Review of Age Estimation Techniques and Growth Models for Shelled Organisms in Marine Animal Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
- Age determination: “age reading,” “growth increments,” “shell analysis,” “sclerochronology,” “isotope analysis,” “microstructure,” and “increment formation”
- Growth modelling: “growth rate,” “growth modelling,” “von Bertalanffy,” “length-at-age,” “size-frequency analysis,” “growth curves”
- Organism and ecosystem context: “bivalves,” “gastropods,” “shelled organisms,” “Marine animal forests,” “ecosystem engineers”
2.2. Data Extraction and Classification
- Age determination methods: including direct techniques (e.g., shell increment analysis and operculum examination), indirect methods (e.g., length-frequency analysis), and advanced approaches (e.g., stable isotope analysis, mark–recapture, and laboratory-based validation).
- Growth models: with emphasis on the use of the von Bertalanffy growth function (VBGF) and other length-at-age modelling frameworks. Parameters, such as asymptotic length (L∞), growth coefficient (K), and theoretical age at zero length (t0), were recorded where available.
- Species-specific growth rates: including raw and modelled shell length data across age classes, with intra- and interspecific variability considered.
2.3. Statistical Analysis
3. Results
3.1. Synthesis of Species and Methodological Scope
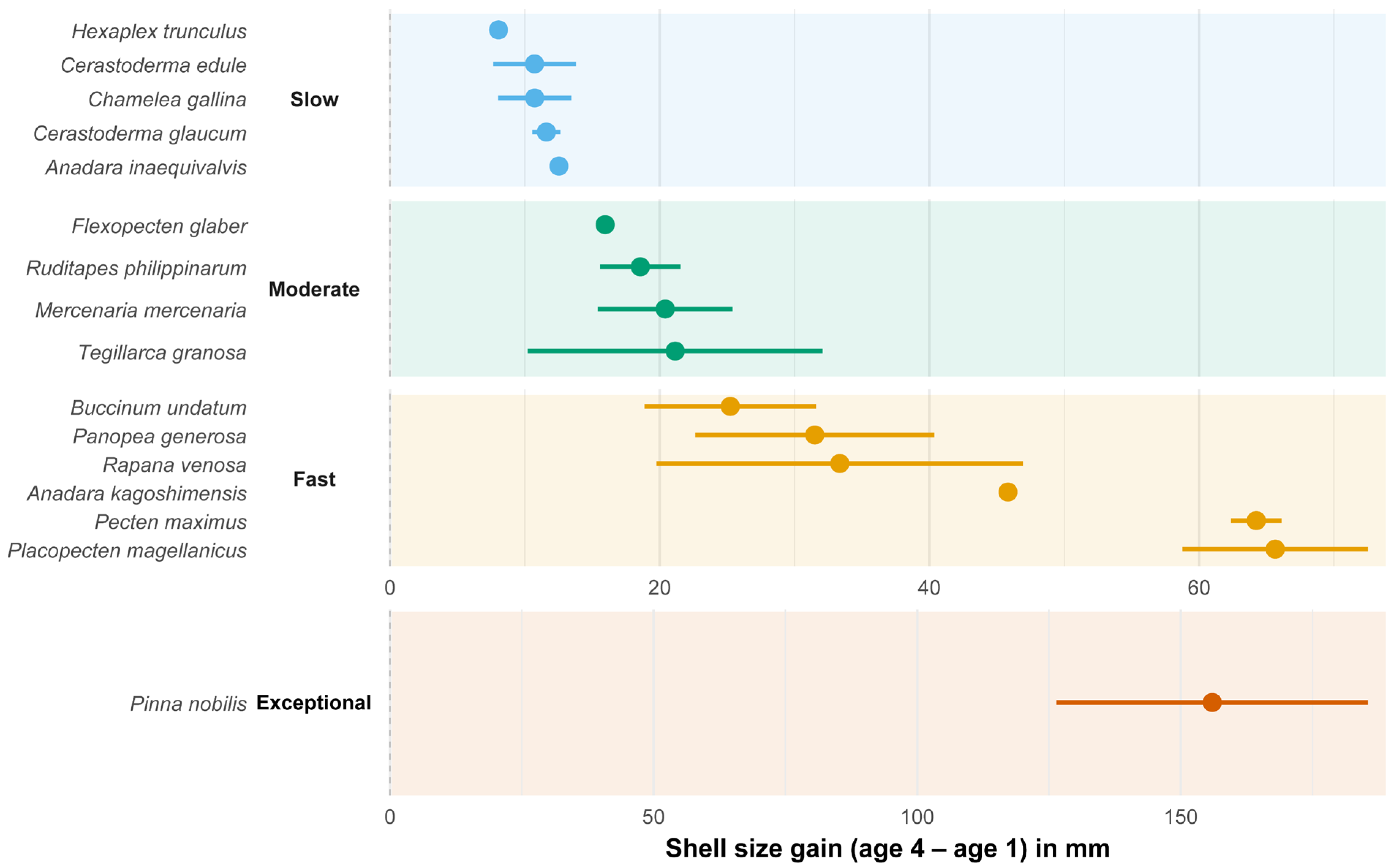
3.2. Comparison of Growth Strategies Across Families
- Slow Growers: A. inaequivalvis, C. glaucum, C. gallina, C. edule, and H. trunculus
- Moderate Growers: T. granosa, M. mercenaria, R. philippinarum, and F. glaber
- Fast Growers: P. magellanicus, P. maximus, A. kagoshimensis, R. venosa, P. generosa, and B. undatum
- Exceptional Grower: P. nobilis
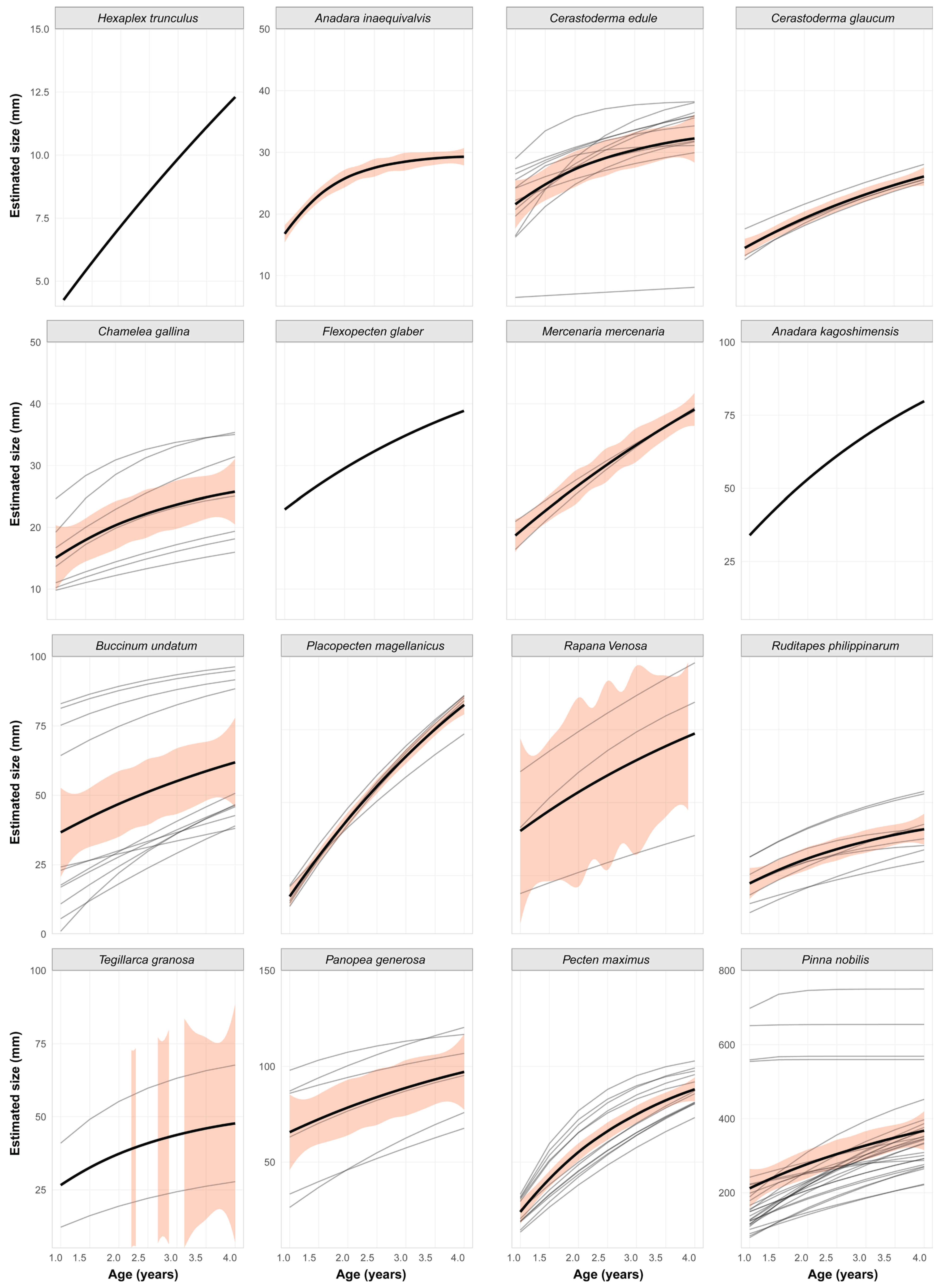
3.2.1. Arcidae
3.2.2. Buccinidae
3.2.3. Cardiidae
3.2.4. Hiatellidae
3.2.5. Muricidae
3.2.6. Pectinidae
3.2.7. Veneridae
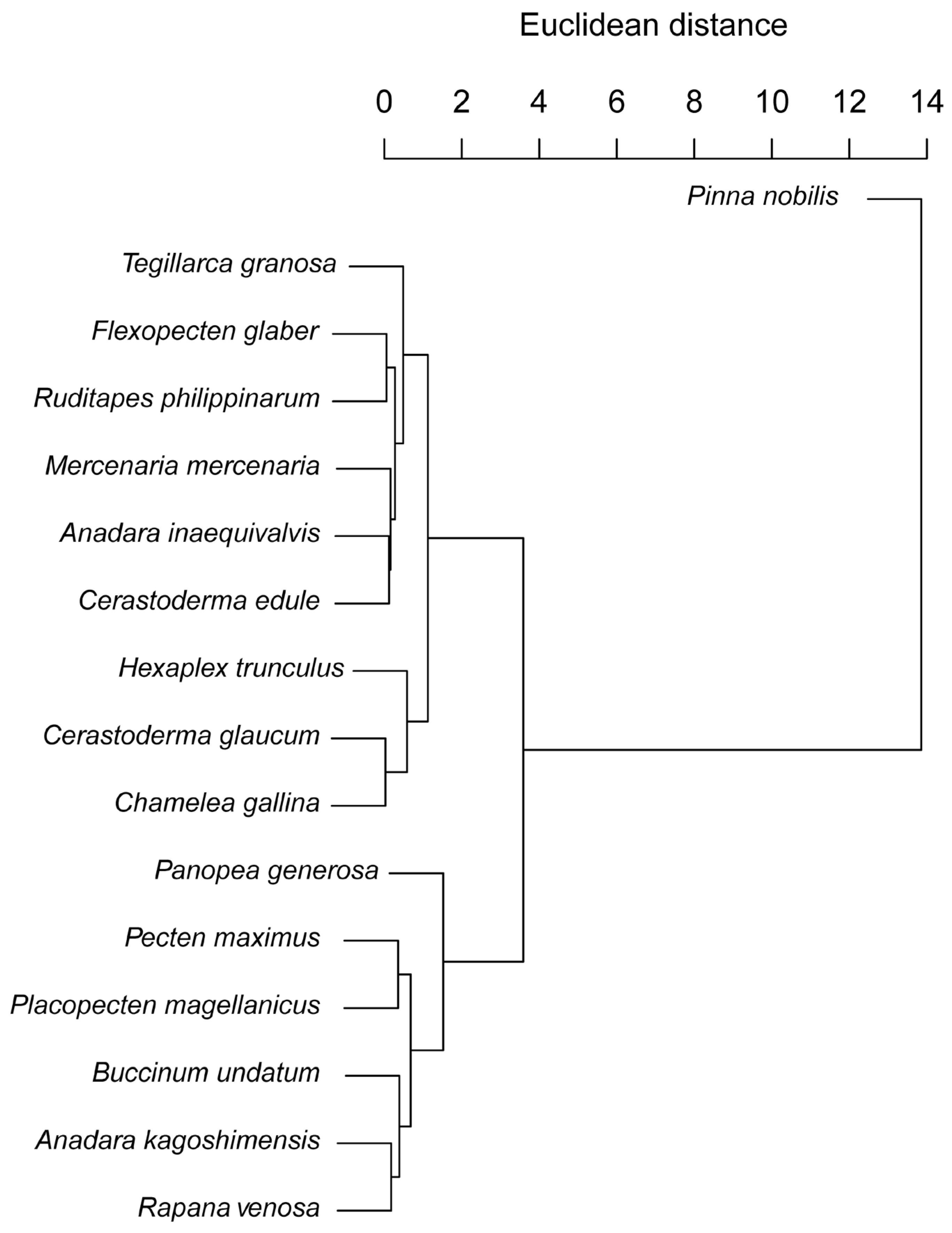
3.3. Hierarchical Clustering of Growth Strategies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orejas, C.; Carreiro-Silva, M.; Mohn, C.; Reimer, J.D.; Samaai, T.; Allcock, A.L.; Rossi, S. Marine Animal Forests of the World: Definition and Characteristics. Res. Ideas Outcomes 2022, 8, e96274. [Google Scholar] [CrossRef]
- Ponti, M.; Cerrano, C.; Wörheide, G. Bridging Knowledge Gaps Between Tropical, Temperate, and Cold-Water Coral Reefs. Book of Abstracts of the 2024 European Coral Reef Symposium; Città della Scienza & Stazione Zoologica Anton Dohrn: Naples, Italy, 2024; 348p. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Fortunato, H. Mollusks: Tools in Environmental and Climate Research. Am. Malacol. Bull. 2015, 33, 310–324. [Google Scholar] [CrossRef]
