1. Introduction
The environmental and socioeconomic problems of abandoned and lost fishing gear, particularly nets (hereafter ghost nets), are of global scale [
1]. Between 500,000 and 1 million tonnes of fishing gear is abandoned, discarded or lost in the ocean every year [
2]. Due to their designed purpose of catching fish, ghost nets pose a chronic impact on marine biodiversity which can last for decades after the gear has become lost in the sea, namely by continuing the trapping and killing of mobile species. Ghost-fished biodiversity (usually referred to as “ghost catch” [
2]) includes not only commercial species but many others, including birds, marine mammals and turtles [
3,
4]. Ghost nets also alter the substrate on which they rest or in which they are ensnarled, significantly affecting shallow and deep benthic ecosystems, such as seagrass and coralligenous habitats [
5,
6,
7,
8,
9].
Even though there is knowledge about the origin of this problem, as well as actions and alternatives to decrease the impacts, it is a problem that only seems to increase with time and is not only due to advances in the fishing industry and fishing rounds but also due to the availability of new, cheaper and more resistant materials with a prolonged utility life [
4]. This type of plastic debris is infamous because of its wide-ranging impacts on ecosystem processes, habitats and organisms by facilitating the settlement and dispersal of invasive species [
10] and incorporating plastics and toxic chemicals into food pathways [
11,
12,
13]. The mortalities associated with ghost nets are linked to several environmental and ecological factors, such as the state of the nets, the topography of the seafloor, currents, the type of habitat, and species’ vulnerability and abundance, among others [
4,
9,
14,
15,
16,
17]. As a result, ghost nets, the most ubiquitous type of lost gear, are considered the deadliest form of marine plastic debris [
6] and an important challenge to management and conservation efforts [
18]. However, not all ghost nets will continue catching and killing indeterminately. The efficiency of the nets in catching mobile species changes with the physical state of the net itself and the increase in fouling that usually “stabilizes” the net by sinking and flattening it to the substrate. However, this process may require decades and there are many possible resting positions along the nets’ total length that could keep harming benthic or other communities.
Dramatic photos and videos of the ghost catch showing many degrees of damage and ensnarement [
19] have contributed to raising awareness of the problem and implementing remedial actions (i.e., removals) that are not always properly regulated and do not always fall under a management action plan. For example, a common approach to the ghost nets is the business-as-usual “search and remove” approach, where the nets are located and soon after retrieved. However, the removals can seriously damage the same marine habitats that they seek to protect [
9,
17,
20]. Despite this being a well-intentioned approach, no aspects except for logistics and depth are considered. Once the ghost nets are located, divers engage in their removal from the substrate, paying little to no attention to the living organisms colonizing the net. The longer the nets have been immersed, the higher the colonization by organisms [
8]. However, the colonization degree may be species-specific and affected by the nets’ material and seasonal environmental fluctuations [
21]. Careless removal activities of “aged” ghost nets usually cause more harm to the marine habitats than is acceptable, and this is mostly due to the destruction of colonizing species of EU importance (e.g., corals and sponges) and the damage to the attachment substrate (e.g., coralligenous communities).
