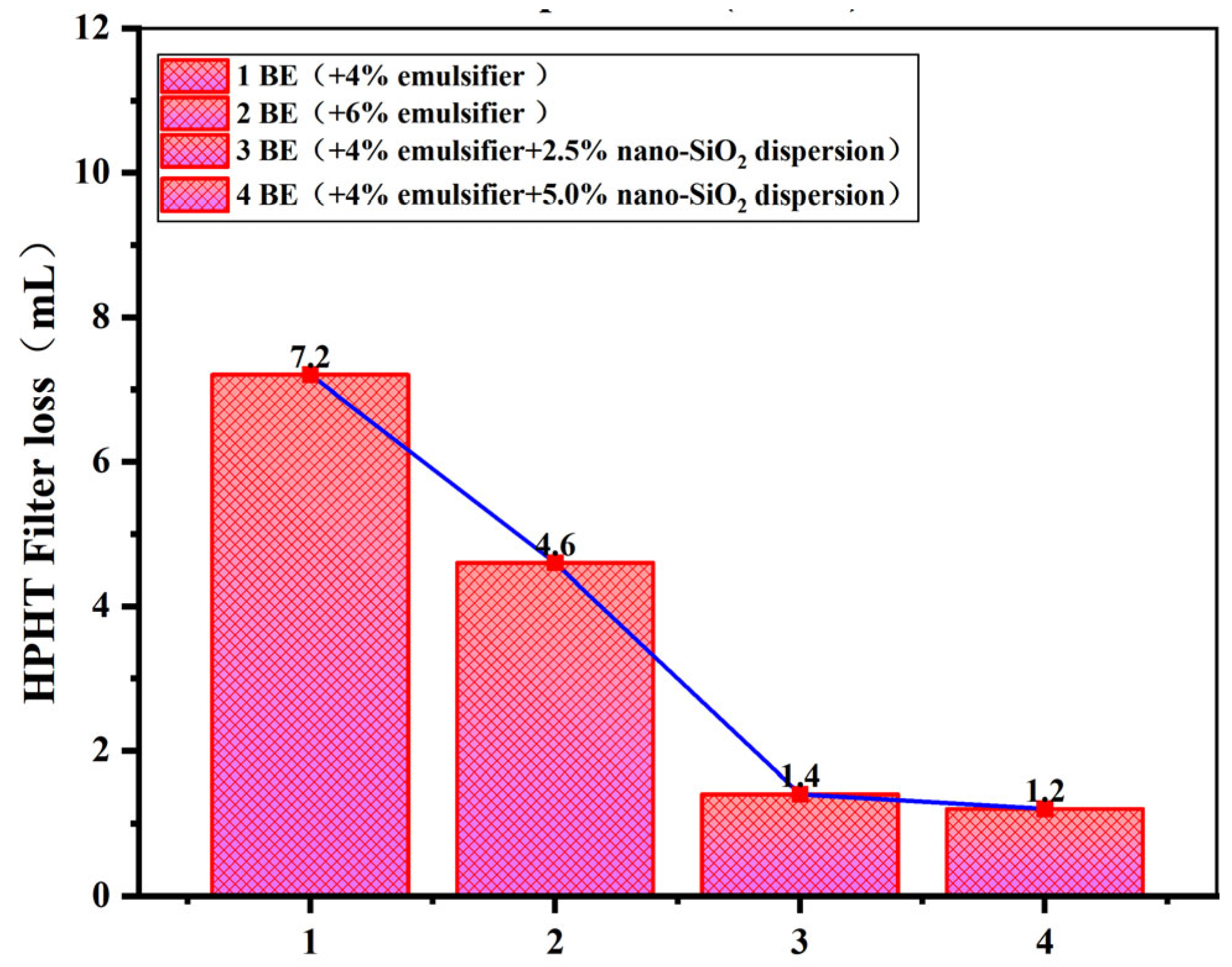
3.3.4. High-Temperature and High-Pressure Filtration Performance of Offshore Drilling Fluid
3.4. Microstructure of Mud Cakes
4. Conclusions
- This study reveals the enhancement mechanism of nano-SiO2 on the rheological behavior of emulsion interfacial films and non-Newtonian fluids at both macro- and micro-scales: nano-SiO2 adsorbs at the oil–water interface, forms a composite film with main and auxiliary emulsifiers to strengthen the mechanical strength of emulsion interfacial films, thereby inhibiting droplet coalescence; via hydrophobic interactions with emulsifiers’ hydrophobic groups, it reduces droplet surface tension and decreases droplet size to improve droplet dispersibility in the oil phase; and forms a network structure around droplets through inter-droplet electrostatic repulsion to enhance emulsion rheological properties and structural stability at elevated temperatures. Furthermore, under shear stress, the nano-SiO2 network structure is disrupted, making the emulsion gradually transition from an expansive fluid to a pseudoplastic fluid and exhibit the characteristic shear-thinning behavior of non-Newtonian fluids.