- ICES. Working Group on Introductions and Transfers of Marine Organisms (WGITMO). In ICES Scientific Reports. Report; International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2022. [Google Scholar] [CrossRef]
- de la Ballina, N.R.; Maresca, F.; Cao, A.; Villalba, A. Bivalve Haemocyte Subpopulations: A Review. Front. Immunol. 2022, 13, 826255. [Google Scholar] [CrossRef]
- Seyhan, K.; Mazlum, E.R.; Emiral, H.; Engin, S.; Demirhan, S. Diel feeding periodicity, gastric emptying, and estimated daily food consumption of whelk (Rapana venosa) in the south eastern Black Sea (Turkey) marine ecosystem. Indian J. Mar. Sci. 2003, 32, 249–251. [Google Scholar]
- Güler, M.; Lök, A. Foraging behaviors of a predatory snail (Hexaplex trunculus) in group-attacking. Turk. J. Fish. Aquat. Sci. 2018, 19, 391–398. [Google Scholar] [CrossRef]
- Moncheva, S.; Namiesnik, J.; Apak, R.; Arancibia-Avila, P.; Toledo, F.; Kang, S.-G.; Jung, S.-T.; Gorinstein, S. Rapana venosa as a bioindicator of environmental pollution. Chem. Ecol. 2011, 27, 31–41. [Google Scholar] [CrossRef]
- Roméo, M.; Gharbi-Bouraoui, S.; Gnassia-Barelli, M.; Dellali, M.; Aïssa, P. Responses of Hexaplex (Murex) trunculus to selected pollutants. Sci. Total Environ. 2006, 359, 135–144. [Google Scholar] [CrossRef]
- Alkan, N.; Alkan, A. Elemental Compositions of Rapana venosa (Mollusca: Muricidae) from the Eastern Black Sea Region of Turkey: Toxicology Health Risk Assessment. Anal. Lett. 2023, 56, 504–516. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Hoellein, T.J. Bivalve Impacts in Freshwater and Marine Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 183–208. [Google Scholar] [CrossRef]
- Otter, L.M.; Agbaje, O.B.A.; Kilburn, M.R.; Lenz, C.; Henry, H.; Trimby, P.; Hoppe, P.; Jacob, D.E. Insights into architecture, growth dynamics, and biomineralization from pulsed Sr-labelled Katelysia rhytiphora shells (Mollusca, Bivalvia). Biogeosciences 2019, 16, 3439–3455. [Google Scholar] [CrossRef]
- Chahouri, A.; Yacoubi, B.; Moukrim, A.; Banaoui, A. Bivalve molluscs as bioindicators of multiple stressors in the marine environment: Recent advances. Cont. Shelf Res. 2023, 264, 105056. [Google Scholar] [CrossRef]
- Hollyman, P.R.; Laptikhovsky, V.V.; Richardson, C.A. Techniques for Estimating the Age and Growth of Molluscs: Gastropoda. J. Shellfish Res. 2018, 37, 773–782. [Google Scholar] [CrossRef]
- Frank, P.W. Growth rates and longevity of some gastropod mollusks on the coral reef at Heron Island. Oecologia 1969, 2, 232–250. [Google Scholar] [CrossRef]
- Haskin, H.H. Age determination in molluscs. Trans. N. Y. Acad. Sci. 1954, 16, 300–304. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Bizikov, V.A.; Doubleday, Z.A.; Laptikhovsky, V.V.; Lishchenko, F.V.; Perales-Raya, C.; Hollyman, P.R. Techniques for Estimating the Age and Growth of Molluscs: Cephalopoda. J. Shellfish Res. 2018, 37, 783–792. [Google Scholar] [CrossRef]