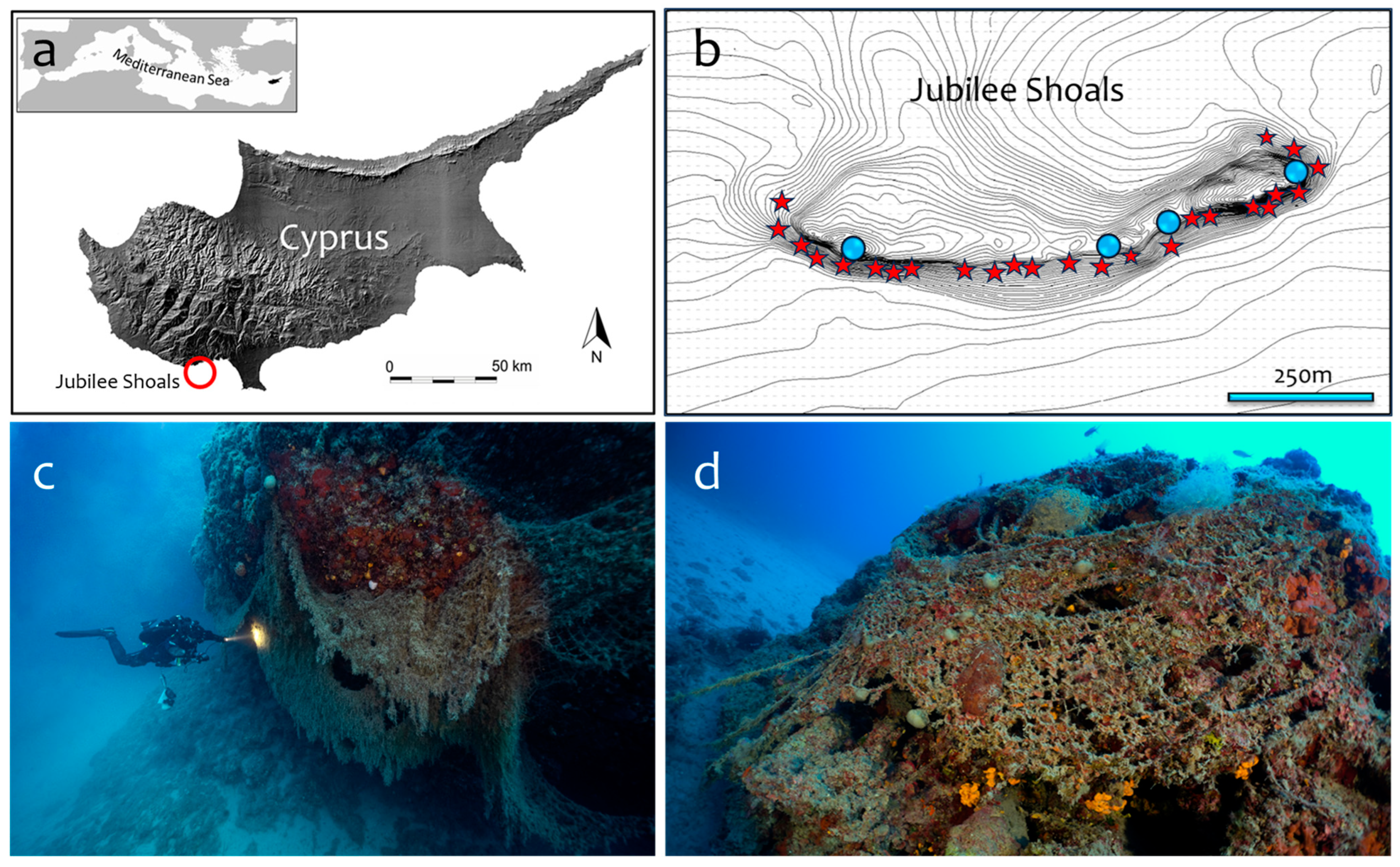
In Cyprus, ghost nets are found almost everywhere around the island, threatening marine life and scuba diving activities and obstructing in-use fishing gear and navigation. The resilience of the marine habitats of Cyprus is already compromised by climate change and human activities. For example, marine heat waves have significantly impacted the marine biodiversity of the island by producing seawater warming which lasts for months, probably facilitating the spread of diseases and killing en masse sponges and other species [
22,
23]. Regrettably, ghost nets have become a permanent feature of the seascape and a pervasive source of deterioration of the already stressed-out marine communities of Cyprus. Hundredths of meters of massive, derelict nets of different ages and lengths, hanging and smothering benthic substrates, were confirmed during ecological surveys of coralligenous habitats and submarine caves in the Jubilee Shoals area, Cyprus [
24]. In this study, our goal is to introduce an evidence-based approach assisting environmental managers in deciding whether they must (or not) remove particular ghost nets from the shoals and elsewhere.
The Gear Removal Index (GRI) is a decision support tool originally developed to guide the selective removal of ghost fishing gear based on ecological criteria [
17]. First applied in studies across the central and western Mediterranean, the GRI enables field practitioners to evaluate the ecological value of ghost nets and determine whether removal is advisable, discouraged or neutral. The index incorporates field-based observations of net structure, fouling community composition and environmental context to assign priority scores. Its application is based on the principle that not all ghost nets are equal in ecological impact—some may serve as a substrate for sensitive benthic communities, while others may pose immediate hazards with little ecological value. The original GRI has been used in protected areas and biodiversity hotspots such as marine caves and coralligenous habitats, where unselective removal can cause significant ecological disturbance. The framework promotes a more strategic and evidence-based approach to marine debris mitigation, shifting the focus from purely visual or logistical criteria to ecological function and risk.