- The emulsion formulation containing 4% emulsifier and 2.5% nano-SiO2 dispersion was selected as optimal. Compared to the 4% emulsifier formulation, it reduced the average droplet size by 42.1% while only decreasing the centrifugal oil separation rate by 26%. It also exhibited excellent rheological properties and pronounced shear-thinning behavior at elevated temperatures. The introduction of 2.5% nano-SiO2 dispersion significantly reduced the interfacial tension between oil and water, decreased the contact angle by 55.7%, and lowered the zeta potential well below the stability threshold of −60 mV. This indicates enhanced overall emulsion performance and improved emulsion stability.
- After incorporating bentonite and various additives to form offshore oil-based drilling fluids (OBDFs), nano-SiO2 promotes the compact arrangement of solid particles, significantly enhancing the overall viscosity and structural stability of the system. Compared to conventional offshore oil-based drilling fluid formulations, the addition of nano-SiO2 enables the formation of a denser mud cake, resulting in superior thermal stability and filtration performance. Filtration loss is reduced by 69.5%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Yang, X.; Wang, R.; Li, H. Environmentally friendly and temperature resistant water-based drilling fluids with highly inhibitory synthetic nanocellulose polymers for deep sea drilling. Geoenergy Sci. Eng. 2024, 241, 213196. [Google Scholar] [CrossRef]
- Qi, Y.; Gao, Y.; Zhang, L.; Chen, H. Study of the formation of hydrates with NaCl, methanol additive, and quartz sand particles. J. Mar. Sci. Eng. 2024, 12, 364. [Google Scholar] [CrossRef]
- Seyyedattar, M.; Zendehboudi, S.; Butt, S. Technical and non-technical challenges of development of offshore petroleum reservoirs: Characterization and production. Nat. Resour. Res. 2020, 29, 2147–2189. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Z.; Lee, K.; Wang, R. Managing deepsea oil spills through a systematic modeling approach. J. Environ. Manag. 2024, 360, 121118. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, J.; Chang, X.; Li, Q. Novel polymeric organic gelator as lost circulation material for oil-based drilling fluids. Geoenergy Sci. Eng. 2023, 231, 212414. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Sun, J.; Guo, Y. Multi-ring side groups copolymer as an effective filtration control additive for water-based drilling fluids under high temperature and salt contamination. Geoenergy Sci. Eng. 2023, 225, 211690. [Google Scholar] [CrossRef]
- Guo, J.; Gu, B.; Lv, H.; Zhang, Y. Improved fracture permeability evaluation model for granite reservoirs in marine environments: A case study from the South China Sea. J. Mar. Sci. Eng. 2024, 12, 1868. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Y.; Gong, Q.; Li, H. Fluid flow simulation for predicting bottomhole pressure that considers wellbore storage effects under shut-in conditions in deepwater drilling. J. Mar. Sci. Eng. 2024, 13, 22. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Song, C.; Zhang, L. Seepage–diffusion mechanism of gas kick considering the filtration loss of oil-based muds during deepwater drilling. J. Mar. Sci. Eng. 2024, 12, 2035. [Google Scholar] [CrossRef]