- Dürrani, Ö.; Ateşşahin, T.; Eroğlu, M.; Düşükcan, M. Morphological variations of an invasive cyprinid fish (Carassius gibelio) in lentic and lotic environments inferred from the body, otolith, and scale shapes. Acta Zool. 2023, 104, 458–472. [Google Scholar] [CrossRef]
- Ridgway, I.D.; Richardson, C.A.; Austad, S.N. Maximum shell size, growth rate, and maturation age correlate with longevity in bivalve molluscs. J. Gerontol. Ser. A 2010, 66, 183–190. [Google Scholar] [CrossRef]
- Punt, A.E.; Huang, T.; Maunder, M.N. Review of integrated size-structured models for stock assessment of hard-to-age crustacean and mollusc species. ICES J. Mar. Sci. 2013, 70, 16–33. [Google Scholar] [CrossRef]
- Başusta, N.; Dürrani, Ö.; Bat, L.; Başusta, A.; Dağtekin, M.; Seyhan, K. Modelling growth and population parameters of the invasive gastropod Rapana venosa (Muricidae) in the Black Sea using statolith-based ageing. Fish. Sci. 2025, 1–15. [Google Scholar] [CrossRef]
- Lasda Bergman, E.M. Finding Citations to Social Work Literature: The Relative Benefits of Using Web of Science, Scopus, or Google Scholar. J. Acad. Librariansh. 2012, 38, 370–379. [Google Scholar] [CrossRef]
- Cruz-Vásquez, R.; Rodríguez-Domínguez, G.; Alcántara-Razo, E.; Aragón-Noriega, E.A. Estimation of Individual Growth Parameters of the Cortes Geoduck Panopea globosa from the Central Gulf of California using a Multimodel Approach. J. Shellfish Res. 2012, 31, 725–732. [Google Scholar] [CrossRef]
- Cleveland, W.S.; Devlin, S.J. Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: http://www.R-project.org/ (accessed on 21 August 2025).
- Posit Team. RStudio: Integrated Development Environment for R Posit Software; PBC: Boston, MA, USA, 2025; Available online: https://www.posit.co (accessed on 21 August 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Galili, T. dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]
- Palomares, M.L.D.; Pauly, D. SeaLifeBase. World Wide Web Electronic Publication. 2024. Available online: www.sealifebase.org (accessed on 17 August 2025).
- WoRMS Editorial Board. World Register of Marine Species. 2025. Available online: https://www.marinespecies.org/imis.php?dasid=1447&doiid=170 (accessed on 17 August 2025).
- Kapeller, R. European Mollusks: Database and Determination Key [Photograph]. 2025. Available online: https://www.rkapeller.eu/index_en.html (accessed on 21 August 2025).
- Trausel, J.; Slieker, F. Malacopics: Mollusk Image Database; Naturalis Biodiversity Center: South Holland, The Netherlands, 2025; Available online: https://malacopics.nl/ (accessed on 21 August 2025).
