Hydrochemistry of Blackwaters in a Shoreline Zone of São Paulo State, Brazil
Abstract
1. Introduction
2. Study Area
3. Sampling Points
4. Analytical Methods
5. Results and Discussion
6. Conclusions
- -
- a high level of dissolved iron corresponding to 1.9 mg/L in one blackwater stream monitored at Bertioga municipality, São Paulo State, Brazil, thus contributing to the natural fertilization of the studied shoreline zone;
- -
- coherent results for such a blackwater stream in terms of color, turbidity, organic matter, organic carbon, biochemical oxygen demand, and phosphate, beyond the iron concentration;
- -
- strong influence of ocean backward currents on the hydrochemical composition of the Itaguaré River and Una River blackwaters, especially in the lower reaches of the rivers closer to the mouth;
- -
- accentuated increase in electrical conductivity (EC) and dissolved concentration of sodium, potassium, calcium, magnesium, chloride, sulfate, and, consequently, total dissolved solids in the lower reaches of Itaguaré and Una rivers;
- -
- possible implications for the blackwater’s use in the cultivation of some crops, as demonstrated by the EC vs. SAR (sodium adorption ratio) diagram for salinity hazard classification.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, C.H. The Alfred Russel Wallace Page. Available online: https://people.wku.edu/charles.smith/wallace/chsarwin.htm (accessed on 10 July 2025).
- Raby, P. Alfred Russel Wallace: A Life; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Duncan, W.P.; Fernandes, M.N. Physicochemical characterization of the white, black, and clearwater rivers of the Amazon Basin and its implications on the distribution of freshwater stingrays (Chondrichthyes, Potamotrygonidae). Pan-Am. J. Aquat. Sci. 2010, 5, 454–464. [Google Scholar]
- Forel, F.A. A new classification of the color types to study the lake waters. Arch. Phys. Nat. Sci./Phys. Nat. Hist. Soc. Geneva 1890, 6, 25. (In French) [Google Scholar]
- Ule, W. Determining the water color in lakes: Minor announcements. Justus Perthe’s Geogr. Rep. 1892, 38, 70–71. (In German) [Google Scholar]
- Ye, M.; Sun, Y. Review of the Forel-Ule Index based on in situ and remote sensing methods and application in water quality assessment. Environ. Sci. Pollut. Res. 2022, 29, 13024–13041. [Google Scholar] [CrossRef]
- Wozniak, S.B.; Meler, J. Modelling water colour characteristics in an optically complex nearshore environment in the Baltic Sea; Quantitative interpretation of the Forel-Ule scale and algorithms for the remote estimation of seawater composition. Remote Sens. 2020, 12, 2852. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhang, W.; Cao, C.; Zhang, F.; Shen, Q.; Zhang, X.; Zhang, B. A dataset of remote-sensed Forel-Ule Index for global inland waters during 2000–2018. Sci. Data 2021, 8, e26. [Google Scholar] [CrossRef]
- Hutchinson, G.E. A Treatise on Limnology; Chapman and Hall: London, UK, 1957; Volume 1. [Google Scholar]
- Pitarch, J.; Van der Woerd, H.J.; Brewin, R.J.W.; Zielinski, O. Optical properties of Forel-Ule water types deduced from 15 years of global satellite ocean color observations. Remote Sen. Environ. 2019, 231, e111249. [Google Scholar] [CrossRef]
- Van der Woerd, H.J.; Wernand, M.R. True colour classification of natural waters with medium-spectral resolution satellites: SeaWiFS, MODIS, MERIS and OLCI. Sensors 2015, 15, 25663–25680. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.E. Remote sensing of tropical blackwater rivers: A method for environmental water quality analysis. Appl. Geogr. 1993, 13, 153–168. [Google Scholar] [CrossRef]
- Lehmann, M.K.; Nguyen, U.; Allan, M.; Van der Woerd, H.J. Colour classification of 1486 lakes across a wide range of optical water types. Remote Sens. 2018, 10, 1273. [Google Scholar] [CrossRef]
- Gardner, J.R.; Yang, X.; Topp, S.N.; Ross, M.R.V.; Altenau, E.H.; Pavelsky, T.M. The color of rivers. Geophys. Res. Lett. 2021, 48, e2020GL088946. [Google Scholar] [CrossRef]
- Lai, Y.; Zhanf, J.; Li, W.; Song, Y. Water quality monitoring of large reservoirs in China based on water color change from 1999 to 2021. J. Hydrol. 2024, 633, e130988. [Google Scholar] [CrossRef]
- Jerome, J.H.; Bukata, R.P.; Whitfield, P.H.; Rousseau, N. Colours of natural waters: 1. Factors controlling the dominant wavelength. Northwest Sci. 1994, 68, 43–52. [Google Scholar]
- Marinho, R.R.; Martinez, J.-M.; Oliveira, T.C.S.; Moreira, W.P.; Carvalho, L.A.S.; Moreira-Turcq, P.; Harmel, T. Estimating the colored dissolved organic matter in the Negro River, Amazon Basin, with in situ remote sensing data. Remote Sens. 2024, 16, 613. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, Z.; Zhu, Y.; Zhang, L.; Fang, X. Identification for water quality based on color characteristics. IOP Conf. Ser. Earth Environ. Sci. 2022, 983, e012075. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Blackwater Rivers. Available online: https://agrovoc.fao.org/browse/agrovoc/ar/page/?clang=vi&uri=c_4f5b9967 (accessed on 10 July 2025).
- Weatherstem Scholar. Blackwater Rivers. Available online: https://learn.weatherstem.com/modules/learn/lessons/178/20.html (accessed on 10 July 2025).