- Gautam, S.; Guria, C.; Rajak, V.K. A state of the art review on the performance of high-pressure and high-temperature drilling fluids: Towards understanding the structure–property relationship of drilling fluid additives. J. Pet. Sci. Eng. 2022, 213, 110318. [Google Scholar] [CrossRef]
- Mao, H.; Yang, Y.; Zhang, H.; Li, J. A critical review of the possible effects of physical and chemical properties of subcritical water on the performance of water-based drilling fluids designed for ultra-high temperature and ultra-high pressure drilling applications. J. Pet. Sci. Eng. 2020, 187, 106795. [Google Scholar] [CrossRef]
- Sun, J.; Yang, J.; Bai, Y.; Zhao, L. Research progress and development of deep and ultra-deep drilling fluid technology. Pet. Explor. Dev. 2024, 51, 1022–1034. [Google Scholar] [CrossRef]
- Ofei, T.N.; Lund, B.; Saasen, A.; Sangesland, S. The effect of oil–water ratio on rheological properties and sag stability of oil-based drilling fluids. J. Energy Resour. Technol. 2022, 144, 073008. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, A.; Mandal, A. Formation, characteristics and oil industry applications of nanoemulsions: A review. J. Pet. Sci. Eng. 2021, 206, 109042. [Google Scholar] [CrossRef]
- Hao, X.; Elakneswaran, Y.; Shimokawara, M.; Chen, H. Impact of the temperature, homogenization condition, and oil property on the formation and stability of crude oil emulsion. Energy Fuels 2024, 38, 979–994. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. An experimental study of stability of oil–water emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Bera, B.; Khazal, R.; Schroën, K. Coalescence dynamics in oil-in-water emulsions at elevated temperatures. Sci. Rep. 2021, 11, 10990. [Google Scholar] [CrossRef]
- Siddig, O.; Mahmoud, A.A.; Elkatatny, S. A review of the various treatments of oil-based drilling fluids filter cakes. J. Pet. Explor. Prod. Technol. 2022, 12, 365–381. [Google Scholar] [CrossRef]
- Patel, A.D.; Growcock, F.B. Reversible invert emulsion drilling fluids: Controlling wettability and minimizing formation damage. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 31 May–1 June 1999; p. SPE-54764-MS. [Google Scholar]
- Kamal, M.S.; Hussain, S.M.S.; Fogang, L.T.; Malik, I.A. Development of polyoxyethylene zwitterionic surfactants for high-salinity high-temperature reservoirs. J. Surfact. Deterg. 2019, 22, 795–806. [Google Scholar] [CrossRef]
- Long, L.; Xianguang, X.; Rongchao, C.; Li, H. Application of innovative high-temperature high-density oil-based drilling fluid technology in the efficient exploration and development of ultra-deep natural gas resources in West China. In Proceedings of the International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 18–20 February 2025; p. IPTC-18600-MS. [Google Scholar]
- Shen, Z.; Zhang, H.; Yu, X.; Li, J. Experimental optimization of high-temperature-resistant and low oil–water ratio high-density oil-based drilling fluid. Processes 2023, 11, 1129. [Google Scholar] [CrossRef]
- Hou, J.; Huang, T.; Alotaibi, M.; AlSofi, A. Long-term thermal stability of ionic surfactants for improving oil production at high-salinity high-temperature conditions. ACS Omega 2024, 9, 11976–11986. [Google Scholar] [CrossRef] [PubMed]
- Hamill, R.D.; Petersen, R.V. Effects of aging and surfactant concentration on the rheology and droplet size distribution of a nonaqueous emulsion. J. Pharm. Sci. 1966, 55, 1268–1274. [Google Scholar] [CrossRef]