- Acarli, S.; Lok, A.; Yigitkurt, S. Growth and Survival of Anadara Inaequivalvis (Bruguiere, 1789) in Sufa Lagoon, Izmir, Turkey. Isr. J. Aquac.-Bamidgeh 2012, 64, 1–7. [Google Scholar] [CrossRef]
- Dağtekin, M. The invasive mollusk Rapana venosa (Mollusca: Neogastropoda: Muricidae) in the mid-southern Black Sea: Distribution, growth, and stock structure. Acta Ichthyol. Pisc. 2023, 53, 191–199. [Google Scholar] [CrossRef]
- Mirzaei, M.R.; Yasin, Z.; Shau Hwai, A.T. Length-weight relationship, growth and mortality of Anadara granosa in Penang Island, Malaysia: An approach using length-frequency data sets. J. Mar. Biol. Assoc. UK 2015, 95, 381–390. [Google Scholar] [CrossRef]
- Narasimham, K. Biology of the blood clam Anadara granosa (Linnaeus) in Kakinada Bay. J. Mar. Biol. Assoc. India 1988, 30, 137–150. [Google Scholar]
- Ponsero, A.; Dabouineau, L.; Allain, J. Modelling of common European cockle Cerastoderma edule fishing grounds aimed at sustainable management of traditional harvesting. Fish. Sci. 2009, 75, 839–850. [Google Scholar] [CrossRef]
- Jelesias, J.I.P.; Navarro, E. Shell growth of the cockle Cerastoderma edule in the Mundaca estuary (north Spain). J. Molluscan Stud. 1990, 56, 229–238. [Google Scholar] [CrossRef]
- Maia, F.; Barroso, C.M.; Gaspar, M.B. Biology of the common cockle Cerastoderma edule (Linnaeus, 1758) in Ria de Aveiro (NW Portugal): Implications for fisheries management. J. Sea Res. 2021, 171, 102024. [Google Scholar] [CrossRef]
- Mahony, K.E.; Egerton, S.; Lynch, S.A.; Blanchet, H.; Goedknegt, M.A.; Groves, E.; Savoye, N.; de Montaudouin, X.; Malham, S.K.; Culloty, S.C. Drivers of growth in a keystone fished species along the European Atlantic coast: The common cockle Cerastoderma edule. J. Sea Res. 2022, 179, 102148. [Google Scholar] [CrossRef]
- Gam, M.; de Montaudouin, X.; Bazairi, H. Population dynamics and secondary production of the cockle Cerastoderma edule: A comparison between Merja Zerga (Moroccan Atlantic Coast) and Arcachon Bay (French Atlantic Coast). J. Sea Res. 2010, 63, 191–201. [Google Scholar] [CrossRef]
- Mohammad, S.; Mohallal, M.; Mohammed, S.; Attia, M. Age and Growth of the Cockles Cerastoderma glaucum and Papyridea papyracea in Lake Timsah, Suez Canal. Catrina Int. J. Environ. Sci. 2006, 1, 25–32. [Google Scholar]
- Calderon-Aguilera, L.E.; Aragón-Noriega, E.A.; Hand, C.M.; Moreno-Rivera, V.M. Morphometric Relationships, Age, Growth, and Mortality of the Geoduck Clam, Panopea generosa, Along the Pacific Coast of Baja California, Mexico. J. Shellfish Res. 2010, 29, 319–326. [Google Scholar] [CrossRef]
- Aragón-Noriega, E.A.; Calderon-Aguilera, L.E.; Pérez-Valencia, S.A. Modeling Growth of the Cortes Geoduck Panopea globosa from Unexploited and Exploited Beds in the Northern Gulf of California. J. Shellfish Res. 2015, 34, 119–127. [Google Scholar] [CrossRef]
- Hidalgo-De-La-Toba, J.A.; González-peláez, S.S.; Morales-Bojórquez, E.; Bautista-Romero, J.J.; Lluch-Cota, D.B. Geoduck Panopea generosa Growth at Its Southern Distribution Limit in North America using a Multimodel Inference Approach. J. Shellfish Res. 2015, 34, 91–99. [Google Scholar] [CrossRef]
- Hidalgo-de-la-Toba, J.A.; Vadopalas, B.; Lluch-Cota, D.B.; Morales-Bojórquez, E.; Bautista-Romero, J.J.; González-Peláez, S.S. Individual growth profiling improves growth modelling in the geoduck clam Panopea generosa. ICES J. Mar. Sci. 2020, 78, 112–124. [Google Scholar] [CrossRef]
- Todorova, V.R.; Panayotova, M.D.; Bekova, R.I.; Prodanov, B.K. Recovery of Flexopecten glaber (Linnaeus, 1758) (Bivalvia: Pectinidae) in the Bulgarian Black Sea waters: Recent distribution, population characteristics and future perspectives for protection and commercial utilization. Acta Zool. Bulg. 2022, 74, 437–444. [Google Scholar]
- Chauvaud, L.; Patry, Y.; Jolivet, A.; Cam, E.; Le Goff, C.; Strand, Ø.; Charrier, G.; Thébault, J.; Lazure, P.; Gotthard, K.; et al. Variation in Size and Growth of the Great Scallop Pecten maximus along a Latitudinal Gradient. PLoS ONE 2012, 7, e37717. [Google Scholar] [CrossRef]
- MacDonald, B.; Thompson, R. Intraspecific variation in growth and reproduction in latitudinally differentiated populations of the giant scallop Placopecten magellanicus (Gmelin). Biol. Bull. 1988, 175, 361–371. [Google Scholar] [CrossRef]
- García-March, J.R.; Hernandis, S.; Vázquez-Luis, M.; Prado, P.; Deudero, S.; Vicente, N.; Tena-Medialdea, J. Age and growth of the endangered fan mussel Pinna nobilis in the western Mediterranean Sea. Mar. Environ. Res. 2020, 153, 104795. [Google Scholar] [CrossRef] [PubMed]
- Garcia-March, J.R.; Marquez-Aliaga, A.; Wang, Y.-G.; Surge, D.; Kersting, D.K. Study of Pinna nobilis growth from inner record: How biased are posterior adductor muscle scars estimates? J. Exp. Mar. Biol. Ecol. 2011, 407, 337–344. [Google Scholar] [CrossRef]
- Nebot Colomer, E.; VÁZquez-Luis, M.; GarcÍA-March, J.R.; Deudero, S. Population Structure and Growth of the Threatened Pen Shell, Pinna rudis (Linnaeus, 1758) in a Western Mediterranean Marine Protected Area. Mediterr. Mar. Sci. 2016, 17, 785–793. [Google Scholar] [CrossRef]
- Hernandis, S.; Tena-Medialdea, J.; Téllez, C.; López, D.; Prado, P.; García-March, J.R. Suspended culture of Pinna rudis enhances survival and allows the development of a seasonal growth model for Mediterranean Pinnids. Aquaculture 2021, 543, 736964. [Google Scholar] [CrossRef]
- Gaspar, M.B.; Pereira, A.M.; Vasconcelos, P.; Monteiro, C.C. Age and growth of Chamelea gallina from the Algarve Coast (southern Portugal): Influence of seawater temperature and gametogenic cycle on growth rate. J. Molluscan Stud. 2004, 70, 371–377. [Google Scholar] [CrossRef]
- Bargione, G.; Vasapollo, C.; Donato, F.; Virgili, M.; Petetta, A.; Lucchetti, A. Age and Growth of Striped Venus Clam Chamelea gallina (Linnaeus, 1758) in the Mid-Western Adriatic Sea: A Comparison of Three Laboratory Techniques. Front. Mar. Sci. 2020, 7, 582703. [Google Scholar] [CrossRef]
- Boltacheva, N.; Mazlumyan, S. The growth and longevity of Chamelea gallina (Mollusca, Veneridae) in the Black Sea. Vestn. Zool. 2003, 37, 71–74. [Google Scholar]
- Delgado, M.; Silva, L.; Moura, P.; Sánchez-Leal, R.F.; Gaspar, M. Variaciones en los Índices de Crecimiento de Chamelea gallina (Mollusca: Bivalvia) en las Poblaciones del sur de Europa y su Relación con Variables Ambientales; Centro Oceanográfico de Cádiz: Cádiz, Spain, 2014. [Google Scholar]