- Flotemersch, J.E.; Blocksom, K.A.; Herlihy, A.T.; Kaufmann, P.R.; Mitchell, R.M.; Peck, D.V. Distribution and characteristics of blackwater rivers and streams of the contiguous United States. Water Resour. Res. 2024, 60, e2023WR035529. [Google Scholar] [CrossRef]
- Amazon Waters Alliance. Blackwater Rivers. Available online: https://en.aguasamazonicas.org/waters/river-types/blackwater-rivers (accessed on 10 July 2025).
- Anwana, E.D.; Ogbemudia, F.O.; Ita, R.E.; Sunday, P.E. Influence of hydrological variables on macrophytes in a black water river ecosystem. J. Appl. Sci. Envir. Manag. 2021, 25, 151–158. [Google Scholar] [CrossRef]
- Thieme, M.L.; Abell, R.; Stiassny, M.L.; Skelton, P.; Lehner, B.; Teugels, G.G. Freshwater Ecoregions of Africa and Madagascar: A Conservation Assessment; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Alkhatib, M.; Jennerjahn, T.C.; Samiaji, J. Biogeochemistry of the Dumai River estuary, Sumatra, Indonesia, a tropical black-water river. Limno. Oceanogr. 2007, 52, 2410–2417. [Google Scholar] [CrossRef]
- Britannica. Black River. Available online: https://www.britannica.com/place/Vietnam (accessed on 10 July 2025).
- Grzybkowska, M.; Temech, A.; Dukowska, M. Impact of long-term alternations of discharge and spate on the chironomid community in the lowland Widawka River (Central Poland). Hydrobiologia 1996, 324, 107–115. [Google Scholar] [CrossRef]
- Heikkinen, K. Organic matter, iron and nutrient transport and nature of dissolved organic matter in the drainage basin of a boreal humic river in northern Finland. Sci. Total Environ. 1994, 152, 81–89. [Google Scholar] [CrossRef]
- Benke, A.C.; Wallace, J.B. High secondary production in a Coastal Plain river is dominated by snag invertebrates and fueled mainly by amorphous detritus. Freshw. Biol. 2015, 60, 236–255. [Google Scholar] [CrossRef]
- Meyer, J.L. A blackwater perspective on riverine ecosystems. BioScience 1990, 40, 643–651. [Google Scholar] [CrossRef]
- Zampella, R.A.; Laidig, K.J.; Lowe, R.L. Distribution of diatoms in relation to land use and pH in blackwater Coastal Plain streams. Env. Mgmt. 2007, 39, 369–384. [Google Scholar] [CrossRef]
- Zampella, R.A.; Bunnell, J.F.; Procopio, N.A.; Bryson, D.E. Macroinvertebrate assemblages in blackwater streams draining forest land and active and abandoned cranberry bogs. Wetlands 2008, 28, 390–400. [Google Scholar] [CrossRef]
- Ríos-Villamizar, E.A.; Piedade, M.T.F.; da Costa, J.G.; Adeney, J.M.; Junk, W.J. Chemistry of different Amazonian water types for river classification: A preliminary review. WIT Trans. Ecol. Environ. 2014, 178, 17–28. [Google Scholar]
- Vegas-Vilarrúbia, T.; Paolini, J.; Herrera, R. A physico-chemical survey of blackwater rivers from the Orinoco and the Amazon basins in Venezuela. Arch. Hydrobiol. 1988, 111, 491–506. [Google Scholar] [CrossRef]
- Horbe, A.M.C.; Santos, A.G.S. Chemical composition of black-watered rivers in the western Amazon region (Brazil). J. Braz. Chem. Soc. 2009, 20, 119–1126. [Google Scholar] [CrossRef]
- Küchler, I.L.; Miekeley, N.; Forsberg, B.R. A contribution to the chemical characterization of rivers in the Rio Negro basin, Bazil. J. Braz. Chem. Soc. 2000, 11, 286–292. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Botero, W.G.; Santos, F.A.; Sargentini, E., Jr.; Rocha, J.C.; Santos, A. Influence of seasonality on the interaction of mercury with aquatic humic substances extracted from the middle Negro River basin (Amazon). J. Braz. Chem. Soc. 2012, 23, 1711–1718. [Google Scholar] [CrossRef]
- Por, F.D.; Shimizu, G.Y.; Prado-Por, M.S.A.; Tòha, F.A.L.; Oliveira, I.R. The blackwater river estuary of Rio Una do Prelado (São Paulo, Brazil): Preliminary hydrobiological data. Rev. Hydrobiol. Trop. 1984, 17, 215–258. [Google Scholar]
- Fraley, K.M.; Haynes, T.B.; López, J.A. Under-ice population density estimation of Alaska Blackfish. N. Am. J. Fish. Manag. 2018, 38, 454–461. [Google Scholar] [CrossRef]
- Largier, J.L. Structure and mixing in the Palmiet estuary, South Africa. Afr. J. Mar. Sci. 1986, 4, 139–152. [Google Scholar] [CrossRef]
- Benedetti, M.M.; Raber, M.J.; Smith, M.S.; Leonard, L.A. Mineralogical indicators of alluvial sediment sources in the Cape Fear River Basin, North Carolina. Phys. Geogr. 2006, 27, 258–281. [Google Scholar] [CrossRef]
- Clair, T.A.; Freedman, B. Patterns and importance of dissolved organic carbon in four acidic brownwater streams in Nova Scotia, Canada. Water Air Soil Pollut. 1986, 31, 139–147. [Google Scholar] [CrossRef]
- Stahle, D.W.; Cleaveland, M.K.; Hehr, J.G. A 450-year drought reconstruction for Arkansas, United States. Nature 1985, 316, 530–532. [Google Scholar] [CrossRef]
- McKinley, T.R. Survey of Northern Pike in Lakes of Soldotna Creek Drainage, 2002; Alaska Department of Fish and Game, Division of Sport Fish, Research and Technical Services: Juneau, AK, USA, 2013. [Google Scholar]
- Waldo, A.J. Operational Plan: Crooked Creek Chinook Salmon Enhancement Project, 2019–2021; Alaska Department of Fish and Game, Regional Operational Plan ROP: Soldotna, AK, USA, 2019; Volume 2. [Google Scholar]
- American Rivers. Blackwater River Named Among America’s most Endangered Rivers of 2024. Available online: https://www.americanrivers.org/media-item/blackwater-river-named-among-americas-most-endangered-rivers-of-2024/ (accessed on 10 July 2025).