- Xia, X.; Ma, J.; Liu, F.; Chen, H. A novel demulsifier with strong hydrogen bonding for effective breaking of water-in-heavy oil emulsions. Int. J. Mol. Sci. 2023, 24, 14805. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, Z.; Cui, J. Carbonation resistance of marine concrete containing nano-SiO2 under the action of bending load. J. Mar. Sci. Eng. 2023, 11, 637. [Google Scholar] [CrossRef]
- Binks, B.P.; Rodrigues, J.A. Enhanced stabilization of emulsions due to surfactant-induced nanoparticle flocculation. Langmuir 2007, 23, 7436–7439. [Google Scholar] [CrossRef]
- Pal, N.; Mandal, A. Oil recovery mechanisms of Pickering nanoemulsions stabilized by surfactant–polymer–nanoparticle assemblies: A versatile surface energies’ approach. Fuel 2020, 276, 118138. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Pickering emulsions: A novel tool for cosmetic formulators. Cosmetics 2022, 9, 68. [Google Scholar] [CrossRef]
- Eftekhari, M.; Schwarzenberger, K.; Javadi, A.; Eckert, K. The influence of negatively charged silica nanoparticles on the surface properties of anionic surfactants: Electrostatic repulsion or the effect of ionic strength? Phys. Chem. Chem. Phys. 2020, 22, 2238–2248. [Google Scholar] [CrossRef]
- Katende, A.; Boyou, N.V.; Ismail, I.; Chung, D.Z.; Sagala, F.; Hussein, N.; Ismail, M.S. Improving the performance of oil based mud and water based mud in a high temperature hole using nanosilica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 645–673. [Google Scholar] [CrossRef]
- Foroozesh, J.; Kumar, S. Nanoparticles behaviors in porous media: Application to enhanced oil recovery. J. Mol. Liq. 2020, 316, 113876. [Google Scholar] [CrossRef]
- L’Estimé, M.; Schindler, M.; Shahidzadeh, N.; Bonn, D. Droplet size distribution in emulsions. Langmuir 2024, 40, 275–281. [Google Scholar] [CrossRef]
- Schott, H. Saturation adsorption at interfaces of surfactant solutions. J. Pharm. Sci. 1980, 69, 852–854. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Wang, B.; Wang, N. pH-responsive non-Pickering emulsion stabilized. Langmuir 2020, 36, 15230–15239. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Su, J.; Shi, T.; Guo, R.; Peng, J. Effect evaluation of nanosilica particles on O/W emulsion properties. Geofluids 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Zhang, K.; Ni, O. Rheological properties and stability of highly concentrated water-in-oil emulsions with different emulsifiers. J. Dispers. Sci. Technol. 2015, 36, 549–555. [Google Scholar] [CrossRef]
- Sherman, P. Changes in the rheological properties of emulsions on aging, and their dependence on the kinetics of globule coagulation. J. Colloid Interface Sci. 1963, 22, 2531–2537. [Google Scholar] [CrossRef]
- Wei, L.; Bi, H.; Zhao, J.; Dong, H.; Wang, D.; Zhang, X. The influence of the microstructure of W/O emulsion of waxy crude oil on its rheology. Adv. Chem. Eng. Sci. 2019, 9, 299–316. [Google Scholar] [CrossRef]
- Kroger, M. Effect of nanoparticle hydrophobicity on the rheology of highly concentrated emulsions. Appl. Rheol. 2013, 23, 62835. [Google Scholar]
- Liu, L.; Pu, X.; Tao, H.; Chen, K.; Guo, W.; Luo, D.; Ren, Z. Pickering emulsion stabilized by organoclay and intermediately hydrophobic nanosilica for high-temperature conditions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125694. [Google Scholar] [CrossRef]