- Dalgiç, G.; Okumuş, İ.; Karayücel, S. The effect of fishing on growth of the clam Chamelea gallina (Bivalvia: Veneridae) from the Turkish Black Sea coast. J. Mar. Biol. Assoc. U.K. 2010, 90, 261–265. [Google Scholar] [CrossRef]
- Moura, P.; Garaulet, L.; Vasconcelos, P.; Chainho, P.; Costa, J.; Gaspar, M. Age and growth of a highly successful invasive species: The manila clam Ruditapes philippinarum (Adams & Reeve, 1850) in the Tagus estuary (Portugal). Aquat. Invasions 2017, 12, 133–146. [Google Scholar] [CrossRef]
- Yoon, H.-S.; An, Y.-K.; Kim, S.-T.; Choi, S.-D. Age and growth of the short necked Ruditapes philippinarum on the south coast of Korea. Korean J. Malacol. 2011, 27, 1–7. [Google Scholar] [CrossRef]
- Humphreys, J.; Caldow, R.W.G.; McGrorty, S.; West, A.D.; Jensen, A.C. Population dynamics of naturalised Manila clams Ruditapes philippinarum in British coastal waters. Mar. Biol. 2007, 151, 2255–2270. [Google Scholar] [CrossRef]
- Fan, D.; Zhang, A.; Yang, Z.; Sun, X. Observations on shell growth and morphology of the bivalve Ruditapes philippinarum. Chin. J. Oceanol. Limnol. 2007, 25, 322–329. [Google Scholar] [CrossRef]
- Çolakoğlu, S.; Palaz, M. Some population parameters of Ruditapes philippinarum (Bivalvia, Veneridae) on the southern coast of the Marmara Sea, Turkey. Helgol. Mar. Res. 2014, 68, 539–548. [Google Scholar] [CrossRef]
- Sugiura, D.; Kikuya, N. Validation of the age estimation method using the shell section of the Manila clam Ruditapes philippinarum in Mutsu Bay, northern Japan. Aquac. Sci. 2017, 65, 193–202. [Google Scholar]
- Chung, E.-Y.; Ryou, D.-K.; Lee, J.-H. Gonadal development, age and growth of the shortnecked clam, Ruditapes philippinarum (Pelecypoda: Veneridae), on the coast of Kimje, Korea. Korean J. Malacol. 1994, 10, 38–48. [Google Scholar]
- Park, J.-S.; Kim, S.-Y. Growth status of Ruditapes philippinarum in Komso bay. J. Fish. Mar. Sci. Educ. 2009, 21, 230–236. [Google Scholar]
- Shelmerdine, R.L.; Adamson, J.; Laurenson, C.H.; Leslie, B. Size variation of the common whelk, Buccinum undatum, over large and small spatial scales: Potential implications for micro-management within the fishery. Fish. Res. 2007, 86, 201–206. [Google Scholar] [CrossRef]
- Santarelli, L.; Gros, P. Détermination de l’âge et de la croissance de Buccinum undatum L. (Gasteropoda: Prosobranchia) à l’aide des isotopes stables de la coquille et de l’ornementation operculaire. Oceanol. Acta 1985, 8, 221–229. [Google Scholar]
- Fahy, E.; Yalloway, G.; Gleeson, P. Appraisal of the Whelk Buccinum undatum Fishery of the Southern Irish Sea with Proposals for a Management Strategy; Department of the Marine: Singapore, 1995.
- Lahbib, Y.; Abidli, S.; Trigui El Menif, N. Laboratory Study of the Intracapsular Development and Juvenile Growth of the Banded Murex, Hexaplex trunculus. J. World Aquacult. Soc. 2010, 41, 18–34. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Barroso, C.M.; Gaspar, M.B. Morphometric relationships and relative growth of Hexaplex trunculus and Bolinus brandaris (Gastropoda: Muricidae) from the Ria Formosa lagoon (southern Portugal). J. Mar. Biol. Assoc. UK 2016, 96, 1417–1425. [Google Scholar] [CrossRef]
- Kasapoğlu, N. Population structure and shell dimension of the invasive veined whelk (Rapana venosa). J. Fish. 2021, 9, 91205. [Google Scholar] [CrossRef]
- Sağlam, N.E.; Sağlam, C.; Sağlam, Y.D. Determination of some population parameters of the veined rapa whelk (Rapana venosa) in the Central Black Sea. J. Mar. Biol. Assoc. UK 2015, 95, 123–129. [Google Scholar] [CrossRef]
- Choi, J.-D.; Ryu, D.-K. Age and growth of purple whelk, Rapana venosa (Gastropoda: Muricidae) in the west sea of Korea. Korean J. Malacol. 2009, 25, 189–196. [Google Scholar]
- Bitter, M.C.; Kapsenberg, L.; Gattuso, J.P.; Pfister, C.A. Standing genetic variation fuels rapid adaptation to ocean acidification. Nat. Commun. 2019, 10, 5821. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau, P.E.; Butlin, R.K.; Brönmark, C.; Edgell, T.C.; Hoverman, J.T.; Hollander, J. What can aquatic gastropods tell us about phenotypic plasticity? A review and meta-analysis. Heredity 2015, 115, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Mele, I.; McGill, R.A.R.; Thompson, J.; Fennell, J.; Fitzer, S. Ocean acidification, warming and feeding impacts on biomineralization pathways and shell material properties of Magallana gigas and Mytilus spp. Mar. Environ. Res. 2023, 186, 105925. [Google Scholar] [CrossRef]
- Morán, G.A.; Martínez, J.J.; Reyna, P.B.; Martín, J.; Malits, A.; Gordillo, S. Identifying environmental drivers of shell shape and size variation in a widely distributed marine bivalve along the Atlantic Patagonian coast. Zool. Anz. 2022, 299, 49–61. [Google Scholar] [CrossRef]
- Palomares, M.L.D.; Pauly, D. von Bertalanffy growth parameters of non-fish marine organisms. Fish. Cent. Res. Rep. 2008, 16, 137. [Google Scholar]
- Alfredo, H.-L.; David, A.R. Growth of fishes, crustaceans and molluscs: Estimation of the von Bertalanffy, Logistic, Gompertz and Richards curves and a new growth model. Mar. Ecol. Prog. Ser. 2004, 282, 237–244. [Google Scholar] [CrossRef]
- Carter, R.M. On the biology and palaeontology of some predators of bivalved mollusca. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1968, 4, 29–65. [Google Scholar] [CrossRef]
- Bayne, B.L. Phenotypic flexibility and physiological tradeoffs in the feeding and growth of marine bivalve molluscs. Integr. Comp. Biol. 2004, 44, 425–432. [Google Scholar] [CrossRef]
- Prieto, D.; Tamayo, D.; Urrutxurtu, I.; Navarro, E.; Ibarrola, I.; Urrutia, M.B. Nature more than nurture affects the growth rate of mussels. Sci. Rep. 2020, 10, 3539. [Google Scholar] [CrossRef]
- Grüss, A.; Biggs, C.R.; Heyman, W.D.; Erisman, B. Protecting juveniles, spawners or both: A practical statistical modelling approach for the design of marine protected areas. J. Appl. Ecol. 2019, 56, 2328–2339. [Google Scholar] [CrossRef]
- MacKenzie, C.L. The History, Present Condition, and Future of the Molluscan Fisheries of North and Central America and Europe; US Department of Commerce: Washington, DC, USA, 1997; Volume 127.