This study presents the first application and refinement of the Gear Removal Index (GRI) in the eastern Mediterranean, with a specific focus on the ecologically significant reef complex of the Jubilee Shoals, Cyprus. While the original GRI has been previously applied in other Mediterranean contexts, this study introduces a modified version (GRI+), incorporating region-specific ecological considerations, notably the presence of non-indigenous species (NIS) and the structural complexity of habitats such as submarine caves. The main aim of this study was to evaluate whether ghost nets should be removed based on the composition of fouling communities, net structure and ecological sensitivity of the surrounding habitat. This study did not test a formal statistical hypothesis, but it was guided by the expectation that nets supporting early successional, opportunistic species (e.g., green algae) would be prioritized for removal, while nets with “mature” biodiverse assemblages (e.g., Rhodophyta, Porifera) would be candidates for conservation. In doing so, this study demonstrates the operational value of the GRI+ as a decision-making tool for targeted net removal in biodiversity-sensitive marine environments.
4. Discussion
It is only relatively recently (mid 1980s) that the ghost nets issue has gained international recognition [
4], and this might account for the current lack of a systematic management approach to deal with the problem and the many different responses from environmental managers and the general public. Ghost nets, particularly nets that have been recently lost, are deadly effective for the sole reason that they were designed to “capture and kill” and they will continue doing that in most cases [
9,
33]. As a result, removal of those nets is the most common approach. However, removal of nets without taking into account ecological factors and careful evaluation is certainly not the best approach [
9,
17,
20]. The importance of implementing an evidence-based protocol to help managers decide whether ghost nets should be removed or not was demonstrated in this study, using the important marine habitats of the Jubilee Shoals as an example.
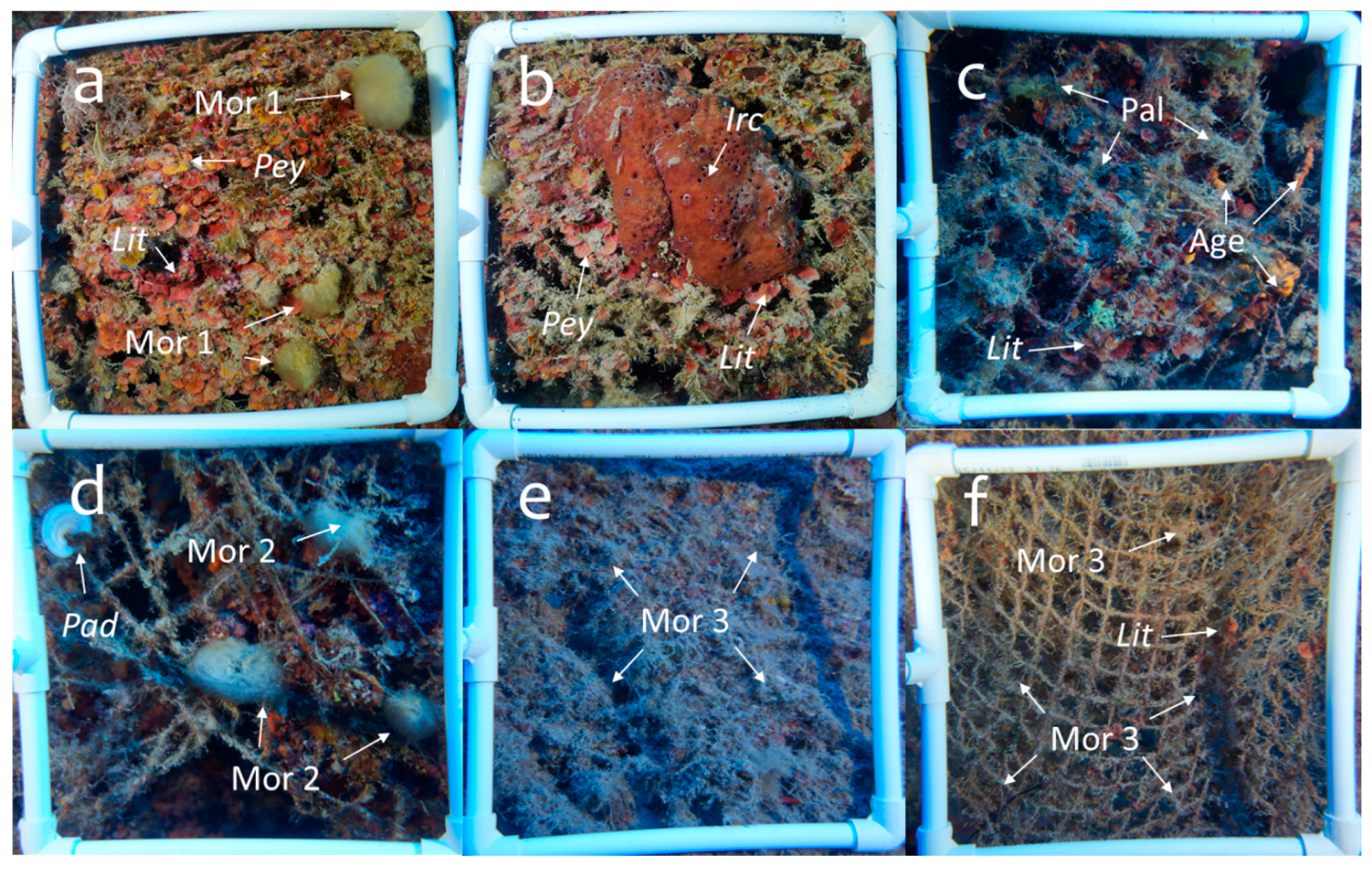
Analysis of the communities fouling the nets was necessary, and the percentages of cover show a strong prevalence of opportunistic algal colonization, particularly by green algae. The significant presence of Rhodophyta (calcareous algae) on some nets indicates a more established fouling community of species of conservation importance [
8,
9,
34], which was reflected in the GRI+ values and removal priorities. While green algae dominance was evident in our results, this pattern deviates from regional baselines. In similar coralligenous habitats in Cyprus, and in the same Jubilee Shoals area (especially at comparable depths), Rhodophyta, in particular crustose coralline algae, are typically the dominant group [