- Ahuja, A.; Iqbal, A.; Iqbal, M.; Lee, J.W.; Morris, J.F. Rheology of hydrate-forming emulsions stabilized by surfactant and hydrophobic silica nanoparticles. Energy Fuels 2018, 32, 5877–5884. [Google Scholar] [CrossRef]
- Liu, R.; Lu, Y.; Pu, W.; Lian, K.; Sun, L.; Du, D.; Song, Y.; Sheng, J.J. Low-energy emulsification of oil-in-water emulsions with self-regulating mobility via a nanoparticle surfactant. Ind. Eng. Chem. Res. 2020, 59, 18396–18411. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Creaming stability of flocculated monodisperse oil-in-water emulsions. J. Colloid Interface Sci. 2000, 225, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Yegya Raman, A.K.; Aichele, C.P. Demulsification of surfactant-stabilized water-in-oil (cyclohexane) emulsions using silica nanoparticles. Energy Fuels 2018, 32, 8121–8130. [Google Scholar] [CrossRef]
- Cao, R.; Wang, C.; Zhou, C.; Liu, Y.; Yin, Y.; Chen, H.; Li, F.; Zhou, W.; Xu, M.; Yang, W. Polymeric surfactant (PIBSA-X) facilitates the formation of a water-in-oil emulsion reactor for the preparation of ultrasmall nanosilica. ACS Omega 2023, 8, 44647–44658. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Lee, H.; Yi, S.; Lee, J.H.; Kim, J.W. Hydrophobically modified silica nanolaces-armored water-in-oil Pickering emulsions with enhanced interfacial attachment energy. J. Colloid Interface Sci. 2023, 641, 376–385. [Google Scholar] [CrossRef]
- Tian, S.; Gao, W.; Liu, Y.; Kang, W.; Yang, H. Effects of surface modification Nano-SiO2 and its combination with surfactant on interfacial tension and emulsion stability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124682. [Google Scholar] [CrossRef]
- Keane, R.K.; Hong, W.; He, W.; Teale, S.; Bancroft, R.; Dinsmore, A.D. Adsorption of hydrophilic silica nanoparticles at oil–water interfaces with reversible emulsion stabilization by ion partitioning. Langmuir 2022, 38, 2821–2831. [Google Scholar] [CrossRef]
- Parizad, A.; Shahbazi, K.; Tanha, A.A. SiO2 nanoparticle and KCl salt effects on filtration and thixotropical behavior of polymeric water based drilling fluid: With zeta potential and size analysis. Results Phys. 2018, 9, 1656–1665. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Yuan, S. Hydrophilic silica nanoparticles in O/W emulsion: Insights from molecular dynamics simulation. Molecules 2022, 27, 8407. [Google Scholar] [CrossRef]
- Prakash, V.; Sharma, N.; Bhattacharya, M. Effect of silica nanoparticles on the rheological and HTHP filtration properties of environment friendly additive in water-based drilling fluid. J. Pet. Explor. Prod. Technol. 2021, 11, 4253–4267. [Google Scholar] [CrossRef]
- Li, Y.; Xia, C.; Liu, X. Water-based drilling fluids containing hydrophobic nanoparticles for minimizing shale hydration and formation damage. Heliyon 2023, 9, e22990. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.H.; Bilgesu, H. Investigation of rheological and filtration properties of water-based drilling fluids using various anionic nanoparticles. In Proceedings of the SPE Western Regional Meeting, Bakersfield, CA, USA, 23–27 April 2017; p. D041S015R005. [Google Scholar]
- Ghazali, N.A.; Naganawa, S.; Masuda, Y. The effect of high-temperature environment on the rheology and filtration properties of Rhizophora spp. tannin-lignosulfonate as bio-based additive in water-based drilling fluid. J. Pet. Explor. Prod. Technol. 2024, 14, 727–743. [Google Scholar] [CrossRef]