- Krachler, R.; Krachler, R.; Valda, A.; Keppler, B.K. Natural iron fertilization of the coastal ocean by “blackwater rivers”. Sci. Total Environ. 2019, 656, 952–958. [Google Scholar] [CrossRef]
- Bertioga City Hall. Facts of Bertioga. Available online: https://conhecabertioga.webnode.page/ (accessed on 10 July 2025). (In Portuguese).
- IBGE (Brazilian Geographical and Statistical Institute). São Sebastião Data. Available online: https://cidades.ibge.gov.br/brasil/sp/sao-sebastiao/panorama (accessed on 10 July 2025). (In Portuguese)
- SMA (Secretary of Environment, Infrastructure, and Logistics of São Paulo State). State Park Serra do Mar—São Sebastião Nucleus. Available online: https://guiadeareasprotegidas.sp.gov.br/ap/parque-estadual-serra-do-mar-nucleo-sao-sebastiao/ (accessed on 10 July 2025). (In Portuguese)
- Forestry Foundation. State Park Restinga of Bertioga. Available online: https://fflorestal.sp.gov.br/parque-estadual-restinga-de-bertioga/ (accessed on 10 July 2025). (In Portuguese)
- Ebert, H. Geology of Southern Brazil do Brasil. Meridional; UFRGS (Federal University of Rio Grande do Sul): Porto Alegre, RS, Brazil, 1971. (In Portuguese) [Google Scholar]
- Almeida, F.F.M.; Hasui, Y.; Brito Neves, B.B.; Fuck, R.A. Brazilian structural provinces. In Proc. VIII Geology Symposium of Northeast Brazil; SBG (Brazilian Geological Society), Ed.; SBG (Brazilian Geological Society): Campina Grande, Brazil, 1977; pp. 363–391. (In Portuguese) [Google Scholar]
- Heilbron, M.; Ribeiro, A.; Valeriano, C.M.; Paciullo, F.; Almeida, J.C.H.; Trow, R.; Tupinambá, M.; Silva, L.G.E. The Ribeira belt. In São Francisco Craton, Eastern Brazil Tectonic Genealogy of a Miniature Continent; Heilbron, M., Cordani, U.G., Alkmin, F., Eds.; Springer: Berlin, Germany, 2017; Volume 1, pp. 277–304. [Google Scholar]
- Campos Neto, M.C.; Figueiredo, M.C.H. The Serra do Mar tectonic domain: Pattern of deformation and tectonic evolution of a segment of the Neoproterozoic Ribeira belt, SE Brazil. J. S. Am. Earth Sci. 1995, 8, 235–258. [Google Scholar]
- Campos Neto, M.C. Orogenic systems from southwestern Brazil: An approach based on tectonic setting of superposed orogenies. Precambrian Res. 2000, 97, 25–60. [Google Scholar]
- IPT(Technological Researches Institute of São Paulo State). Geological Map of São Paulo State; IPT: São Paulo, Brazil, 1981. [Google Scholar]
- Almeida, F.F.M.; Hasui, Y. The Precambrian in Brazil; Edgard Blücher: São Paulo, Brazil, 1984. [Google Scholar]
- Barreto, G.S. Geology and Tectonics of the Western Portion of São Sebastião Island (SP) and Comparison with Surrounding Continental Areas. Ph.D. Thesis, USP-São Paulo University, São Paulo, Brazil, 2012. (In Portuguese). [Google Scholar]
- Ross, J.L.S.; Moroz, I.C. Geomorphological mapping of the coastal plain between Bertioga and São Sebastião channel (SP). Geogr. Dep. Mag. (USP) 1997, 11, 51–66. (In Portuguese) [Google Scholar]
- Rossi, M. Factors Shaping the Coastal Landscape: The Guaratuba Basin, São Paulo, Brazil. Ph.D. Thesis, USP-São Paulo University, São Paulo, Brazil, 1999. (In Portuguese). [Google Scholar]
- Baraldo, K.B. Characterization and Comparison of Estuaries from Itaguaré and Guaratuba Rivers (Bertioga, SP) Based on Geo-physico-Chemical Parameters, Bathymetry, and Bottom Imaging. Master’s Dissertation, UNESP-São Paulo State University, São Vicente, Brazil, 2018. (In Portuguese). [Google Scholar]
- Hina, S.; Zahid, M.; Baloch, I.H.; Pasha, T.S. Water Analysis Handbook, 2nd ed.; Hach Company: Loveland, CO, USA, 1992. [Google Scholar]
- Hina, S.; Zahid, M.; Baloch, I.H.; Pasha, T.S. Water Analysis Handbook, 4th ed.; Hach Company: Loveland, CO, USA, 2000. [Google Scholar]
- Allison, L.E. Organic carbon. In Methods of Soil Analysis, Part 2; Black, C.A., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA, 1965; pp. 1367–1378. [Google Scholar]
- Boyd, C.E. Influence of organic matter on some characteristics of aquatic soils. Hydrobiologia 1970, 36, 17–21. [Google Scholar] [CrossRef]
- Durridge. RAD7 Radon Detector-User Manual; Durridge Co., Inc.: Bedford, MA, USA, 2009. [Google Scholar]
- Durridge. RAD7—RAD H2O Radon in Water Accessory-Owner’s Manual; Durridge Co., Inc.: Bedford, MA, USA, 2009. [Google Scholar]
- Osmond, J.K.; Cowart, J.B. The theory and uses of natural uranium isotopic variations in hydrology. At. Energy Rev. 1976, 14, 621–679. [Google Scholar]
- Ivanovich, M.; Harmon, R.S. Uranium Series Disequilibrium: Applications to Environmental Problems, 2nd ed.; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Bonotto, D.M. Radioactivity in Waters: From England to Guarani; Ed. UNESP: São Paulo, Brazil, 2004. (In Portuguese) [Google Scholar]
- Waterloo Hydrogeologic. AquaChem User’s Manual: Water Quality Data Analysis, Plotting & Modeling; Waterloo Hydrogeologic: Waterloo, ON, Canada, 2003. [Google Scholar]
- Piper, A.M.A. A graphic procedure in the geochemical interpretation of water-analyses. Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Schoeller, H. Groundwaters; Masson & Cie: Paris, France, 1962. (In French) [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- van Wirdum, G. Vegetation and Hydrology of Floating Rich-Fens. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 1991. [Google Scholar]
- Science Direct. Water Currents. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/water-currents (accessed on 10 July 2025).