24]. Thus, the observed macroalgal prevalence should not be interpreted as a typical condition. Overall, the trends show that ghost nets categorized with higher GRI+ priorities (Priority 5 = removal is not recommended) support more taxonomically diverse and ecologically important communities (i.e., foundational species of coralligenous habitats), while those with lower priorities (Priority 1 = removals absolutely advised) are primarily colonized by early successional, fast-growing algae. Removal of those nets with significantly important communities will affect the species, causing further ecological damage. As a result, the management approach to the ghost nets issue should be evidence-based, not only driven by passionate or aesthetical reasons; it needs pre-surveys of evaluation, analysis and decision making based on evidence, i.e., targeted solutions [
1,
9]. As such, the direct application of the original GRI to the eastern basin required adaptation to ensure that the outcomes remained ecologically meaningful and context-sensitive. Since this study took place in the eastern Mediterranean, where non-native species are more prevalent than anywhere else in the world’s seas [
26], the GRI was adjusted accordingly. As a result, an additional evaluation factor for non-native species presence was included in the revised index (GRI+).
Given the wide range of negative ecological impacts of ghost nets, the removal of the nets most harmful to biodiversity and those that posed a high safety risk to divers should benefit the general ecosystem of the Jubilee Shoals as well as the non-extractive human activities in the area (e.g., scuba and free diving) [
24]. The removals were performed following a consistent protocol that was demonstrated to the competent authorities and decision makers responsible for the conservation and protection of the shoals. We explicitly made the case that the evidence-based approach is only an aid for decision-making, and that it should be complemented with expert opinion (e.g., divers and marine ecologists). It represents the first step in the implementation of the method in other areas of the Levantine Sea with similar problems [
36,
37], and in the achievement of managing the pervasive issue of ghost nets. However, there are other important methodologies which are recommended for the assessment of the impacts of removals and the subsequent evolution of the benthic communities [
9,
38].
Lastly, it is of utmost importance to emphasize that for the appropriate management of ghost nets, tackling the root of the problem is better than concentrating only on actions to mitigate the impacts [
38,
39]. Fishing activities are the source of the ghost nets; therefore, close collaboration with this sector is required if we want to reduce the amount and frequency of abandoned and lost gear [
4,
40,
41,
42,
43,
44,
45,
46]. Removals will always be essential but must be implemented under a systematic approach and in parallel with preventative measures, such as educational activities, the marking of fishing gear, constant hauling of static gear, early reporting of gear loss and enforcement of regulations for fishing [
47].