Design of Organic Rankine Cycle Recovering Multi-Grade Waste Heat from a Two-Stroke Marine Engine
Abstract
1. Introduction
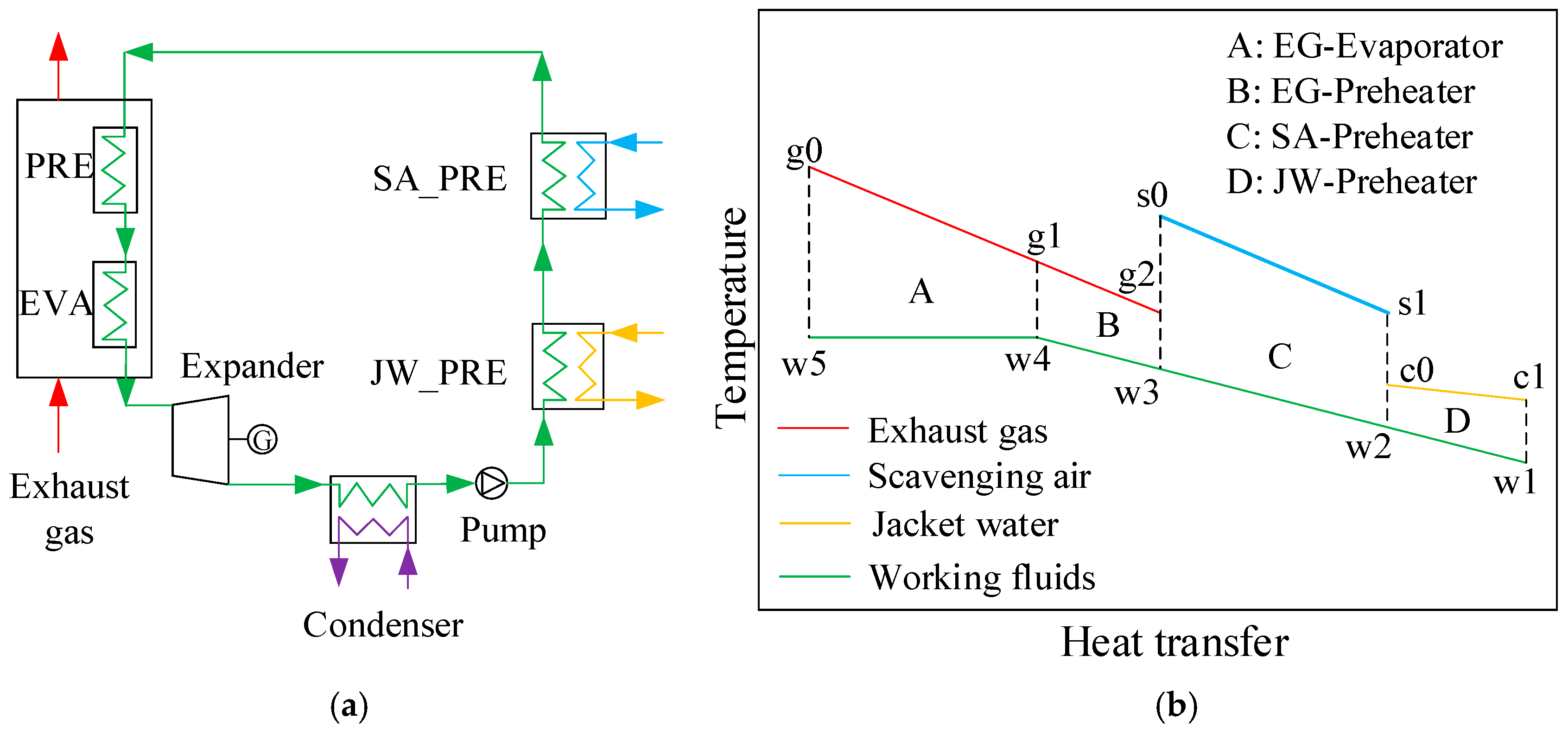
2. System Description
2.1. ORC System
2.2. Thermodynamic Description
- (1)
- Evaporator:
- (2)
- Expander:
- (3)
- Condenser:
- (4)
- Pump:
3. Model Building and Validation
3.1. Model Building
3.2. Model Validation
4. Results and Discussion
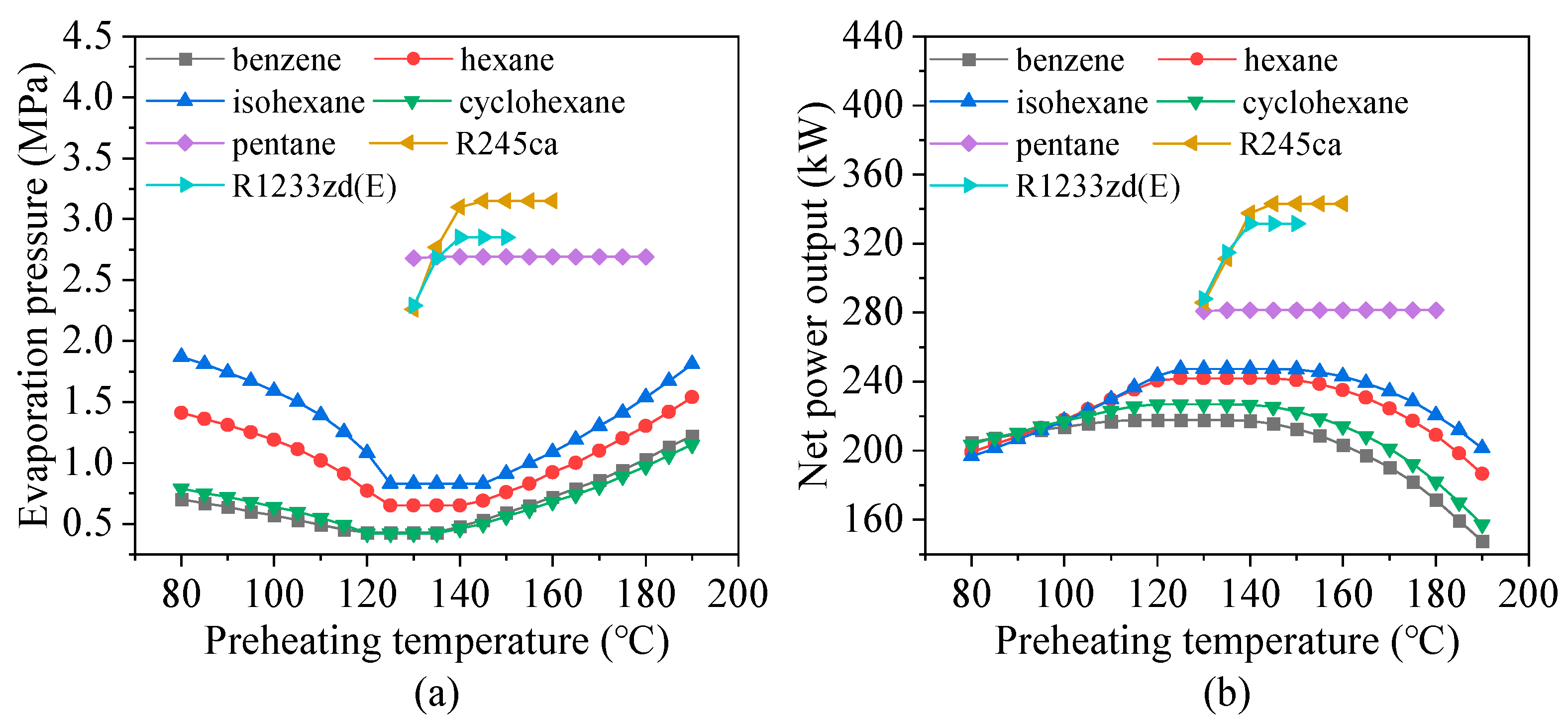
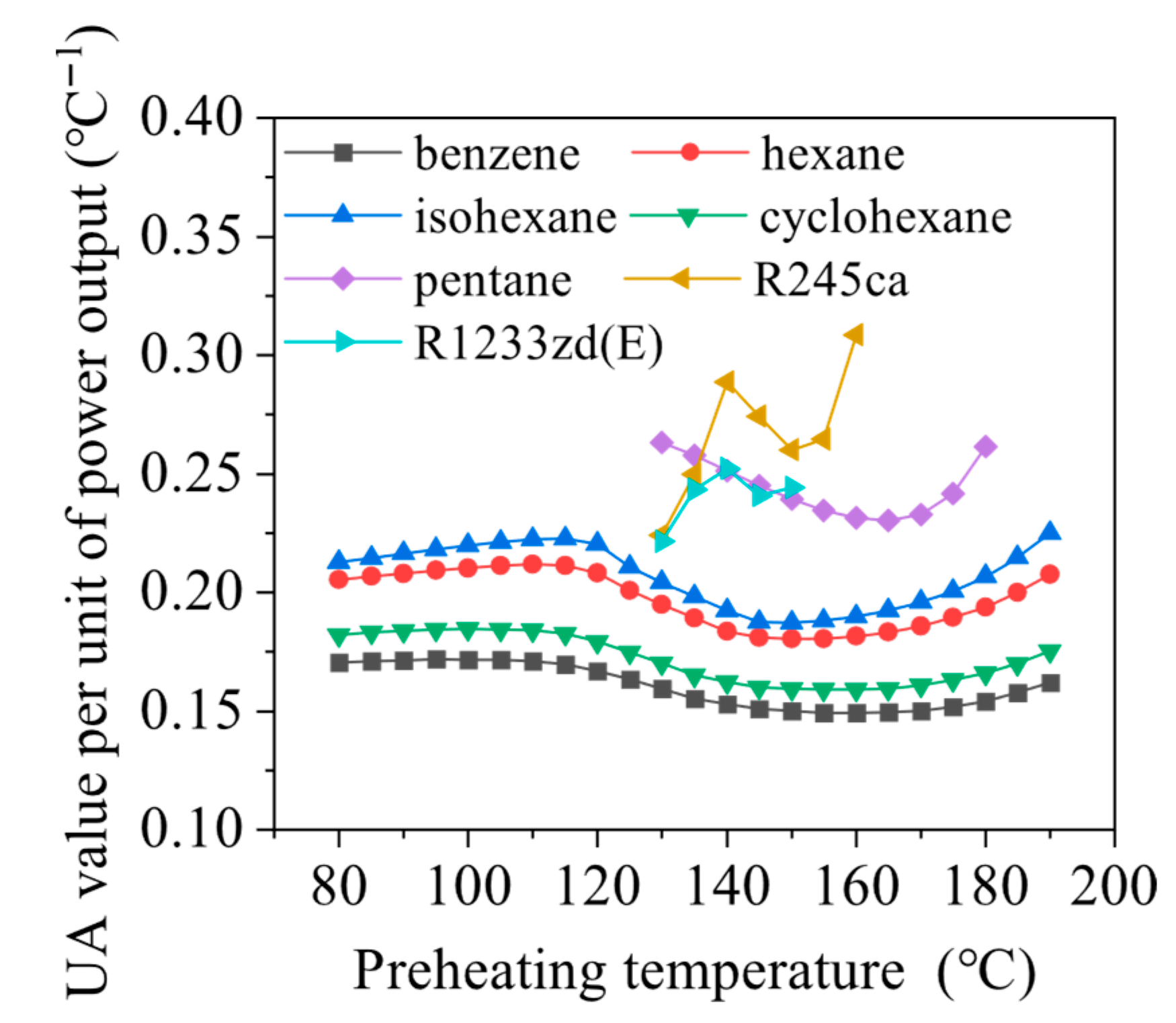
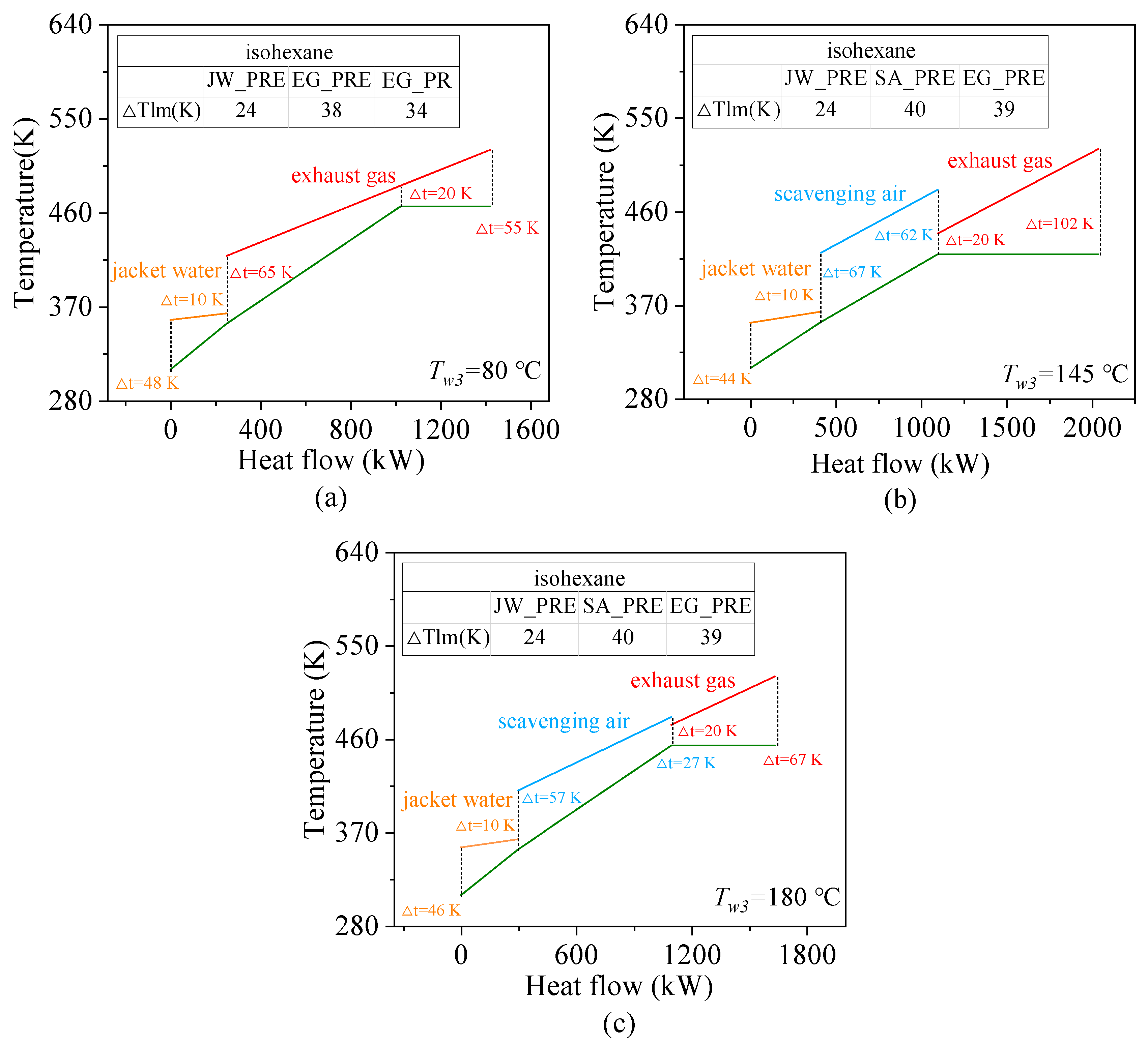
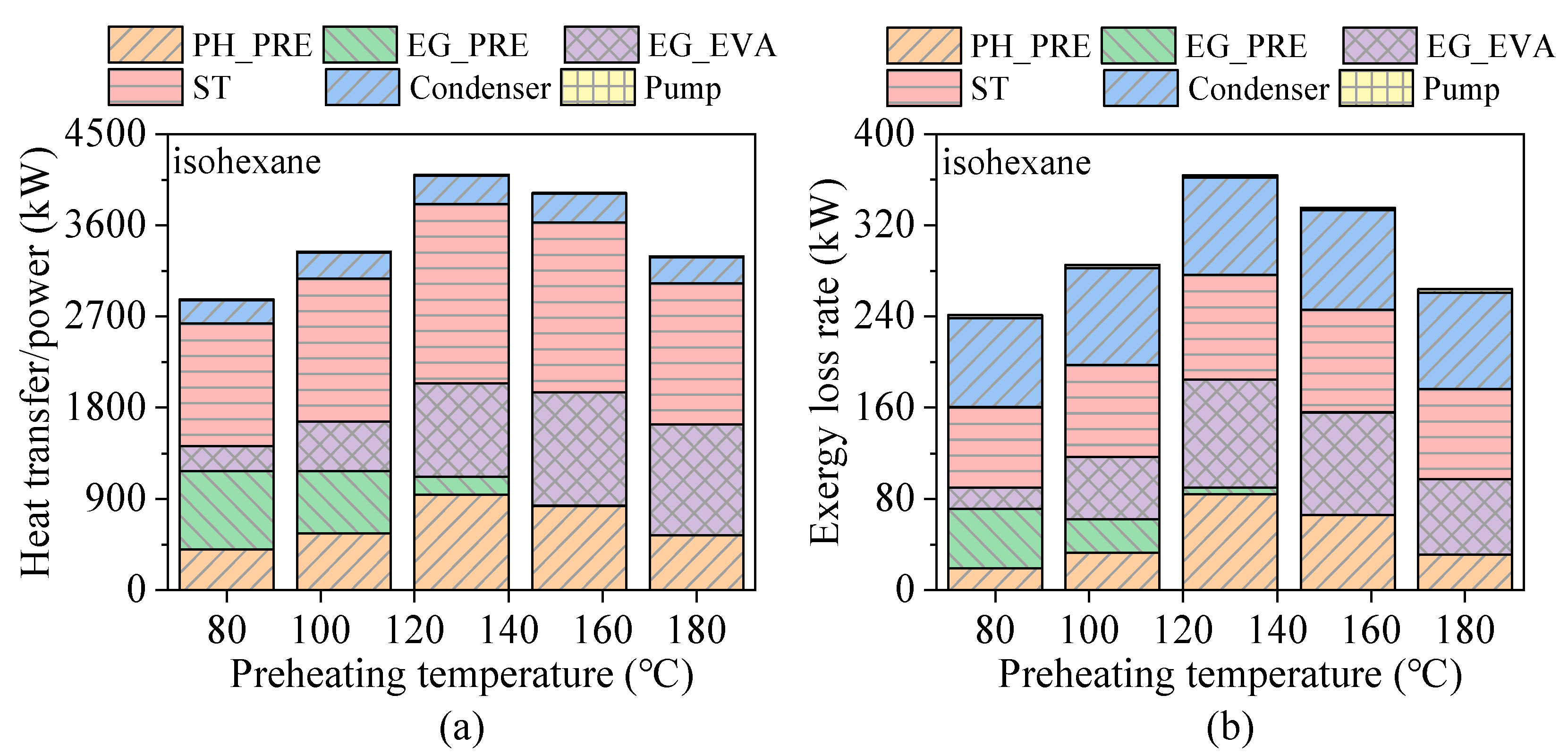
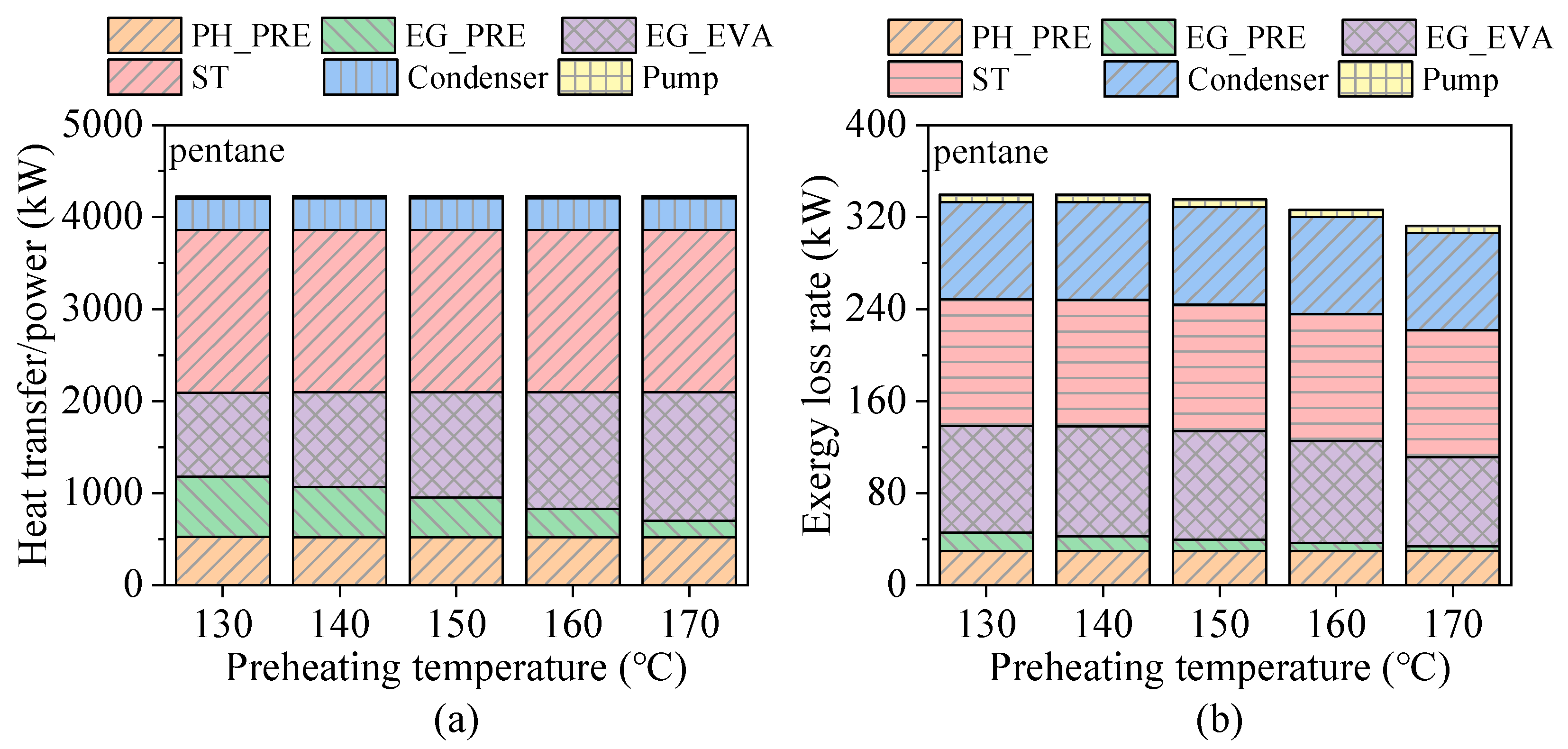
4.1. Effects of Preheating Temperature
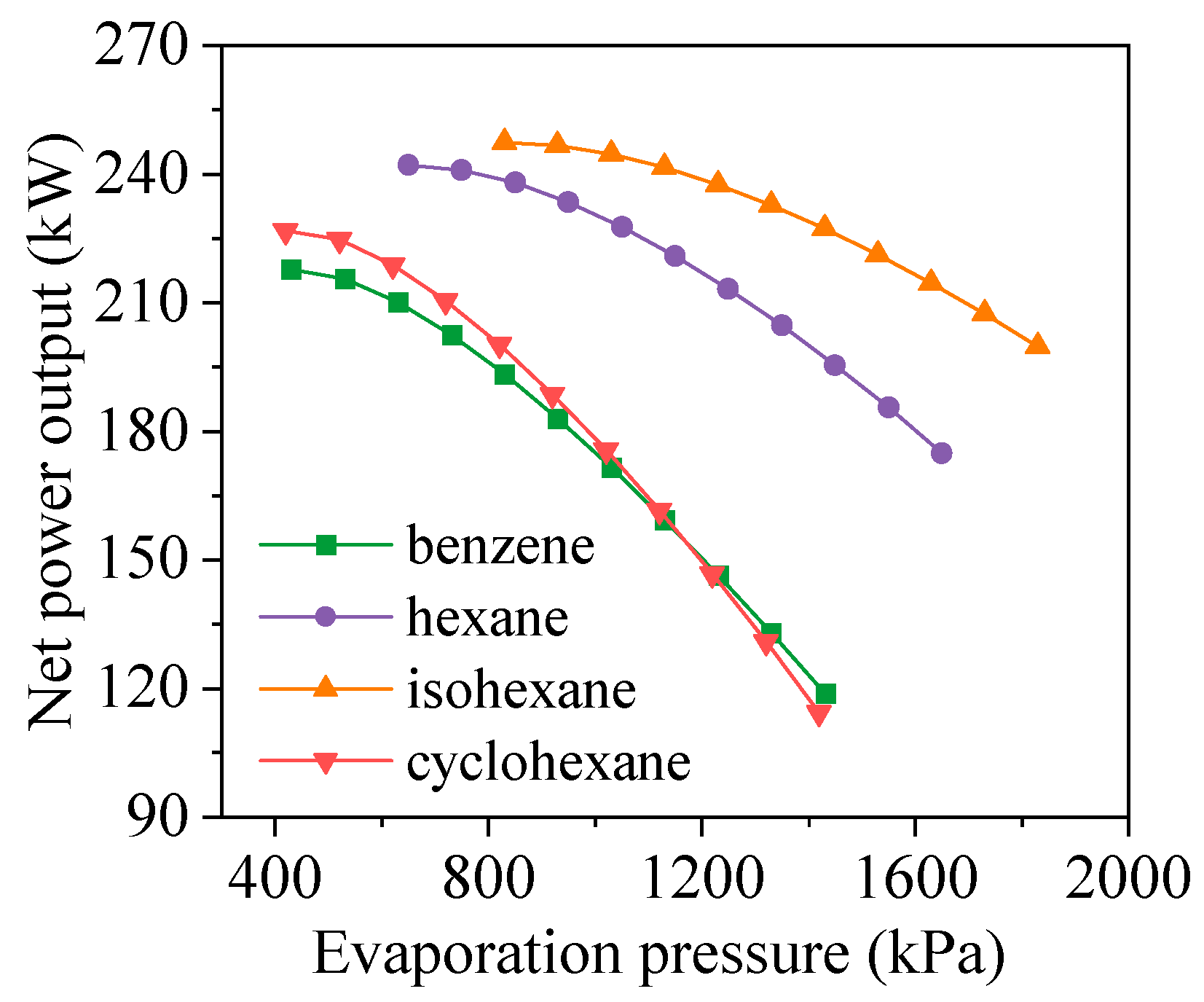
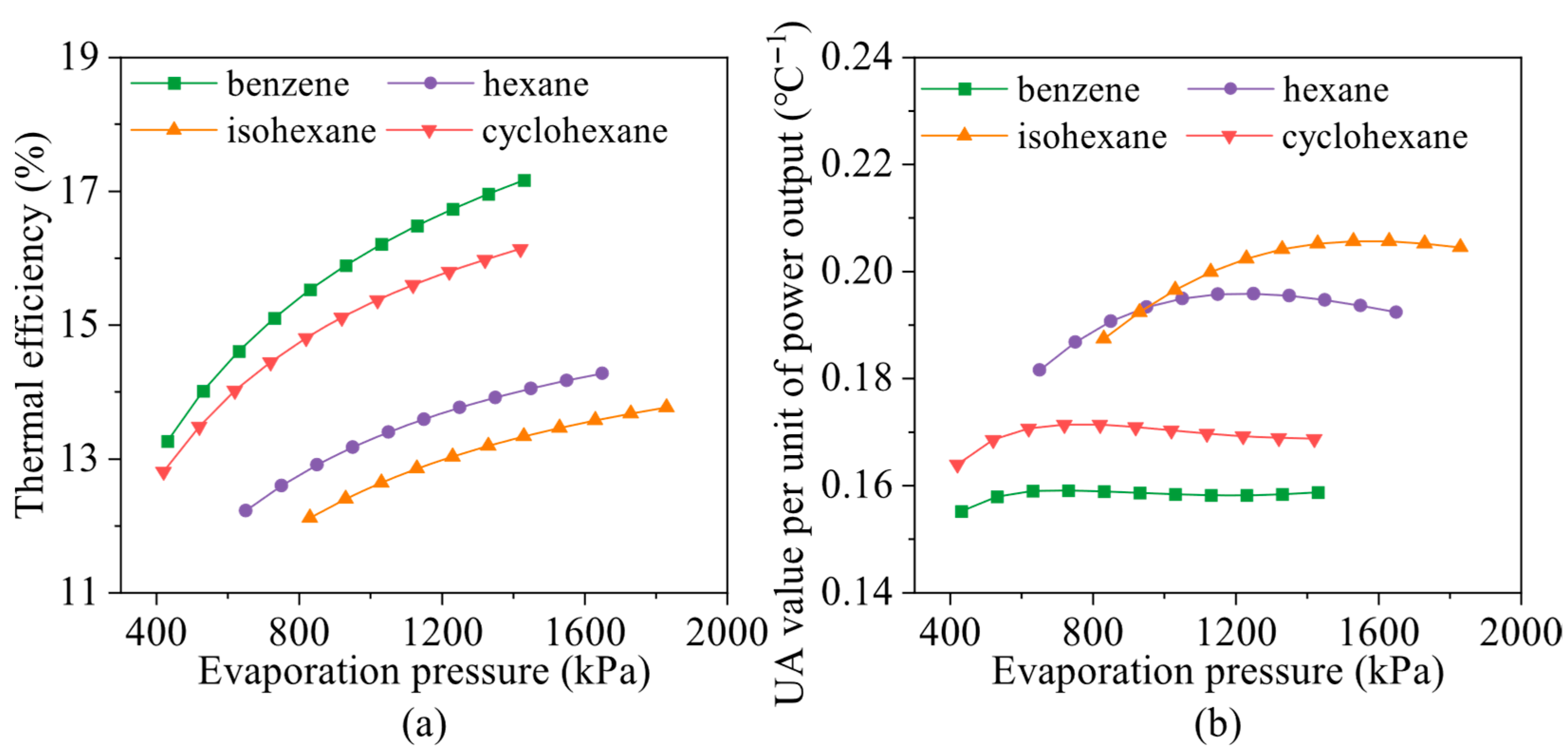
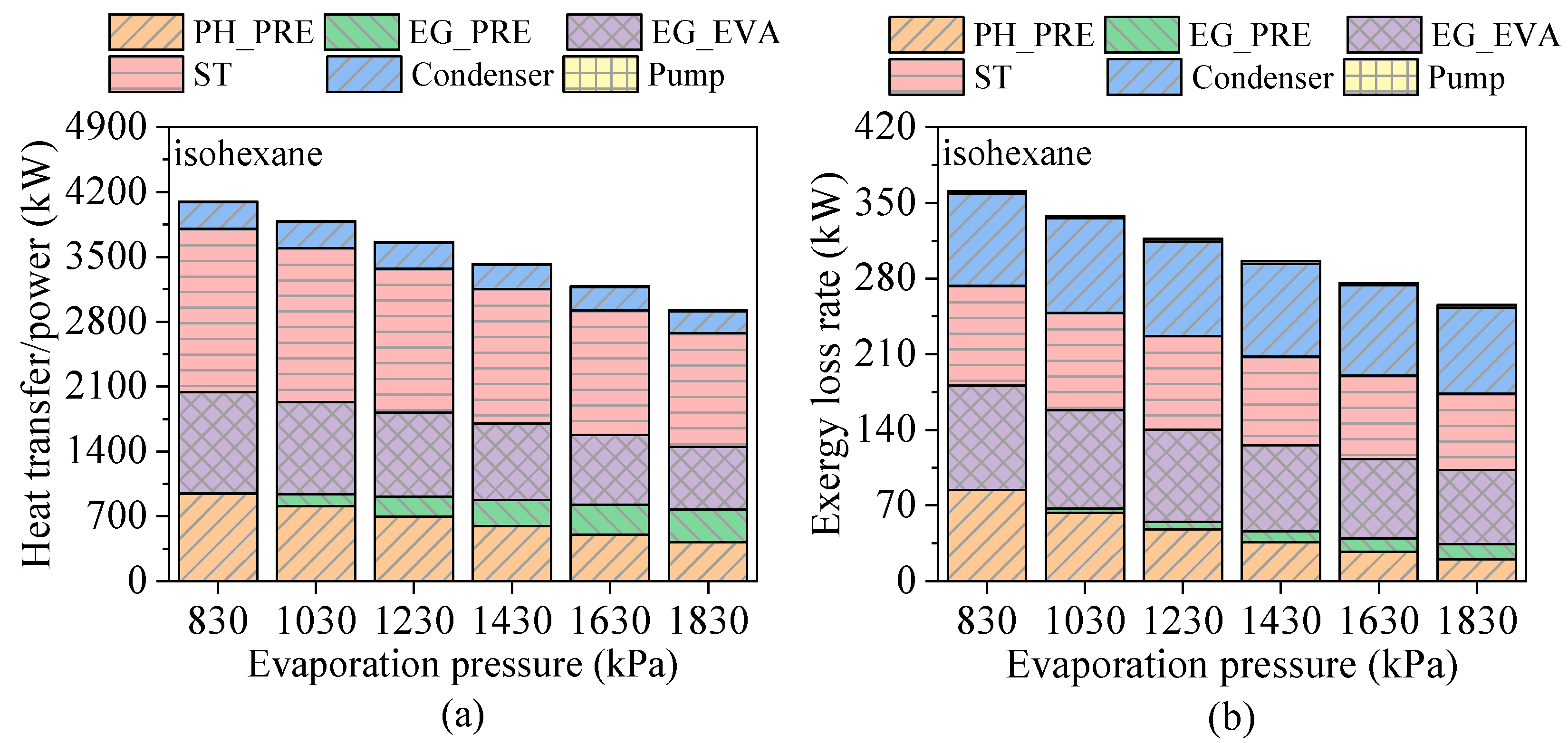
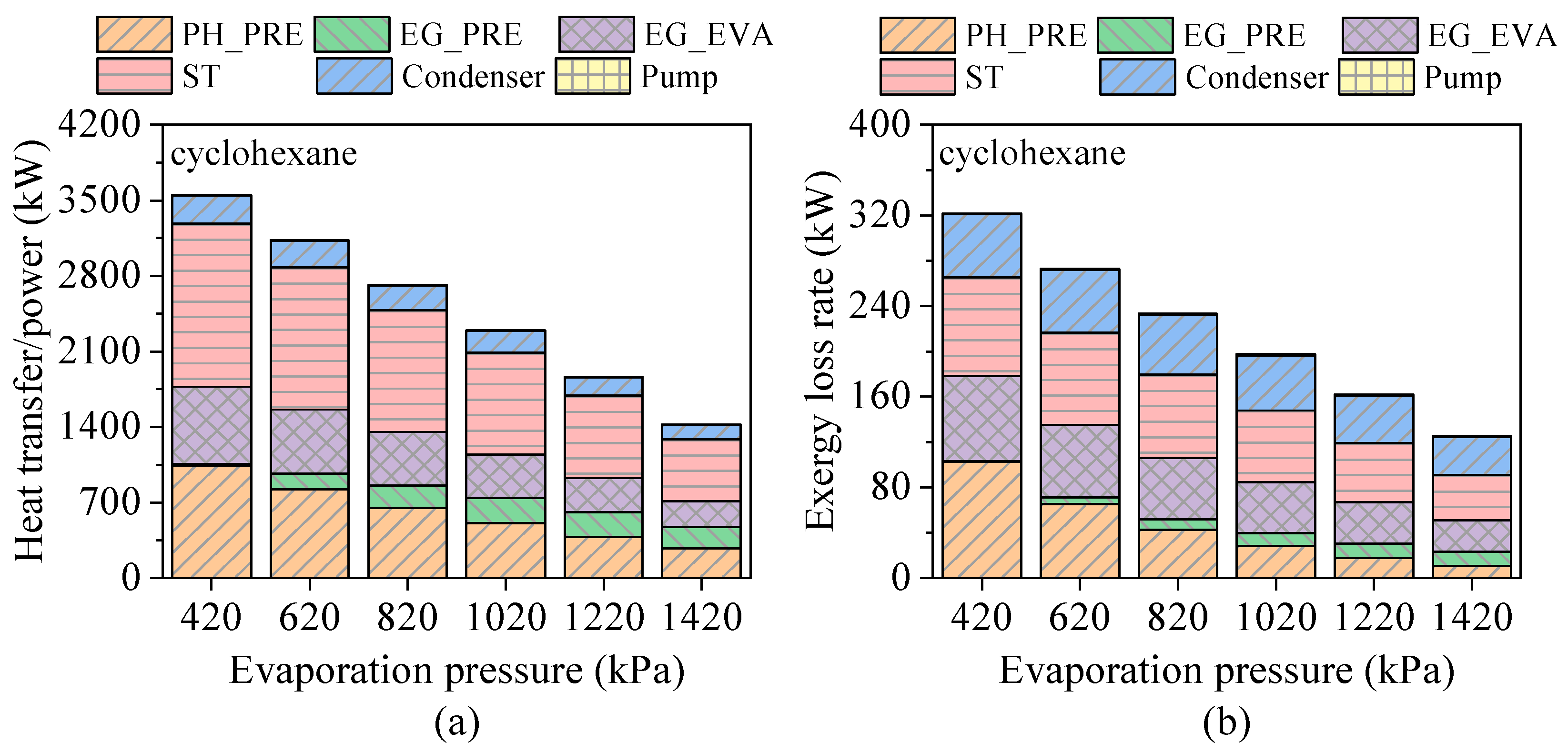