- Bertioga. Tábua de Marés e Solunares. Available online: https://tabuademares.com/br/so-paulo/bertioga (accessed on 10 August 2025). (In Portuguese).
- São Sebastião. Tábua de Marés e Solunares. Available online: https://tabuademares.com/br/so-paulo/sao-sebastiao (accessed on 10 August 2025). (In Portuguese).
- Wilcox, L.V. Classification and use of irrigation waters. In USDA Circular No. 969; U.S. Department of Agriculture: Colorado Springs, CO, USA, 1955. [Google Scholar]
- USSL (U.S. Salinity Laboratory Staff). Diagnosis and improvement of saline and alkali soils. In Handbook No. 60; U.S. Department of Agriculture: Colorado Springs, CO, USA, 1954. [Google Scholar]
- Mirabbasi, R.; Mazloumzadeh, S.M.; Rahnama, M.B. Evaluation of irrigation water quality using Fuzzy Logic. Res. J. Environ. Sci. 2008, 2, 340–352. [Google Scholar]
- Lyerly, P.J.; Longenecker, D.E. Salinity control in irrigation and agriculture. Tex. Agric. Ext. Serv. Bull. 1957, 876, 1–20. [Google Scholar]
- Berner, E.K.; Berner, R.A. The Global Water Cycle; Prentice-Hall: Upper Saddle River, NJ, USA, 1987. [Google Scholar]