- Morton, B. Diet and predation behaviour exhibited by Cominella eburnea (Gastropoda: Caenogastropoda: Neogastropoda) in Princess Royal Harbour, Albany, Western Australia, with a review of attack strategies in the Buccinidae. Molluscan Res. 2006, 26, 39–50. [Google Scholar] [CrossRef]
- Ilano, A.S.; Miranda, R.M.T.; Fujinaga, K.; Nakao, S. Feeding behavior and food consumption of Japanese whelk, Buccinum isaotakii (Neogastropoda: Buccinidae). Fish. Sci. 2005, 71, 342–349. [Google Scholar] [CrossRef]
- Baillie, C.J.; Grabowski, J.H. Factors affecting recruitment, growth and survival of the eastern oyster Crassostrea virginica across an intertidal elevation gradient in southern New England. Mar. Ecol. Prog. Ser. 2019, 609, 119–132. [Google Scholar] [CrossRef]
- Meira, A.; Byers, J.E.; Sousa, R. A global synthesis of predation on bivalves. Biol. Rev. 2024, 99, 1015–1057. [Google Scholar] [CrossRef]
- Vafidis, D.; Antoniadou, C.; Voultsiadou, E.; Chintiroglou, C. Population structure of the protected fan mussel Pinna nobilis in the south Aegean Sea (eastern Mediterranean). J. Mar. Biol. Assoc. UK 2014, 94, 787–796. [Google Scholar] [CrossRef]
- Kozłowski, J.; Teriokhin, A.T. Allocation of energy between growth and reproduction: The Pontryagin Maximum Principle solution for the case of age-and season-dependent mortality. Evol. Ecol. Res. 1999, 1, 423–441. [Google Scholar]
- Haag, W.R.; Rypel, A.L. Growth and longevity in freshwater mussels: Evolutionary and conservation implications. Biol. Rev. 2011, 86, 225–247. [Google Scholar] [CrossRef]
- Bell, M.A.; Aguirre, W.E.; Buck, N.J. Twelve years of contemporary armor evolution in a threespine stickleback population. Evolution 2004, 58, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Félix-Hackradt, F.C.; Hackradt, C.W.; Treviño-Otón, J.; Pérez-Ruzafa, Á.; García-Charton, J.A. Effect of marine protected areas on distinct fish life-history stages. Mar. Environ. Res. 2018, 140, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.C.; Fan, Y.; Baker, G.L. Nutritional Value and Food Safety of Bivalve Molluscan Shellfish. J. Shellfish Res. 2018, 37, 695–708. [Google Scholar] [CrossRef]
- Cooley, S.R.; Lucey, N.; Kite-Powell, H.; Doney, S.C. Nutrition and income from molluscs today imply vulnerability to ocean acidification tomorrow. Fish Fish. 2012, 13, 182–215. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Hopper, G.W.; Kreeger, D.A.; Lopez, J.W.; Maine, A.N.; Sansom, B.J.; Schwalb, A.; Vaughn, C.C. Gains and Gaps in Knowledge Surrounding Freshwater Mollusk Ecosystem Services. Freshw. Mollusk Biol. Conserv. 2023, 26, 20–31. [Google Scholar] [CrossRef]
- Sousa, R. A brief global agenda for advancing the study of molluscs. Front. Ecol. Evol. 2024, 12, 1176380. [Google Scholar] [CrossRef]
- Dinevik, H.; Altenburger, A.; Bluhm, B.A. Slow growth and high longevity characterize the common, large Arctic brittle star, Ophiopleura borealis. Front. Mar. Sci. 2025, 12, 1555911. [Google Scholar] [CrossRef]
- Jobling, M. Bioenergetics: Feed intake and energy partitioning. In Fish Ecophysiology; Rankin, J.C., Jensen, F.B., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 1–44. [Google Scholar]
- Bascinar, N.; Bascinar, N.; Khan, U.; Seyhan, K. Effects of meal and body sizes on gastric evacuation rate in brook trout Salvelinus fontinalis (Mitchill, 1814) fed commercial pellets in group feeding. Indian J. Fish. 2017, 64, 50–54. [Google Scholar] [CrossRef]
- Basçinar, N.; Basçinar, N.S.; Seyhan, K.; Khan, U. The effect of temperature on the rate of gastric evacuation in brook trout Salvelinus fontinalis fed on commercial pellets: Group-feeding. Pak. J. Zool. 2016, 48, 1899–1904. [Google Scholar]
- Jackson, D.J. Mantle Modularity Underlies the Plasticity of the Molluscan Shell: Supporting Data From Cepaea nemoralis. Front. Genet. 2021, 12, 622400. [Google Scholar] [CrossRef]
- Byrne, M.; Foo, S.A.; Ross, P.M.; Putnam, H.M. Limitations of cross- and multigenerational plasticity for marine invertebrates faced with global climate change. Glob. Change Biol. 2020, 26, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Zhang, H.; Zheng, H. Selective breeding of edible bivalves and its implication of global climate change. Rev. Aquac. 2020, 12, 2559–2572. [Google Scholar] [CrossRef]

| Images accessed via WoRMS Editorial Board [31] | From SeaLifeBase [30] | |
|---|---|---|
| Habitats | Feeding Biology | |
 Anadara inaequivalvis [32] | Coastal areas with sandy or muddy substrates, often in brackish waters. | Filter feeder that consumes plankton and organic particles. |
 Anadara kagoshimensis [32] | Shallow marine environments with sandy or muddy bottoms. | Filter feeder feeding on suspended organic matter and plankton. |
 Tegillarca granosa [33] (actual size: 43 mm) | Intertidal and subtidal zones with muddy substrates often found in estuaries. | Filter feeder, primarily consuming phytoplankton. |
 Cerastoderma edule [32] | Sandy or muddy substrates in intertidal zones, common in estuaries and lagoons. | Filter feeder feeding on phytoplankton and organic detritus. |
 Cerastoderma glaucum [33] | Brackish and marine environments, often in lagoons and estuaries. | Filter feeder that consumes plankton and organic matter. |
 Panopea generosa [33] | Deep sandy or muddy substrates in subtidal zones. | Filter feeder placed on plankton and suspended organic material. |
 Flexopecten glaber [33] | Sandy or muddy substrates in shallow subtidal zones. | Filter feeder, primarily consuming phytoplankton. |
 Pecten maximus [32] | Sandy gravel or coarse substrates in subtidal zones. | Filter feeder feeding on phytoplankton and organic particles. |
 Placopecten magellanicus [33] | Deep, sandy, or gravel substrates in cold waters. | Filter feeder that consumes phytoplankton and organic detritus. |
 Pinna nobilis [33] | Seagrass meadows in shallow, sheltered marine environments. | Filter feeder placed on plankton and suspended organic material. |