4.2. Effects of Evaporation Pressure
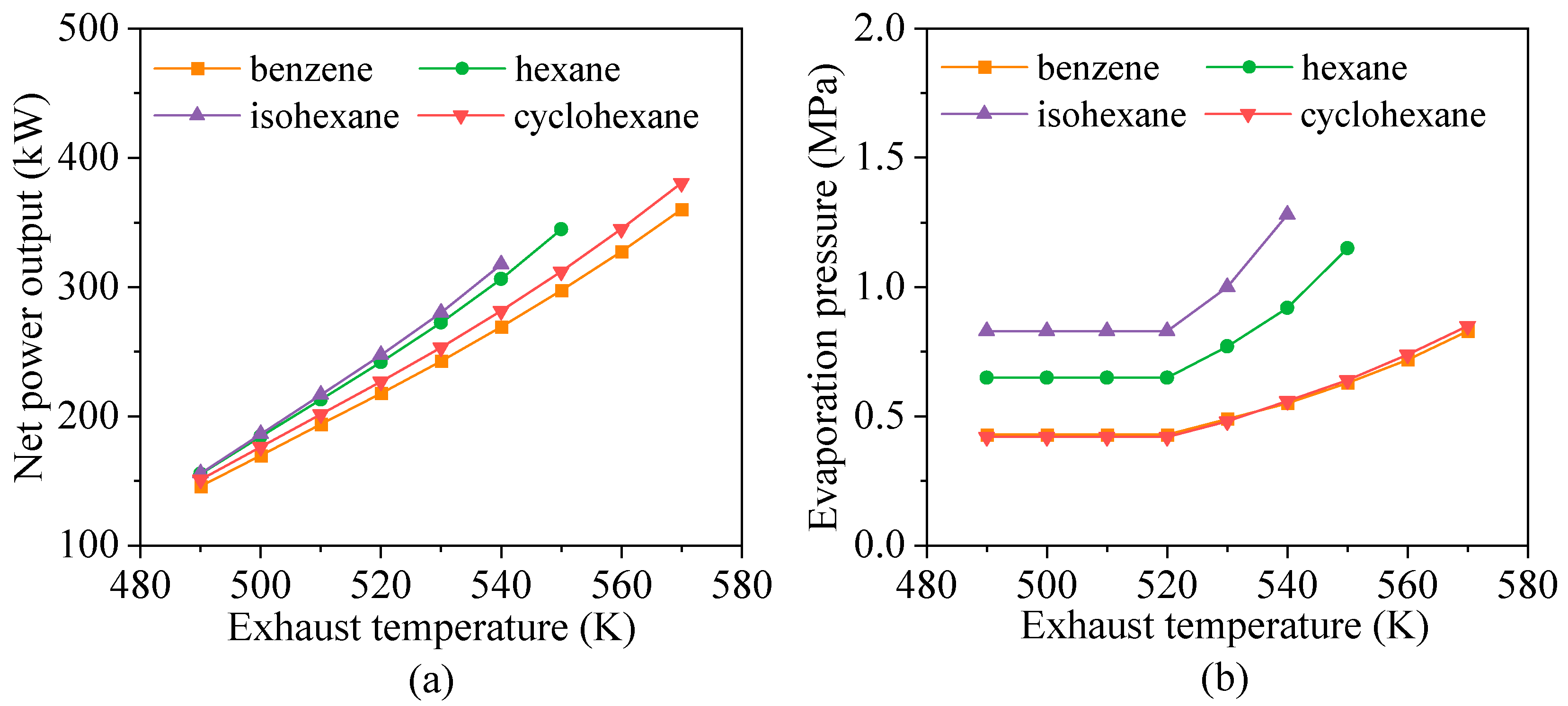
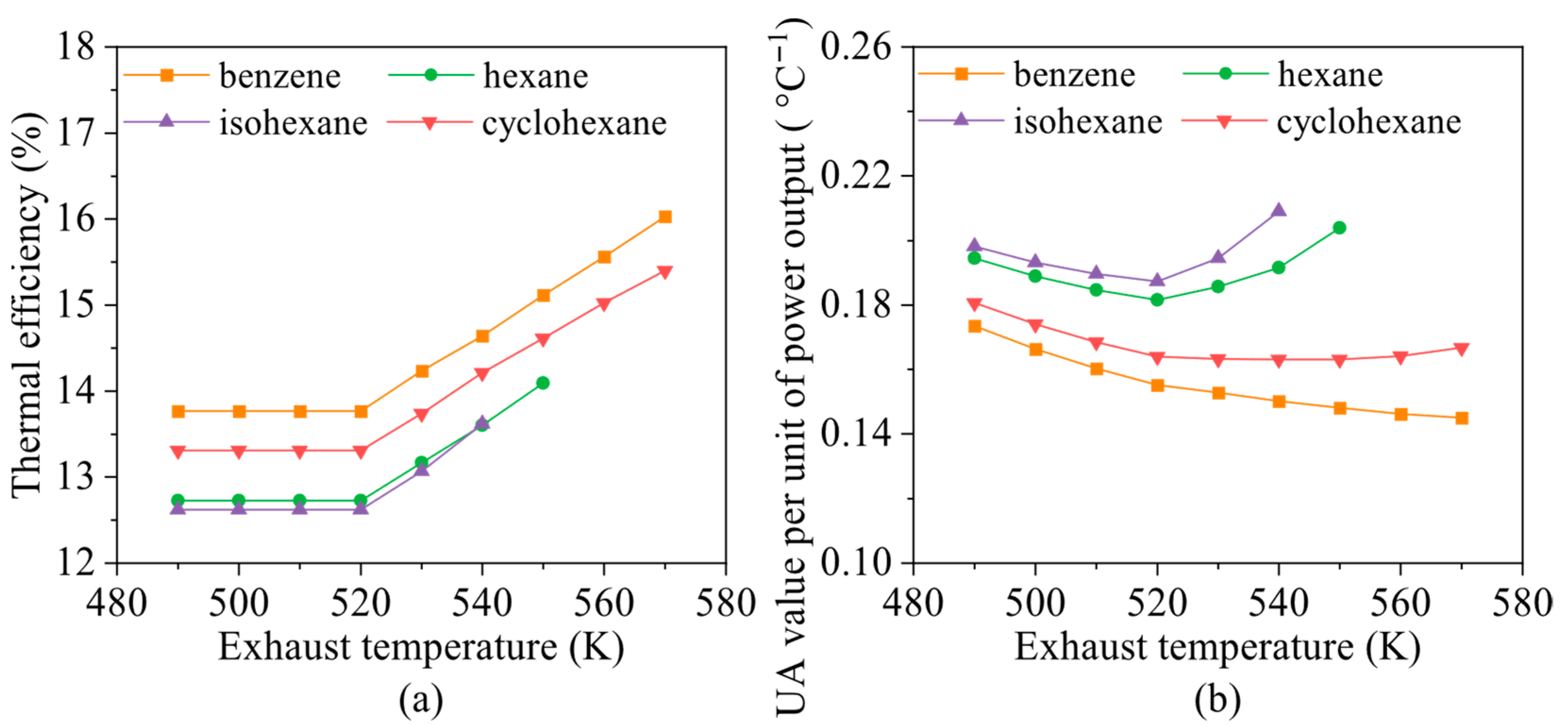
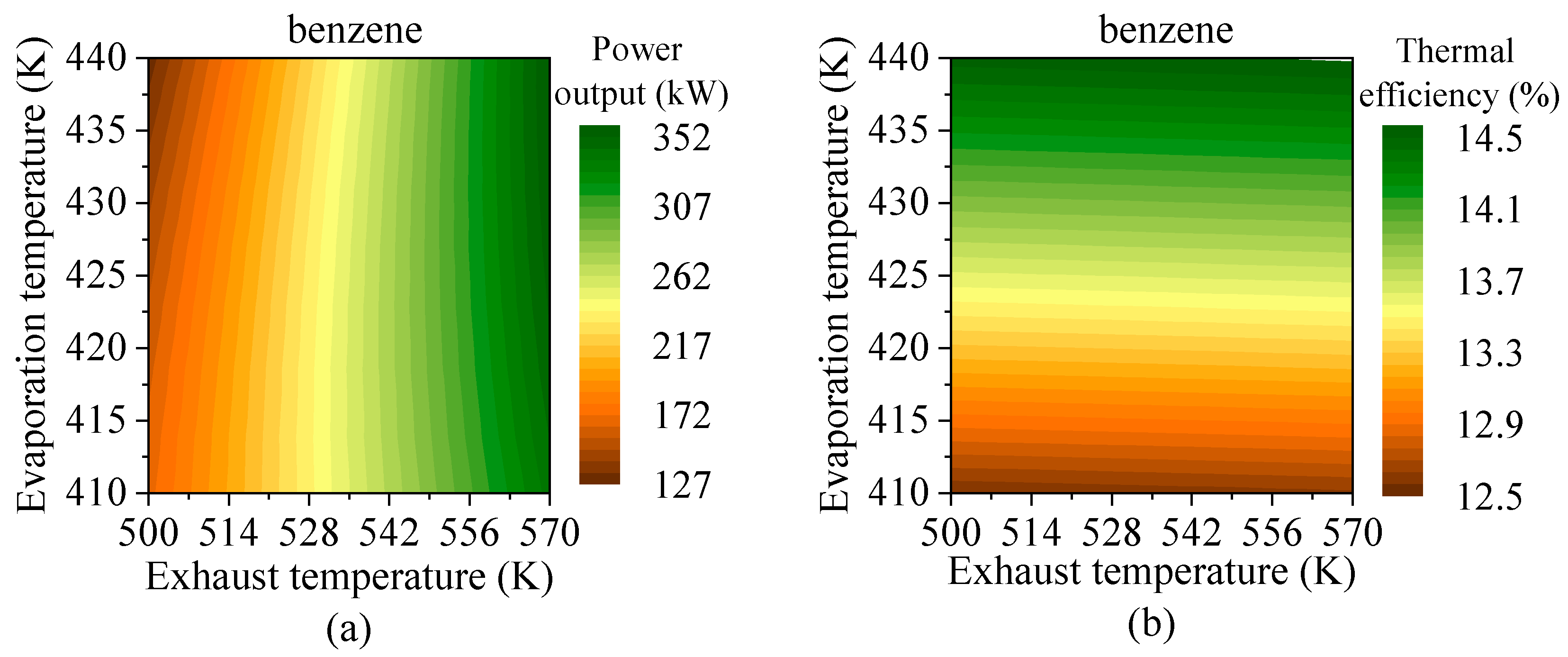
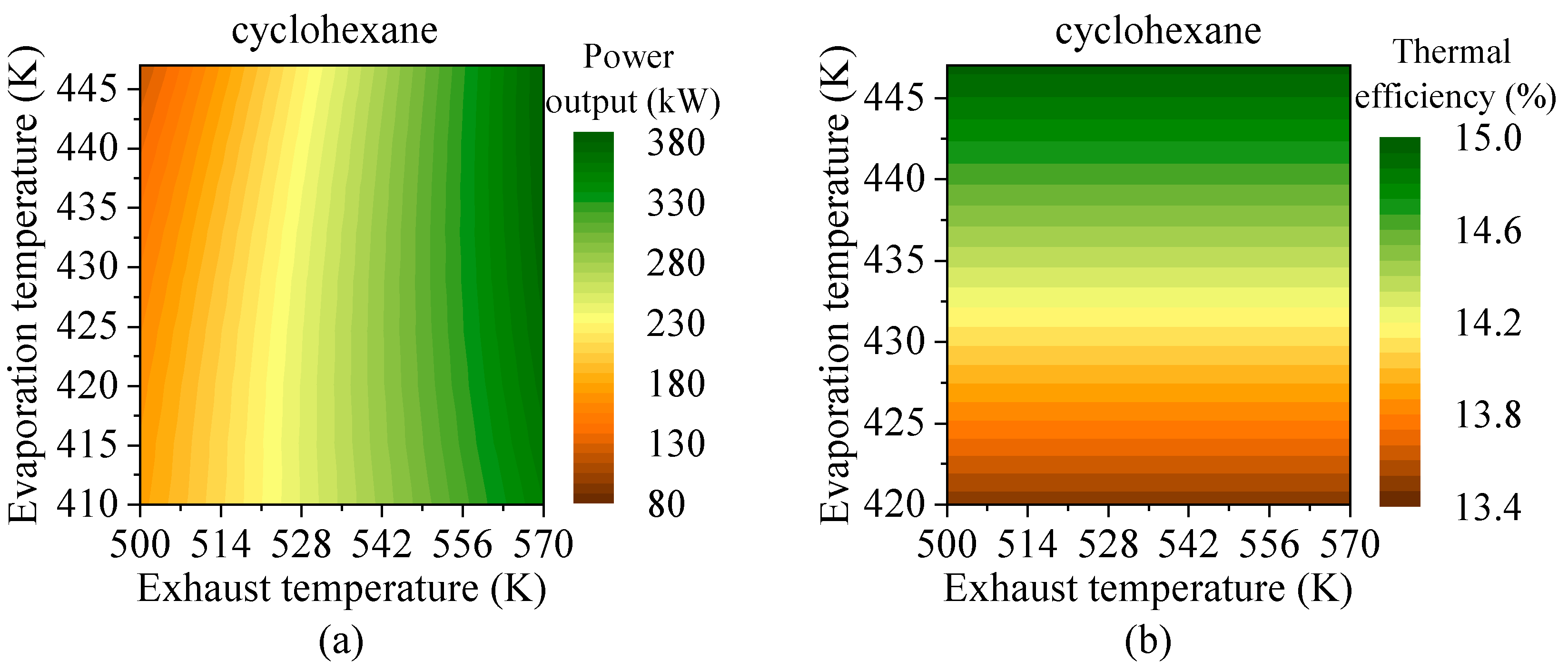
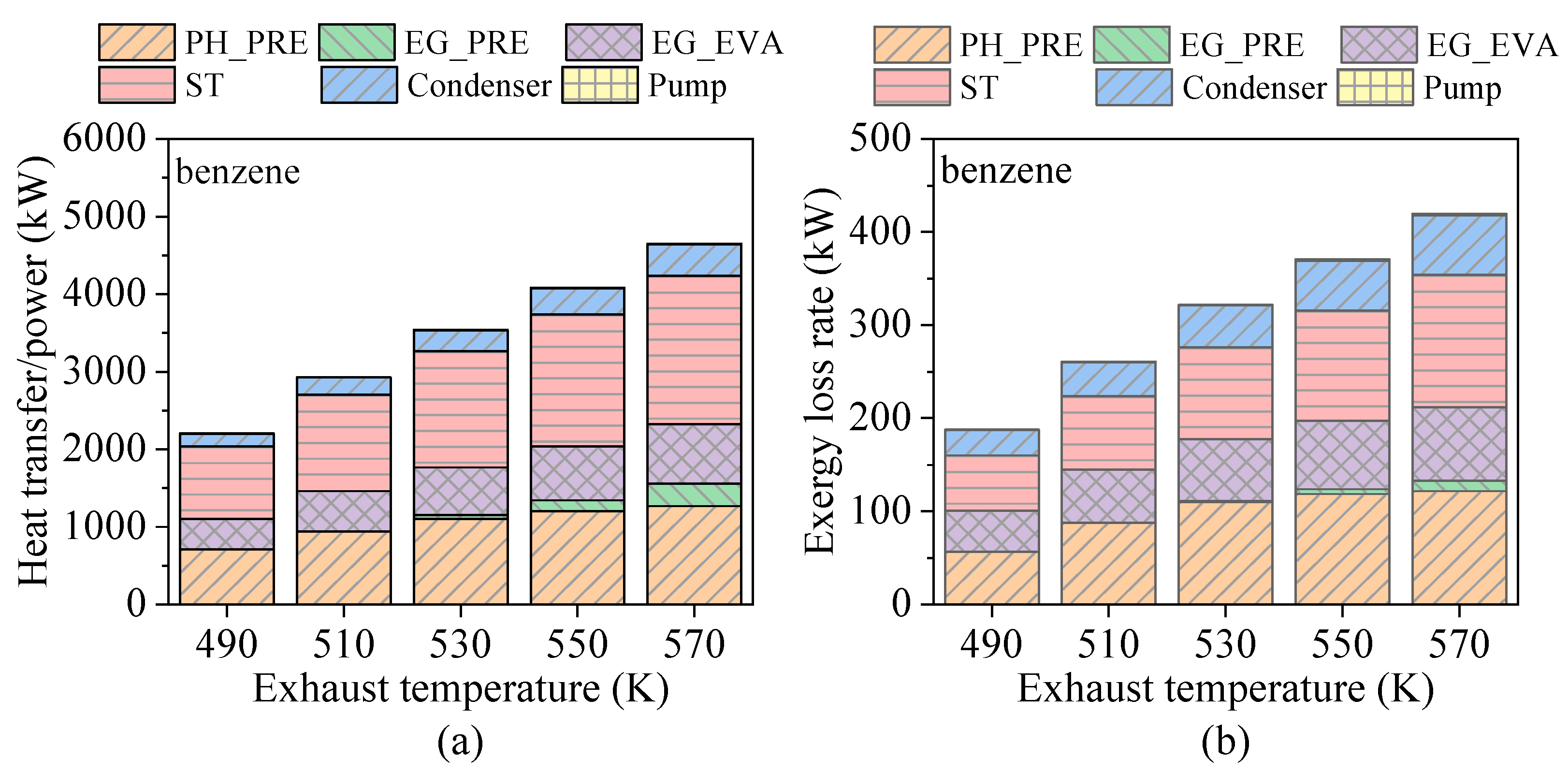
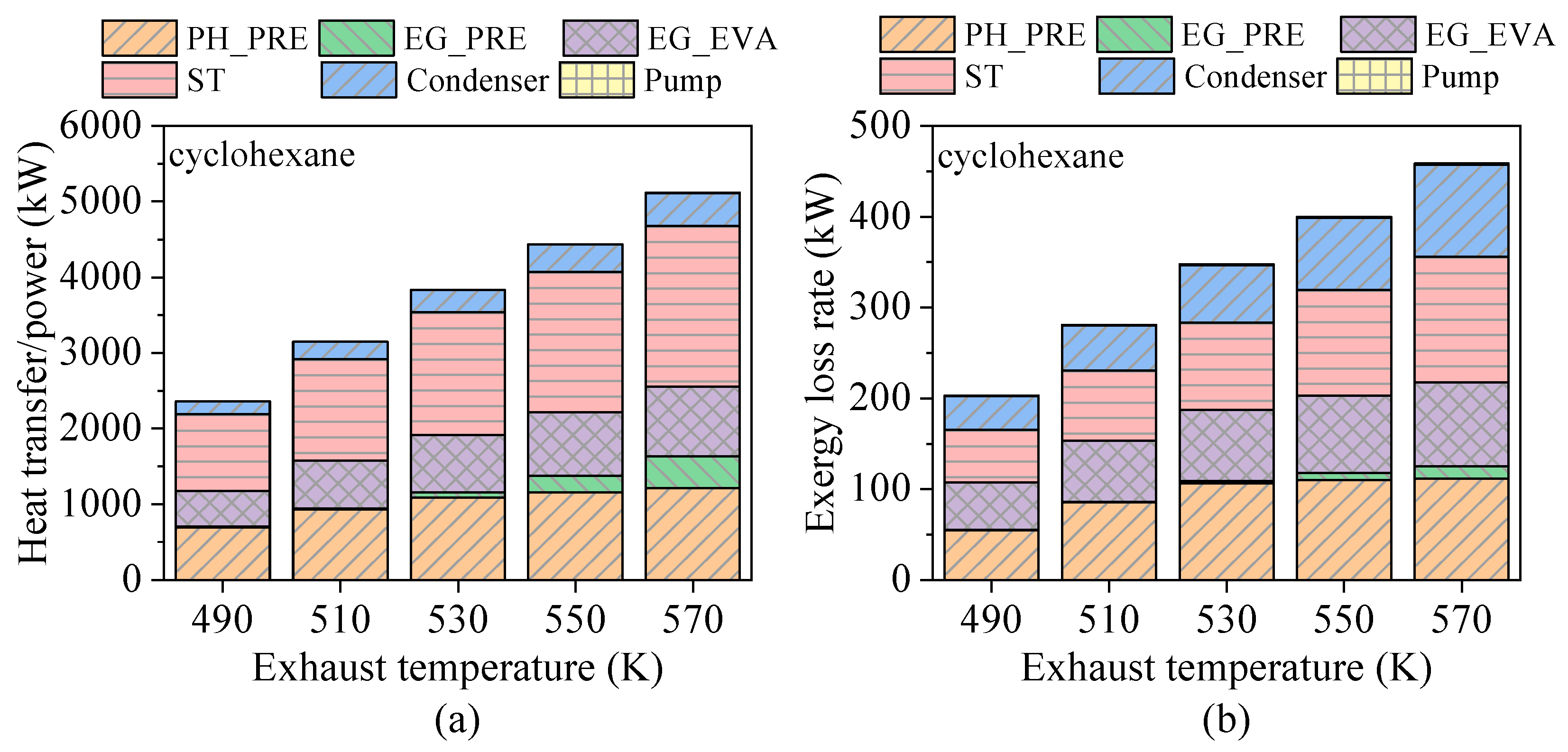
4.3. Effects of Heat Source Conditions
5. Conclusions
- (1)
- Preheating temperature has a significant impact on system performance. For hydrocarbons with higher critical temperatures, the net power output exhibits a peak within the preheating temperature range. In contrast, for fluids with lower critical temperatures, the power output increases continuously until the maximum allowable evaporation pressure is reached. The variations in total energy flow input and exergy loss rate follow trends similar to those of net power output.
- (2)