- Langmuir, D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547–569. [Google Scholar] [CrossRef]
- Rosholt, J.N.; Shields, W.R.; Garner, E.L. Isotope fractionation of uranium in sandstone. Science 1963, 139, 224–226. [Google Scholar] [CrossRef]
- Kigoshi, K. Alpha-recoil 234Th: Dissolution into water and the 234U/238U disequilibrium in nature. Science 1971, 173, 47–48. [Google Scholar] [CrossRef]
- Lou, R.; Lv, Z.; Dang, S.; Su, T.; Li, X. Application of machine learning in ocean data. Multimed. Syst. 2023, 29, 1815–1824. [Google Scholar] [CrossRef]
- Guillou, N.; Chapalain, G. Machine learning methods applied to sea level predictions in the upper part of a tidal estuary. Oceanologia 2021, 63, 531–544. [Google Scholar] [CrossRef]

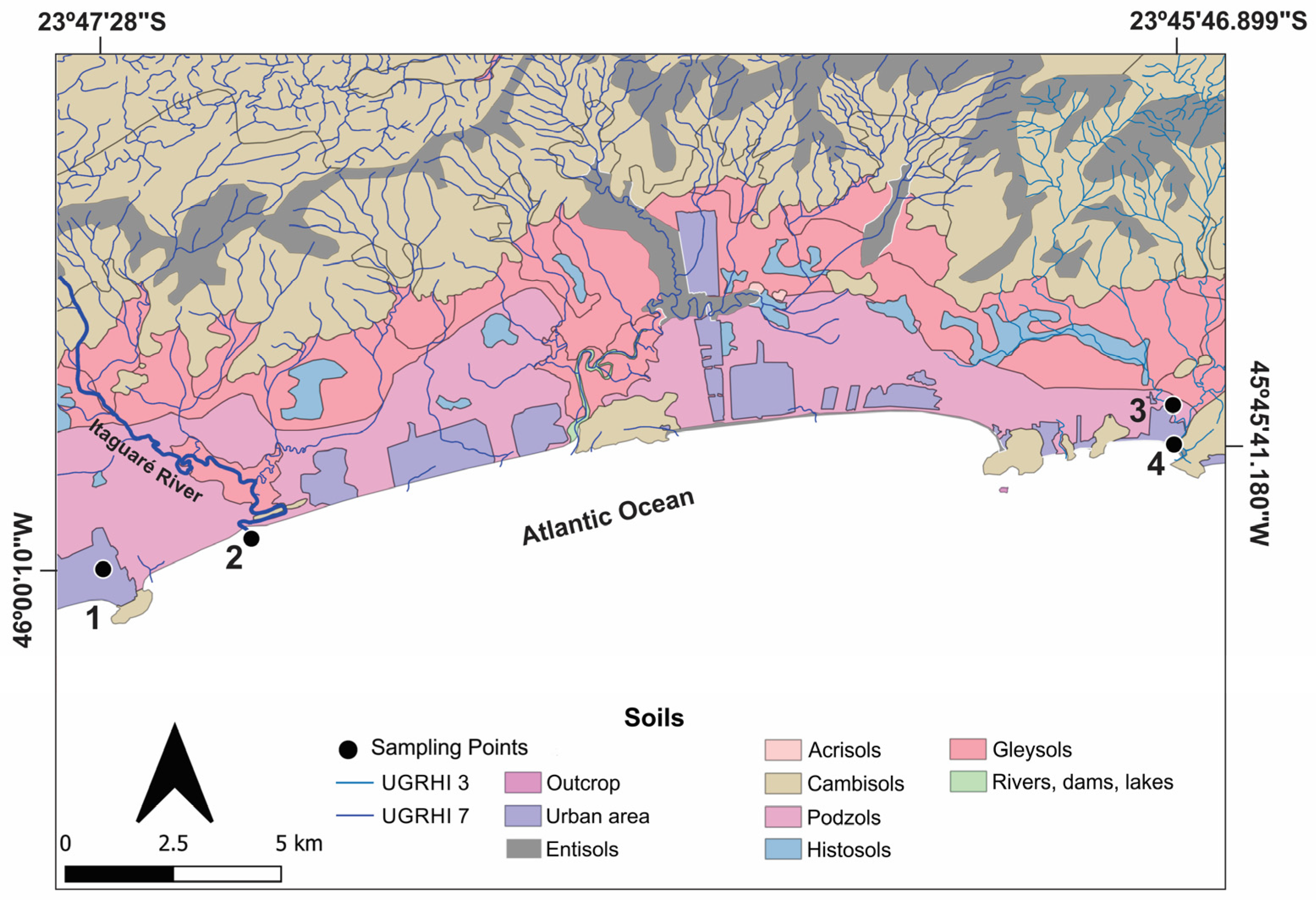





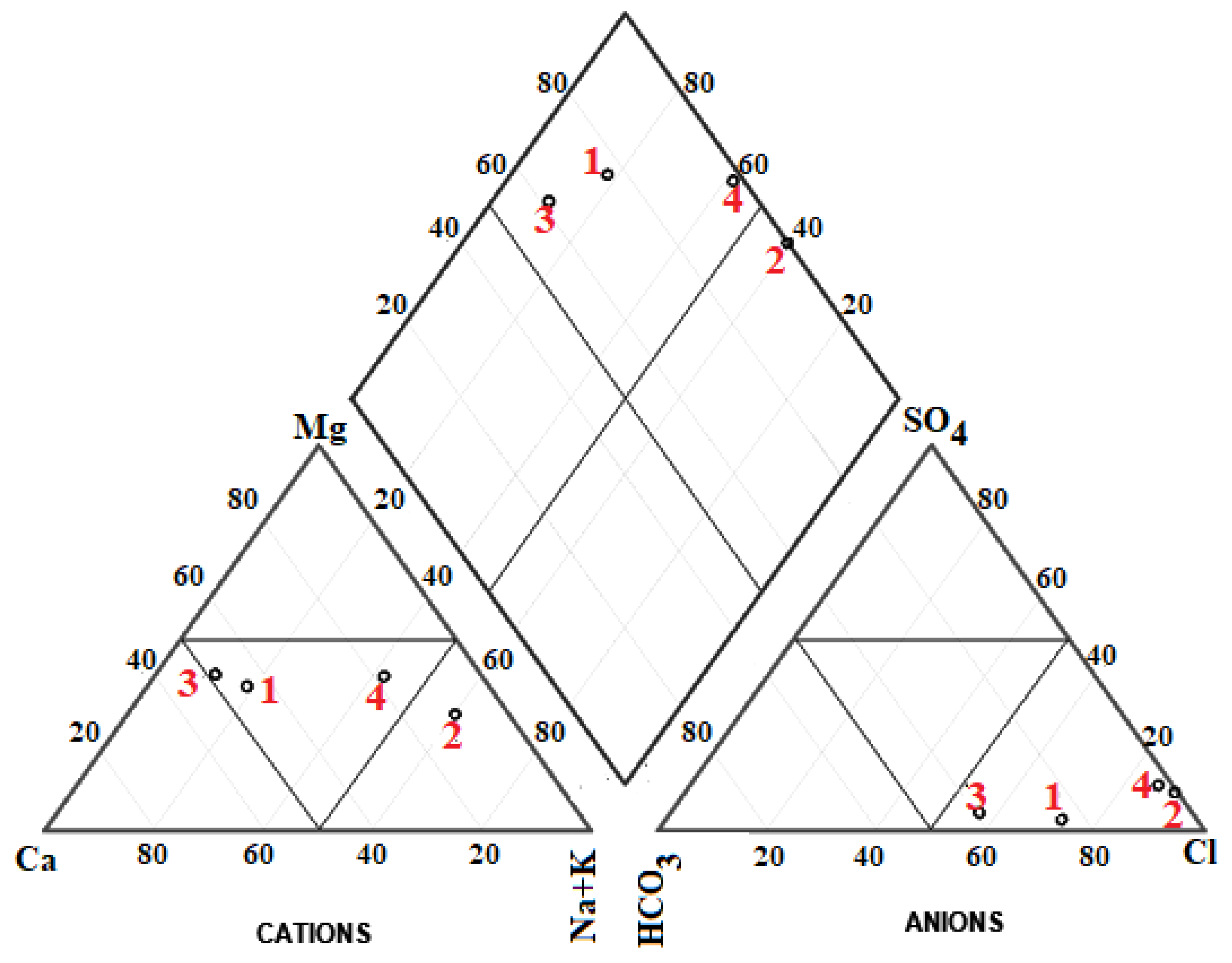

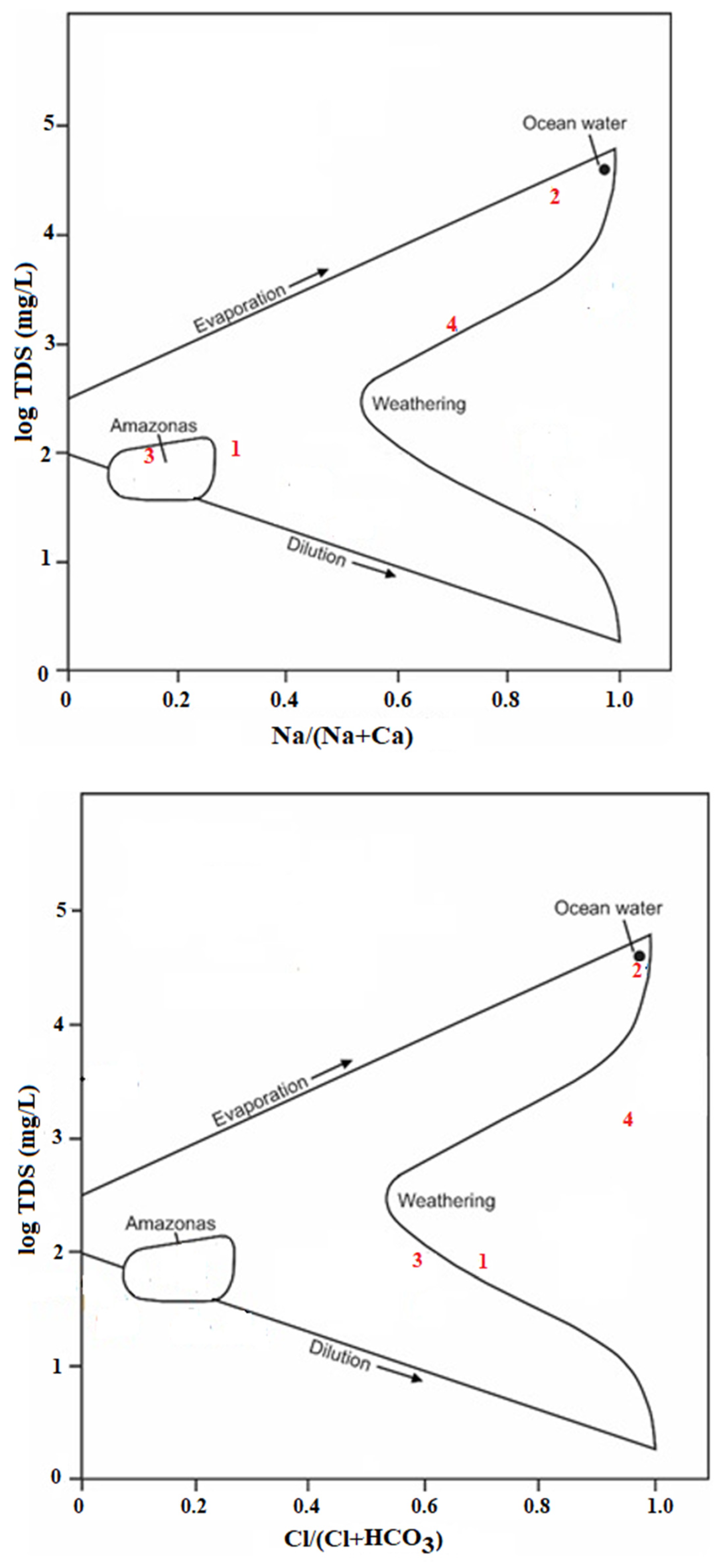

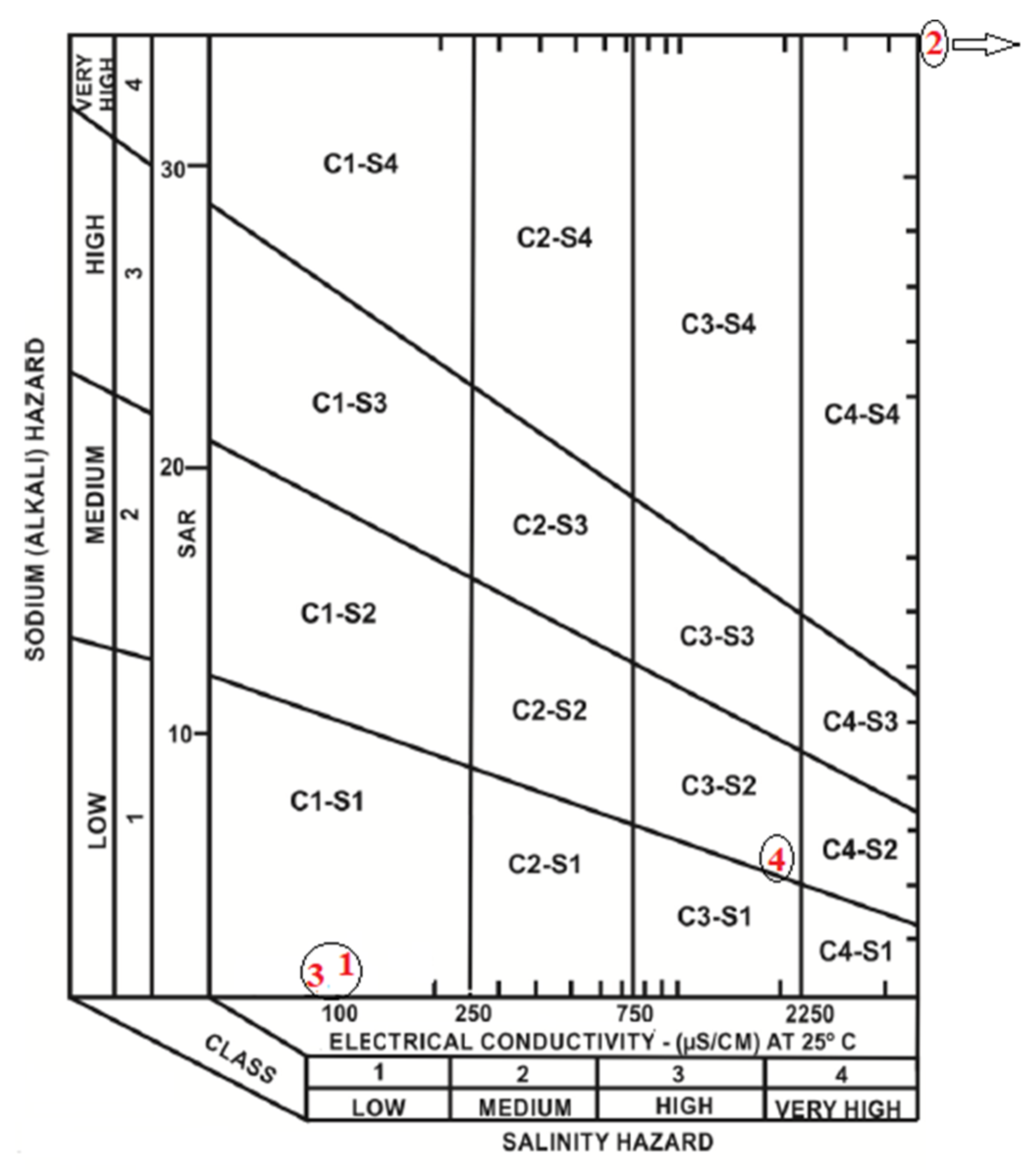
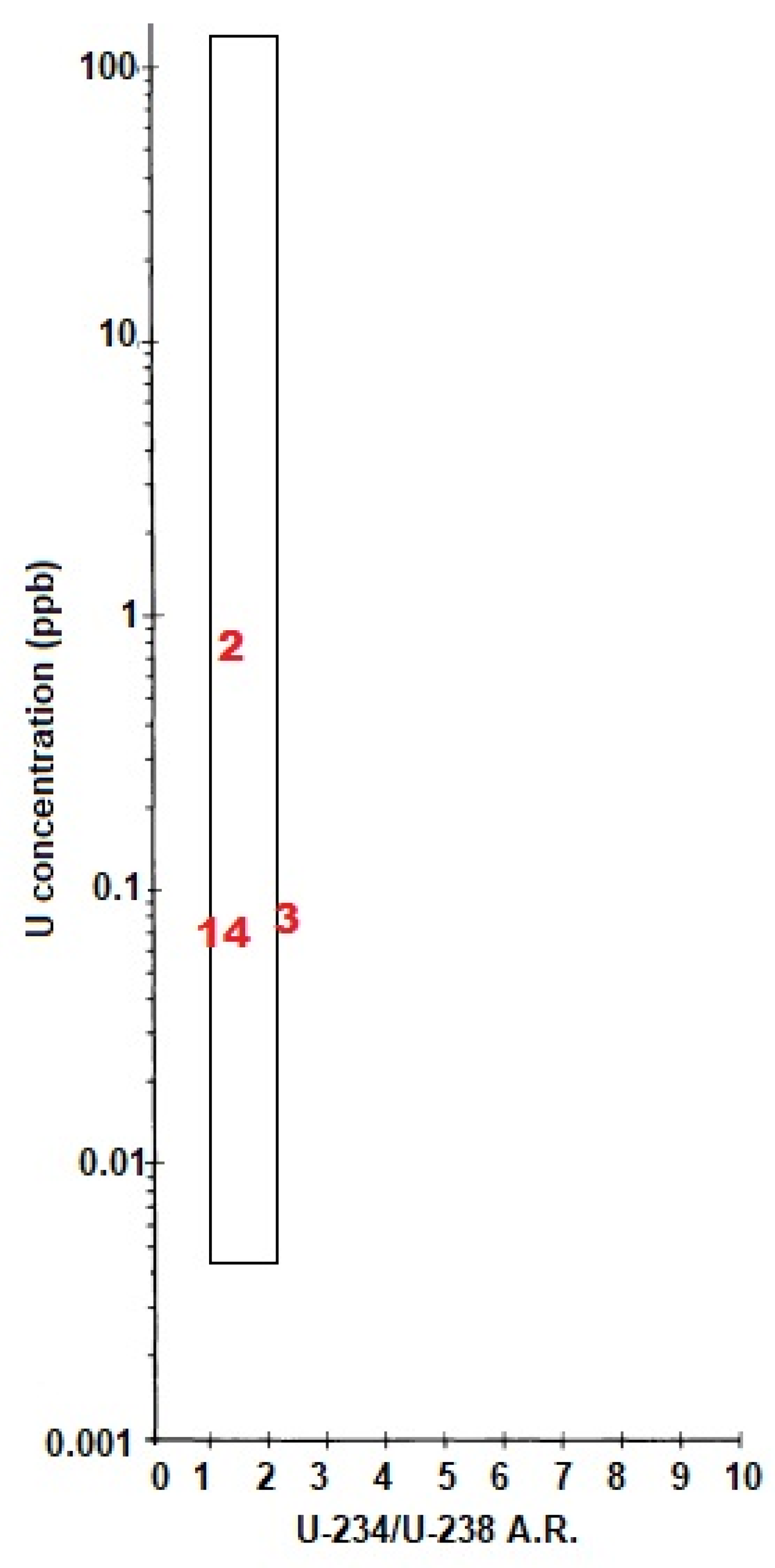
| Parameter | Unit | Analytical Method | Detection Limit | Analytical Uncertainty |
|---|---|---|---|---|
| Color | Pt-Co | Colorimetry | - | - |
| Turbidity | FTU | Colorimetry | - | - |