- Sharma, T.; Kumar, G.S.; Chon, B.H.; Sangwai, J.S. Thermal stability of oil-in-water Pickering emulsion in the presence of nanoparticle, surfactant, and polymer. J. Ind. Eng. Chem. 2015, 22, 324–334. [Google Scholar] [CrossRef]
- Ali, J.A.; Hamadamin, A.B.; Ahmed, S.M.; Mahmood, B.S.; Sajadi, S.M.; Manshad, A.K. Synergistic effect of nanoinhibitive drilling fluid on the shale swelling performance at high temperature and high pressure. Energy Fuels 2022, 36, 1996–2006. [Google Scholar] [CrossRef]
- Contreras, O.; Hareland, G.; Husein, M.; Nygaard, R.; Alsaba, M. Wellbore strengthening in sandstones by means of nanoparticle-based drilling fluids. In Proceedings of the SPE Deepwater Drilling and Completions Conference, Galveston, TX, USA, 10–11 September 2014; p. D011S004R001. [Google Scholar]
- Pang, S.; Xuan, Y.; Zhu, L.; An, Y. Zwitterionic polymer grafted nano-SiO2 as fluid loss agent for high temperature water-based drilling fluids. J. Mol. Liq. 2025, 417, 126542. [Google Scholar] [CrossRef]
- Lai, N.; Fan, W.; Zhang, X.; Liu, L.; Zhou, X.; Chen, S. Temperature-sensitive polymer based nano-SiO2 composite multi-component synergistic improvement of shale stability in water-based drilling fluids. Geoenergy Sci. Eng. 2023, 224, 211498. [Google Scholar] [CrossRef]
- Zhong, H.; Shen, G.; Qiu, Z.; Lin, Y.; Fan, L.; Xing, X.; Li, J. Minimizing the HTHP filtration loss of oil-based drilling fluid with swellable polymer microspheres. J. Pet. Sci. Eng. 2019, 172, 411–424. [Google Scholar] [CrossRef]
- Khalili, P.; Khalifeh, M.; Saasen, A.; Naccache, M. Rheological compatibility of a hardening spacer fluid and oil-based drilling fluid. SPE J. 2023, 28, 2845–2860. [Google Scholar] [CrossRef]




| Treatment Agent | Characteristics | Unit | Dosage |
|---|---|---|---|
| No. 3 white oil | oil phase | mL | 224 |
| Deionized water | liquid phase | mL | 96 |
| Span-80 | Main emulsifier | g | 12.8/19.2 |
| Oleic acid | Co-emulsifier | g | 12.8/19.2 |
| Nano-SiO2 | Nanoparticles | g | 2.4/4.8 |
| Organic soil | lipophilic colloid | g | 4 |
| CaCl2 | salts | g | 24.96 |
| CaO | PH control agent | g | 4 |
| Filter loss reduction agent | Sulfonated asphalt | g | 12 |
| Barite | Aggravating agent | g | 325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, D.; Bao, F.; Yang, D.; Pu, L.; Xu, P. Synergistic Effects of Nano-SiO2 on Emulsion Film Stability and Non-Newtonian Rheology of Offshore Oil-Based Drilling Fluids. J. Mar. Sci. Eng. 2025, 13, 1722. https://doi.org/10.3390/jmse13091722
Peng D, Bao F, Yang D, Pu L, Xu P. Synergistic Effects of Nano-SiO2 on Emulsion Film Stability and Non-Newtonian Rheology of Offshore Oil-Based Drilling Fluids. Journal of Marine Science and Engineering. 2025; 13(9):1722. https://doi.org/10.3390/jmse13091722
Chicago/Turabian StylePeng, Daicheng, Fuhao Bao, Dong Yang, Lei Pu, and Peng Xu. 2025. "Synergistic Effects of Nano-SiO2 on Emulsion Film Stability and Non-Newtonian Rheology of Offshore Oil-Based Drilling Fluids" Journal of Marine Science and Engineering 13, no. 9: 1722. https://doi.org/10.3390/jmse13091722
APA StylePeng, D., Bao, F., Yang, D., Pu, L., & Xu, P. (2025). Synergistic Effects of Nano-SiO2 on Emulsion Film Stability and Non-Newtonian Rheology of Offshore Oil-Based Drilling Fluids. Journal of Marine Science and Engineering, 13(9), 1722. https://doi.org/10.3390/jmse13091722