- Evaporation pressure affects working fluids differently depending on their critical temperature. For hydrocarbons with higher critical temperatures, increasing evaporation pressure leads to a decline in power output but an improvement in thermal efficiency. Due to the constraint of the minimum exhaust gas outlet temperature, both total energy flow input and exergy loss rate decrease monotonically. Meanwhile, the heat transfer rate and exergy destruction rate in the exhaust gas preheater increase with rising evaporation pressure.
- (7)
- Heat source temperature plays a critical role in determining the energy and exergy flows distribution and overall system output. For fluids with high critical temperatures, both energy flow input and exergy loss rate rise with increasing exhaust gas temperature, while the contribution of the preheating section to total heat exchange gradually declines. For instance, in the case of cyclohexane, the net power output increases by approximately 12.3% for every 10 K rise in exhaust temperature.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Specific enthalpy [J/kg] | |
| s | Specific entropy [J/kg K] |
| T | Temperature [K] |
| U | Heat transfer coefficient (kW/m2 K) |
| Exergy loss flow [kW] | |
| A | Area [m2] |
| Exergy destruction rate [kW] | |
| Mass flow rate [kg/s] | |
| Power [kW] | |
| Heat transfer rate [kW] | |
| Pinch point temperature difference [K] | |
| Greek symbols | |
| Efficiency [%] | |
| Subscripts | |
| in | Inlet |
| out | Outlet |
| 0 | Ambient condition |
| wf | Working fluid |
| hs | Heat source |
| cond | Condenser |
| p | Pump |
| exp | Expander |
| cv | Control volume |