 Chamelea gallina [32] | Sandy or muddy substrates in shallow coastal waters. | Filter feeder that consumes phytoplankton and organic particles. |
 Mercenaria mercenaria [32] | Sandy or muddy substrates in estuaries and lagoons. | Filter feeder feeding on phytoplankton and organic detritus. |
 Ruditapes philippinarum [32] | Sandy or muddy substrates in the intertidal and shallow subtidal zones. | Filter feeder that consumes phytoplankton and organic matter. |
 Buccinum undatum [33] | Cold, deep marine waters with sandy or muddy substrates. | Scavenger and predator feeding on carrion and small invertebrates. |
 Hexaplex trunculus [32] | Rocky or sandy substrates in shallow coastal waters. | Carnivorous, preying on bivalves and other molluscs. |
 Rapana venosa [32] | Sandy or muddy substrates in estuaries and shallow coastal waters. | Carnivorous, feeding on bivalves and other molluscs. |
| Species | Age Determination | VBGF | Study Area | References | |||
|---|---|---|---|---|---|---|---|
| Ageing | Method | L∞ (mm) | k | t0 | |||
| Anadara inaequivalvis | Other | Length-frequency | 29.65 | 1.19 | 0.30 | Sufa Lagoon, Türkiye | Acarli et al. [34] |
| Anadara kagoshimensis | Indirect | Length-frequency | 121.78 | 0.25 | −0.33 | Western Black Sea | Dağtekin [35] |
| Tegillarca granosa | Direct | Shell growth ring | 35.40 | 0.37 | −0.14 | Balik Pulau, Malaysia | Mirzaei et al. [36] |
| Indirect | Length-frequency | 73.40 | 0.58 | −0.49 | Kakinada Bay, India | Narasimham [37] | |
| Cerastoderma edule | Direct | Shell growth ring | 34.36 | 0.64 | 0.00 | Bay of Saint-Brieuc, France | Ponsero et al. [38] |
| Direct | Shell growth ring | 28.27 | 0.03 | 8.99 | Mundaka Estuary, Spain | Jelesias and Navarro [39] | |
| Direct | Shell growth ring | 40.70 | 0.74 | 0.30 | Mira Channel, Portugal | Maia et al. [40] | |
| Direct | Shell growth ring | 35.80 | 0.64 | −0.95 | NE Atlantic Coasts (Ireland, Wales, and France) | Mahony et al. [41] | |
| Direct | Shell growth ring | 45.04 | 0.22 | −3.24 | Ireland | ||
| Direct | Shell growth ring | 42.74 | 0.29 | −2.34 | Ireland | ||
| Direct | Shell growth ring | 36.15 | 0.34 | −2.25 | Wales | ||
| Direct | Shell growth ring | 43.24 | 0.40 | −0.63 | Ireland | ||
| Direct | Shell growth ring | 40.74 | 0.47 | −0.40 | Ireland | ||
| Direct | Shell growth ring | 34.29 | 0.34 | −2.07 | France | ||
| Direct | Shell growth ring | 31.20 | 1.43 | −0.05 | Merja Zerga, Morocco; French Atlantic coast | Gam et al. [42] | |
| Direct | Shell growth ring | 38.40 | 1.30 | −0.08 | Arcachon Bay, France; Moroccan Atlantic Coast | ||
| Cerastoderma glaucum | Direct | Shell growth ring | 38.28 | 0.21 | −1.27 | Lake Timsah, Egypt | Mohammad et al. [43] |
| Direct | Shell growth ring | 37.86 | 0.22 | −0.96 | Lake Timsah, Egypt | ||
| Direct | Shell growth ring | 46.45 | 0.15 | −2.17 | S. Sinai Coast, Egypt | ||
| Direct | Shell growth ring | 32.17 | 0.36 | −0.38 | S. Sinai Coast, Egypt | ||
| Panopea generosa | Direct | Shell growth ring | 134.00 | 0.19 | −4.40 | Gulf of California, Mexico | Calderon-Aguilera et al. [44] |
| Direct | Shell growth ring | 163.40 | 0.19 | −2.99 | San Felipe, Mexico | Aragón-Noriega et al. [45] | |
| 157.50 | 0.11 | −1.2 | Puerto Peñasco, Mexico | ||||
| Direct | Shell growth ring | 122.16 | 0.50 | 2.26 | Guaymas-Empalme Bay, Mexico | Cruz-Vásquez et al. [24] | |
| Direct | Shell growth ring | 122.59 | 0.26 | −1.78 | Gulf of California, Mexico | Hidalgo-De-La-Toba et al. [46] | |
| Direct | Shell growth ring | 135.00 | 0.20 | −0.07 | Gulf of California, Mexico | Hidalgo-de-la-Toba et al. [47] | |
| Flexopecten glaber | Other | Mark/recapture | 54.00 | 0.24 | −1.30 | Bulgarian Black Sea | Todorova et al. [48] |
| Pecten maximus | Direct | Shell growth ring | 109.70 | 0.67 | 0.50 | Ría de Vigo, Spain | Chauvaud et al. [49] |
| Direct | Shell growth ring | 155.90 | 0.20 | 0.36 | Austevoll, Norway | ||
| Direct | Shell growth ring | 101.10 | 0.68 | 0.47 | Île de Ré, France | ||
| Direct | Shell growth ring | 103.60 | 0.83 | 0.56 | The Bay of Brest, France | ||
| Direct | Shell growth ring | 108.40 | 0.87 | 0.58 | Bay of Seine, France | ||
| Direct | Shell growth ring | 108.40 | 0.61 | 0.48 | Plymouth, UK | ||
| Direct | Shell growth ring | 143.60 | 0.26 | 0.41 | Holyhead, UK | ||
| Direct | Shell growth ring | 137.00 | 0.25 | 0.40 | Scarborough, UK | ||
| Direct | Shell growth ring | 146.90 | 0.23 | 0.19 | Campbeltown, UK | ||
| Direct | Shell growth ring | 127.20 | 0.28 | 0.42 | Bessaker, Norway | ||
| Direct | Shell growth ring | 133.50 | 0.23 | 0.54 | Brønnøysund, Norway | ||
| Direct | Shell growth ring | 144.50 | 0.24 | 0.56 | Træna, Norway | ||
| Placopecten magellanicus | Other | Nursery/farming | 176.50 | 0.19 | 0.55 | Sunnyside, NL, Canada (10 m) | MacDonald and Thompson [50] |
| Other | Nursery/farming | 158.40 | 0.16 | 0.10 | Sunnyside, NL, Canada (31 m) | ||
| Other | Nursery/farming | 166.90 | 0.21 | 0.51 | St. Andrews, NB, Canada (10 m) | ||
| Other | Nursery/farming | 166.00 | 0.21 | 0.53 | St. Andrews, NB, Canada (31 m) | ||
| Other | Nursery/farming | 155.90 | 0.22 | 0.32 | New Jersey Coast, USA (31 m) | ||
| Pinna nobilis | Direct | Shell growth ring | 439.00 | 0.21 | −0.57 | Es Freus, Spain | García-March et al. [51] |
| Direct | Shell growth ring | 624.00 | 0.19 | −0.05 | W. Mediterranean Sea, Spain | ||
| Direct | Shell growth ring | 587.00 | 0.19 | −0.40 | Tabarca Island, Spain | ||
| Direct | Shell growth ring | 654.00 | 0.15 | −0.95 | Port-Cros, France | ||
| Direct | Shell growth ring | 399.00 | 0.29 | 0.24 | La Olla, Spain | ||
| Direct | Shell growth ring | 582.00 | 0.37 | −0.06 | Mar Menor, Spain | ||
| Direct | Shell growth ring | 456.00 | 0.21 | −0.88 | Moraira, Spain | ||
| Direct | Shell growth ring | 607.00 | 0.24 | 0.12 | El Racó, Spain | ||
| Direct | Shell growth ring | 569.00 | 3.90 | −0.04 | Dénia, Spain | ||
| Direct | Shell growth ring | 560.00 | 4.50 | −0.04 | Île des Embiez, France | ||