| pH | - | Potentiometry | - | ±0.5% |
| Electrical conductivity | µS/cm | Conductometry | - | ±1–10% |
| Suspended solids | mg/L | Absorptometry | - | - |
| Silica | mg/L | Colorimetry | 0.45 | ±0.9% |
| Sodium | mg/L | Flame photometry | 0.1 | ±1% |
| Potassium | mg/L | Flame photometry | 0.05 | ±1% |
| Calcium | mg/L | Colorimetry | 0.02 | ±0.67% |
| Magnesium | mg/L | Colorimetry | 0.006 | ±0.4% |
| Alkalinity | mg/L | Titration | 1 | ±1.5% |
| Chloride | mg/L | Colorimetry | 0.3 | ±3% |
| Nitrate | mg/L | Colorimetry | 0.8 | ±4% |
| Nitrite | mg/L | Colorimetry | 0.0011 | ±1.1% |
| Sulfate | mg/L | Colorimetry | 1 | ±1.8% |
| Phosphate | mg/L | Colorimetry | 0.01 | ±1% |
| Total iron | mg/L | Colorimetry | 0.006 | ±0.6% |
| Tannin + lignin | mg/L | Colorimetry | 0.08 | ±1.6% |
| Organic matter | mg/L | Colorimetry | 100 | ±0.68% |
| Organic carbon | mg/L | Walkley–Black | 58 | ±0.39% |
| Biochemical oxygen demand | mg/L | Potentiometry | 0.2 | ±2.8% |
| Radon | Bq/m3 | Alpha counting | 4 | ±40–50% |
| Uranium (238U) | µg/L | Alpha spectrometry | 0.01 | ±10–15% |