| Abbreviation | |
| IMO | International Maritime Organization |
| ORC | Organic Rankine cycle |
| SRC | Steam Rankine cycle |
| JW | Jacket water |
| PRE | Preheater |
| SA | Scavenging air |
| EG | Exhaust gas |
| EVA | Evaporator |
| HFC | Hydrofluorocarbon |
| HFO | Hydrofluorooefin |
| HC | Hydrocarbon |
| HCFC | Hydrochlorofluorocarbon |
References
- IMO. Fourth IMO Greenhouse Gas Study. 2025. Available online: https://www.imo.org/ (accessed on 21 July 2025).
- IMO. Revised MARPOL Annex VI. 2025. Available online: https://www.imo.org/ (accessed on 21 July 2025).
- ICCT. Long-Term Potential for Increased Shipping Efficiency Through the Adoption of Industry-Leading Practices. Available online: https://www.theicct.org.cn/ (accessed on 21 July 2025).
- Bouman, E.A.; Lindstad, E.; Rialland, A.I.; Strømman, A.H. State-of-the-art technologies, measures, and potential for reducing GHG emissions from shipping—A review. Transp. Res. D Transp. Env. 2017, 52, 408–421. [Google Scholar] [CrossRef]
- MAN Energy Solutions. How to Influence CO2. Available online: https://www.man-es.com (accessed on 21 July 2025).
- MAN Energy Solutions. Thermo Efficiency System for Reduction of Fuel Consumption and CO2 Emission [EB/OL]. Available online: https://www.man-es.com (accessed on 21 July 2025).
- LeBlanc, S. Thermoelectric generators: Linking material properties and systems engineering for waste heat recovery applications. Sustain. Mater. Technol. 2014, 1–2, 26–35. [Google Scholar] [CrossRef]
- Nour Eddine, A.; Chalet, D.; Faure, X.; Aixala, L.; Chessé, P. Optimization and characterization of a thermoelectric generator prototype for marine engine application. Energy 2018, 143, 682–695. [Google Scholar] [CrossRef]
- Aghaali, H.; Ångström, H.-E. A review of turbocompounding as a waste heat recovery system for internal combustion engines. Renew. Sustain. Energy Rev. 2015, 49, 813–824. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, K.; Deng, K. A review of waste heat recovery from the marine engine with highly efficient bottoming power cycles. Renew. Sustain. Energy Rev. 2020, 120, 109611. [Google Scholar] [CrossRef]
- Baldi, F.; Gabrielii, C. A feasibility analysis of waste heat recovery systems for marine applications. Energy 2015, 80, 654–665. [Google Scholar] [CrossRef]
- ICCT. Reducing Greenhouse Gas Emissions from Ships. Available online: https://theicct.org/wp-content/uploads/2021/06/ICCT_GHGfromships_jun2011.pdf (accessed on 15 August 2025).