| Direct | Shell growth ring | 655.00 | 1.90 | −1.78 | Balearic Islands, Spain | ||
| Direct | Shell growth ring | 750.00 | 2.60 | −0.03 | Alfacs Bay, Spain | ||
| Direct | Shell growth ring | 456.00 | 0.21 | −0.88 | Moraira, Spain | García-March et al. [51] | |
| Direct | Shell growth ring | 60.70 | 0.24 | 0.12 | El Racó, Spain | ||
| Direct | Shell growth ring | 569.00 | 0.24 | −0.04 | Dénia, Spain | ||
| Direct | Shell growth ring | 560.00 | 0.30 | 0.28 | Île des Embiez, France | ||
| Direct | Shell growth ring | 655.00 | 0.13 | −1.78 | Balearic Islands, Spain | ||
| Direct | Shell growth ring | 750.00 | 0.18 | −0.03 | Alfacs Bay, Spain | ||
| Direct | Shell growth ring | 631.00 | 0.17 | −0.67 | Site SO, W. Mediterranean * | ||
| Direct | Shell growth ring | 430.00 | 0.23 | −0.47 | Site EO, W. Mediterranean * | ||
| Direct | Shell growth ring | 565.00 | 0.30 | −0.05 | Site LG, W. Mediterranean * | ||
| Direct | Shell growth ring | 560.00 | 0.14 | −3.04 | Moraira Bay and Illa Grossa, Spain | Garcia-March et al. [52] | |
| Direct | Shell growth ring | 573.00 | 0.16 | 0.02 | Moraira Bay and Illa Grossa, Spain | ||
| Direct | Shell growth ring | 452.70 | 0.14 | −3.80 | Cabrera Island, Spain | Nebot Colomer et al. [53] | |
| Direct | Shell growth ring | 452.70 | 0.14 | −0.80 | Cabrera Island, Spain | ||
| Direct | Shell growth ring | 459.40 | 0.15 | −3.44 | Foradada Islet, Spain | ||
| Direct | Shell growth ring | 459.40 | 0.15 | −0.44 | Foradada Islet, Spain | ||
| Other | Nursery/farming | 290.60 | 1.16 | 0.18 | Vila Joiosa, Spain | Hernandis et al. [54] | |
| Chamelea gallina | Direct | Shell growth ring | 37.55 | 0.71 | −0.01 | Portugal Coast and the Mediterranean Sea | Gaspar et al. [55] |
| Direct | Shell growth ring | 43.90 | 0.26 | −0.84 | Ancona, Italy | Bargione et al. [56] | |
| Direct | Shell growth ring | 27.25 | 0.61 | −0.14 | Russian Black Sea | Boltacheva and Mazlumyan [57] | |
| Direct | Shell growth ring | 36.11 | 0.79 | −0.45 | Huelva Coast, Spain | Delgado et al. [58] | |
| Direct | Shell growth ring | 26.60 | 0.22 | −1.21 | Black Sea (closed area) | Dalgiç et al. [59] | |
| Direct | Shell growth ring | 28.88 | 0.21 | −1.29 | Black Sea (non-dredged) | ||
| Direct | Shell growth ring | 26.00 | 0.16 | −1.96 | Black Sea (dredged) | ||
| Mercenaria mercenaria | Direct | Shell growth ring | 84.50 | 0.11 | −1.59 | Narragansett Bay, RI, USA | |
| Direct | Shell growth ring | 80.13 | 0.15 | −0.54 | Southampton Water, UK | ||
| Ruditapes philippinarum | Direct | Shell growth ring | 65.20 | 0.34 | −0.93 | Tagus Estuary, Portugal | Moura et al. [60] |
| Direct | Shell growth ring | 51.01 | 0.17 | −1.07 | Goheung Coast, South Korea | Yoon et al. [61] | |
| Indirect | Length-frequency | 43.32 | 0.70 | −0.27 | Poole Harbour, UK | Humphreys et al. [62] | |
| Direct | Shell growth ring | 60.00 | 0.20 | −0.15 | Jiaozhou Bay, China | Fan et al. [63] | |
| Direct | Shell growth ring | 67.14 | 0.33 | −0.91 | Bandırma Bay, Türkiye | Çolakoğlu and Palaz [64] | |
| Direct | Shell growth ring | 36.90 | 0.74 | −0.24 | Mutsu Bay, Japan | Sugiura and Kikuya [65] | |
| Direct | Shell growth ring | 68.34 | 0.22 | −0.42 | Gimje Coast, South Korea | Chung et al. [66] | |
| Direct | Shell growth ring | 42.83 | 0.46 | −0.59 | Jeonbuk, S. Korea | Park and Kim [67] | |
| Buccinum undatum | Indirect | Length frequency | 101.10 | 0.39 | −3.19 | Shetland, UK | Shelmerdine et al. [68] |
| Indirect | Length-frequency | 102.04 | 0.40 | −3.20 | Shetland, UK | ||
| Indirect | Length-frequency | 104.87 | 0.30 | −2.17 | E. Shetland, UK | ||
| Indirect | Length-frequency | 99.02 | 0.39 | −2.66 | E. Shetland, UK | ||
| Indirect | Length-frequency | 185.67 | 0.03 | −3.65 | W. Shetland, UK | ||
| Indirect | Length-frequency | 157.52 | 0.09 | −0.32 | W. Shetland, UK | ||
| Other | Stable isotopes | 66.05 | 0.39 | 0.96 | S. England Coast, UK | ||
| Other | Stable isotopes | 112.49 | 0.12 | 0.60 | Normano-Breton Gulf, France | Santarelli and Gros [69] | |
| Direct | Operculum | 115.55 | 0.08 | −1.77 | Courtown, Ireland | Fahy et al. [70] | |
| Direct | Operculum | 121.72 | 0.13 | 0.28 | Howth/Kish Bank, Ireland | ||
| Direct | Operculum | 121.66 | 0.11 | −0.36 | S. Irish Sea | ||
| Hexaplex trunculus | Other | Laboratory | 38.27 | 0.09 | −0.31 | Bizerte Lagoon, Tunisia | Lahbib et al. [71] |
| Other | Mark/recapture | - | - | - | Ria Formosa Lagoon, Portugal | Vasconcelos et al. [72] | |
| Rapana venosa | Indirect | Length-frequency | 102.90 | 0.09 | −1.25 | Trabzon, Türkiye | Kasapoğlu [73] |
| Indirect | Length-frequency | 112.35 | 0.31 | −0.49 | Samsun, Türkiye | Sağlam et al. [74] | |
| Direct | Operculum | 199.65 | 0.10 | −2.48 | Yellow Sea, Korea | Choi and Ryu [75] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dürrani, Ö.; Cengiz, Ç.C.; Gabrielczak, H.; Özcan, E.; Varshanidze, M.; Belmonte, G.; Seyhan, K. Review of Age Estimation Techniques and Growth Models for Shelled Organisms in Marine Animal Forests. J. Mar. Sci. Eng. 2025, 13, 1693. https://doi.org/10.3390/jmse13091693
Dürrani Ö, Cengiz ÇC, Gabrielczak H, Özcan E, Varshanidze M, Belmonte G, Seyhan K. Review of Age Estimation Techniques and Growth Models for Shelled Organisms in Marine Animal Forests. Journal of Marine Science and Engineering. 2025; 13(9):1693. https://doi.org/10.3390/jmse13091693
Chicago/Turabian StyleDürrani, Ömerhan, Çağdaş Can Cengiz, Halyna Gabrielczak, Esra Özcan, Madona Varshanidze, Genuario Belmonte, and Kadir Seyhan. 2025. "Review of Age Estimation Techniques and Growth Models for Shelled Organisms in Marine Animal Forests" Journal of Marine Science and Engineering 13, no. 9: 1693. https://doi.org/10.3390/jmse13091693
APA StyleDürrani, Ö., Cengiz, Ç. C., Gabrielczak, H., Özcan, E., Varshanidze, M., Belmonte, G., & Seyhan, K. (2025). Review of Age Estimation Techniques and Growth Models for Shelled Organisms in Marine Animal Forests. Journal of Marine Science and Engineering, 13(9), 1693. https://doi.org/10.3390/jmse13091693