| 234U/238U Activity Ratio | - | Alpha spectrometry | - | ±10–15% |
| Parameter | Unit | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|---|---|
| Color | Pt-Co | 778 | 77 | 59 | 33 |
| Turbidity | FTU | 118 | 4 | 11 | 8 |
| pH | - | 6.35 | 7.30 | 5.40 | 5.95 |
| EC 1 | µS/cm | 102.4 | 16,900 | 92.9 | 2030 |
| SS 2 | mg/L | 22 | 14 | 3 | 5 |
| TDS 3 | mg/L | 102 | 24,157 | 117 | 923 |
| Silica | mg/L | 7.9 | 6.6 | 9.2 | 11 |
| Sodium | mg/L | 12.4 | 9430 | 11.5 | 257 |
| Potassium | mg/L | 1.6 | 187.7 | 0.82 | 10.1 |
| Calcium | mg/L | 29 | 1380 | 49 | 99 |
| Magnesium | mg/L | 15 | 2580 | 25 | 135 |
| Alkalinity 4 | mg/L | 10 | 64 | 10 | 16 |
| Chloride | mg/L | 17 | 9100 | 8.4 | 330 |
| Nitrate | mg/L | 0.80 | 1.40 | 0.6 | 0.6 |
| Nitrite | mg/L | 0.82 | 0.02 | 0.008 | 0.008 |
| Sulfate | mg/L | <1 | 1400 | <1 | 63 |
| Phosphate | mg/L | 0.41 | 0.05 | 0.10 | 0.04 |
| Total iron | mg/L | 1.90 | 0.06 | 0.33 | 0.35 |
| Tannin + lignin | mg/L | 4.4 | 7.1 | 1.0 | 0.6 |
| Organic matter | mg/L | 1700 | 200 | 100 | <100 |
| Organic carbon | mg/L | 988 | 116 | 58 | <58 |
| BOD 5 | mg/L | 0.57 | 0.37 | 0.24 | 0.39 |