- Ringler, J.; Seifert, M.; Guyotot, V.; Hübner, W. Rankine cycle for waste heat recovery of IC engines. SAE Int. J. Engines 2009, 1, 67–76. [Google Scholar] [CrossRef]
- Sprouse, C.; Depcik, C. Review of organic Rankine cycles for internal combustion engine exhaust waste heat recovery. Appl. Therm. Eng. 2013, 51, 711–722. [Google Scholar] [CrossRef]
- Gürgen, S.; Altın, İ. Novel decision-making strategy for working fluid selection in Organic Rankine Cycle: A case study for waste heat recovery of a marine diesel engine. Energy 2022, 252, 124023. [Google Scholar] [CrossRef]
- Li, Y.; Tang, T. Performance Analysis and Optimization of a Series Heat Exchangers Organic Rankine Cycle Utilizing Multi-Heat Sources from a Marine Diesel Engine. Entropy 2021, 23, 906. [Google Scholar] [CrossRef]
- McLinden, M.O.; Kazakov, A.F.; Steven Brown, J.; Domanski, P.A. A thermodynamic analysis of refrigerants: Possibilities and tradeoffs for Low-GWP refrigerants. Int. J. Refrig. 2014, 38, 80–92. [Google Scholar] [CrossRef]
- Liu, W.; Meinel, D.; Wieland, C.; Spliethoff, H. Investigation of hydrofluoroolefins as potential working fluids in organic Rankine cycle for geothermal power generation. Energy 2014, 67, 106–116. [Google Scholar] [CrossRef]
- Wang, E.H.; Zhang, H.G.; Fan, B.Y.; Ouyang, M.G.; Yang, F.Y.; Yang, K.; Wang, Z.; Zhang, J.; Yang, F.B. Parametric analysis of a dual-loop ORC system for waste heat recovery of a diesel engine. Appl. Therm. Eng. 2014, 67, 168–178. [Google Scholar] [CrossRef]
- Fernández, F.J.; Prieto, M.M.; Suárez, I. Thermodynamic analysis of high-temperature regenerative organic Rankine cycles using siloxanes as working fluids. Energy 2011, 36, 5239–5249. [Google Scholar] [CrossRef]
- Larsen, U.; Pierobon, L.; Baldi, F.; Haglind, F.; Ivarsson, A. Development of a model for the prediction of the fuel consumption and nitrogen oxides emission trade-off for large ships. Energy 2015, 80, 545–555. [Google Scholar] [CrossRef]
- Akman, M.; Ergin, S. An investigation of marine waste heat recovery system based on organic Rankine cycle under various engine operating conditions. Proc. IME M J. Eng. Marit. Environ. 2018, 233, 586–601. [Google Scholar] [CrossRef]
- Xu, B.; Rathod, D.; Yebi, A.; Filipi, Z.; Onori, S.; Hoffman, M. A comprehensive review of organic rankine cycle waste heat recovery systems in heavy-duty diesel engine applications. Renew. Sustain. Energy Rev. 2019, 107, 145–170. [Google Scholar] [CrossRef]
- Yang, M.-H.; Yeh, R.-H. Thermodynamic and economic performances optimization of an organic Rankine cycle system utilizing exhaust gas of a large marine diesel engine. Appl. Energy 2015, 149, 1–12. [Google Scholar] [CrossRef]
- Grljušić, M.; Medica, V.; Račić, N. Thermodynamic analysis of a ship power plant operating with waste heat recovery through combined heat and power production. Energies 2014, 7, 7368–7394. [Google Scholar] [CrossRef]
- Song, J.; Gu, C.-W. Performance analysis of a dual-loop organic Rankine cycle (ORC) system with wet steam expansion for engine waste heat recovery. Appl. Energy 2015, 156, 280–289. [Google Scholar] [CrossRef]
- Ahlgren, F.; Mondejar, M.E.; Genrup, M.; Thern, M. Waste heat recovery in a cruise vessel in the baltic sea by using an Organic Rankine Cycle: A Case Study. J. Eng. Gas Turbines Power Trans. ASME 2016, 138, 011702. [Google Scholar] [CrossRef]
- Yuksek, E.; Mirmobin, P. Waste heat utilization of main propulsion engine jacket water in marine application. In Proceedings of the 3rd International Seminar on ORC Power Systems, Bruxelles, Belgium, 12–14 October 2015. [Google Scholar]
- Song, J.; Song, Y.; Gu, C. Thermodynamic analysis and performance optimization of an Organic Rankine Cycle (ORC) waste heat recovery system for marine diesel engines. Energy 2015, 82, 976–985. [Google Scholar] [CrossRef]
- Tayyeban, E.; Deymi-Dashtebayaz, M. Optimizing an expansion engine-based organic rankine cycle system for heat recovery from natural gas engines. Energy Convers. Manag. 2025, 343, 120209. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Shi, L.; Tian, H.; Shu, G. Organic Rankine cycle configuration design method based on the performance of all operating conditions. Energy 2025, 331, 137019. [Google Scholar] [CrossRef]
- Chys, M.; van den Broek, M.; Vanslambrouck, B.; De Paepe, M. Potential of zeotropic mixtures as working fluids in organic Rankine cycles. Energy 2012, 44, 623–632. [Google Scholar] [CrossRef]
- Wang, E. Low and Medium Temperature Power Cycle Systems and Applications; Beijing Institute of Technology Press: Beijing, China, 2021. [Google Scholar]
- Garrido, J.M.; Quinteros-Lama, H.; Mejía, A.; Wisniak, J.; Segura, H. A rigorous approach for predicting the slope and curvature of the temperature-entropy saturation boundary of pure fluids. Energy 2012, 45, 888–899. [Google Scholar] [CrossRef]
- Zhu, S.; Feng, J.; Tang, Y.; Bai, S.; Deng, K. Influence of ambient conditions on the marine two-stroke engine integrated with a bottoming Rankine cycle system: Energy and exergy analyses. Appl. Therm. Eng. 2023, 219, 119601. [Google Scholar] [CrossRef]
- Baldi, F.; Larsen, U.; Gabrielii, C. Comparison of different procedures for the optimisation of a combined Diesel engine and organic Rankine cycle system based on ship operational profile. Ocean. Eng. 2015, 110, 85–93. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Jiang, L.; Zhou, Y.D.; Yu, L.J. An Integrated Comparison for Working Fluids Selection in Both Subcritical and Supercritical ORC. Adv. Mat. Res. 2014, 997, 703–712. [Google Scholar]
- Zhu, S.; Gu, Y.; Yuan, H.; Ma, Z.; Deng, K. Thermodynamic analysis of the turbocharged marine two-stroke engine cycle with different scavenging air control technologies. Energy 2020, 191, 116533. [Google Scholar] [CrossRef]
- Andreasen, J.; Meroni, A.; Haglind, F. A Comparison of Organic and Steam Rankine Cycle Power Systems for Waste Heat Recovery on Large Ships. Energies 2017, 10, 547. [Google Scholar] [CrossRef]




| Working Fluids | Categorization | Critical Temperature (K) | Critical Pressure (kPa) |
|---|---|---|---|
| benzene | HCs | 562.02 | 4907 |
| hexane | HCs | 507.82 | 3034 |
| isohexane | HCs | 497.7 | 3040 |
| cyclohexane | HCs | 553.6 | 4081 |
| pentane | HCs | 469.7 | 3370 |
| R245ca | HFCs | 447.57 | 3941 |
| R1233zd(E) | HFOs | 438.75 | 3571 |
| Type | Two-Stroke, In-Line, Six-Cylinder, Turbocharged, Diesel Engine |
|---|---|
| Bore × Stroke | 340 × 1600 mm |
| Compression ratio | 19.8:1 |
| Connecting rod length | 1600 mm |
| Rated speed and power | 157 r/min @ 4896 kW |
| Displacement volume | 145.3 l/cylinder |
| Parameter | Value | |
|---|---|---|
| Component efficiency | Efficiency of expander (%) | 72 |
| Efficiency of pump (%) | 70 | |
| Efficiency of gear/generator (%) | 93 | |
| Heat exchanger design | Minimum PPTD in the evaporator (°C) | 20 |
| Minimum PPTD in the scavenging air cooler (°C) | 10 | |
| Temperature after the jacket water cooler (°C) | 80 | |
| Condensation temperature (°C) | 35 | |
| Constraints | Maximum evaporation pressure (bar) | |
| Minimum exhaust temperature out of evaporator (°C) | 145 | |
| Minimum preheater outlet scavenging air temperature (°C) | 90 |
| Working Fluids | Critical Temperature (°C) | Critical Pressure (kPa) | Preheating Temperature (°C) | Maximum Power Output (kW) |
|---|---|---|---|---|
| benzene | 288.87 | 4907 | 135 | 217.84 |
| hexane | 234.67 | 3034 | 140 | 242.04 |
| isohexane | 224.55 | 3040 | 145 | 247.44 |
| cyclohexane | 280.45 | 4081 | 135 | 226.91 |
| pentane | 196.55 | 3370 | 180 | 281.38 |
| R245ca | 174.42 | 3941 | 160 | 343.10 |
| R1233zd(E) | 165.6 | 3571 | 150 | 331.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Gu, Y.; Han, S.; Zhao, X.; Tang, Y.; Zhu, S.; Yuan, H.; Wang, G. Design of Organic Rankine Cycle Recovering Multi-Grade Waste Heat from a Two-Stroke Marine Engine. J. Mar. Sci. Eng. 2025, 13, 1679. https://doi.org/10.3390/jmse13091679
Feng J, Gu Y, Han S, Zhao X, Tang Y, Zhu S, Yuan H, Wang G. Design of Organic Rankine Cycle Recovering Multi-Grade Waste Heat from a Two-Stroke Marine Engine. Journal of Marine Science and Engineering. 2025; 13(9):1679. https://doi.org/10.3390/jmse13091679
Chicago/Turabian StyleFeng, Jinfeng, Yuncheng Gu, Shengjun Han, Xunhu Zhao, Yujun Tang, Sipeng Zhu, Hao Yuan, and Guihua Wang. 2025. "Design of Organic Rankine Cycle Recovering Multi-Grade Waste Heat from a Two-Stroke Marine Engine" Journal of Marine Science and Engineering 13, no. 9: 1679. https://doi.org/10.3390/jmse13091679
APA StyleFeng, J., Gu, Y., Han, S., Zhao, X., Tang, Y., Zhu, S., Yuan, H., & Wang, G. (2025). Design of Organic Rankine Cycle Recovering Multi-Grade Waste Heat from a Two-Stroke Marine Engine. Journal of Marine Science and Engineering, 13(9), 1679. https://doi.org/10.3390/jmse13091679