| Radon | Bq/m3 | 93.8 | 46.9 | 318 | 544 |
| Uranium (238U) | µg/L | 0.08 | 0.66 | 0.075 | 0.072 |
| 234U/238U AR 6 | - | 0.92 | 1.17 | 2.79 | 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonotto, D.M.; Lunardi, M.; Goonetilleke, A. Hydrochemistry of Blackwaters in a Shoreline Zone of São Paulo State, Brazil. J. Mar. Sci. Eng. 2025, 13, 1575. https://doi.org/10.3390/jmse13081575
Bonotto DM, Lunardi M, Goonetilleke A. Hydrochemistry of Blackwaters in a Shoreline Zone of São Paulo State, Brazil. Journal of Marine Science and Engineering. 2025; 13(8):1575. https://doi.org/10.3390/jmse13081575
Chicago/Turabian StyleBonotto, Daniel M., Marina Lunardi, and Ashantha Goonetilleke. 2025. "Hydrochemistry of Blackwaters in a Shoreline Zone of São Paulo State, Brazil" Journal of Marine Science and Engineering 13, no. 8: 1575. https://doi.org/10.3390/jmse13081575
APA StyleBonotto, D. M., Lunardi, M., & Goonetilleke, A. (2025). Hydrochemistry of Blackwaters in a Shoreline Zone of São Paulo State, Brazil. Journal of Marine Science and Engineering, 13(8), 1575. https://doi.org/10.3390/jmse13081575








