Evaluating the Trophic Structure of an Artificial Macroalgal Bed of Eisenia bicyclis Using C and N Stable Isotopes
Abstract
1. Introduction
2. Materials and Methods
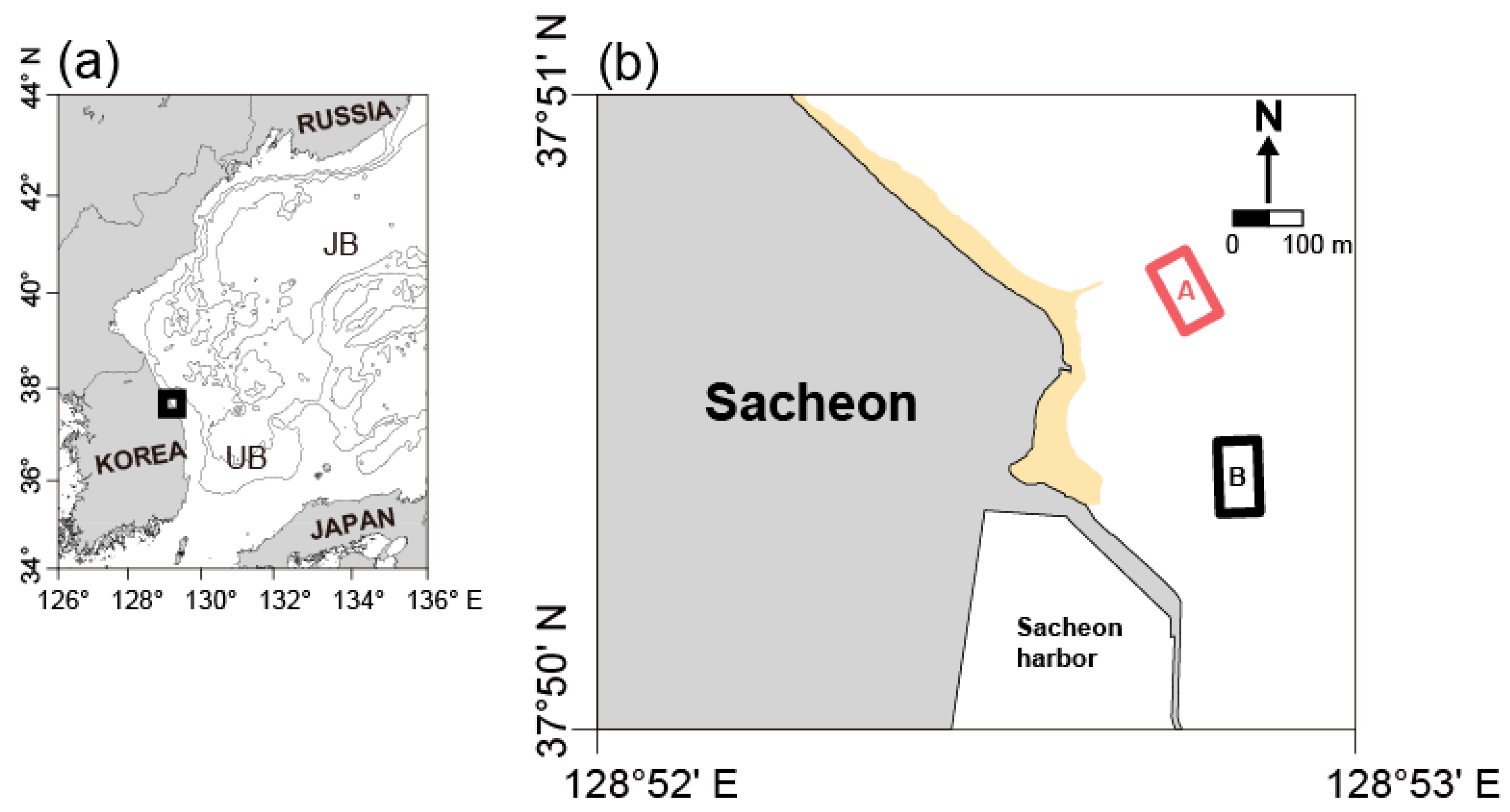
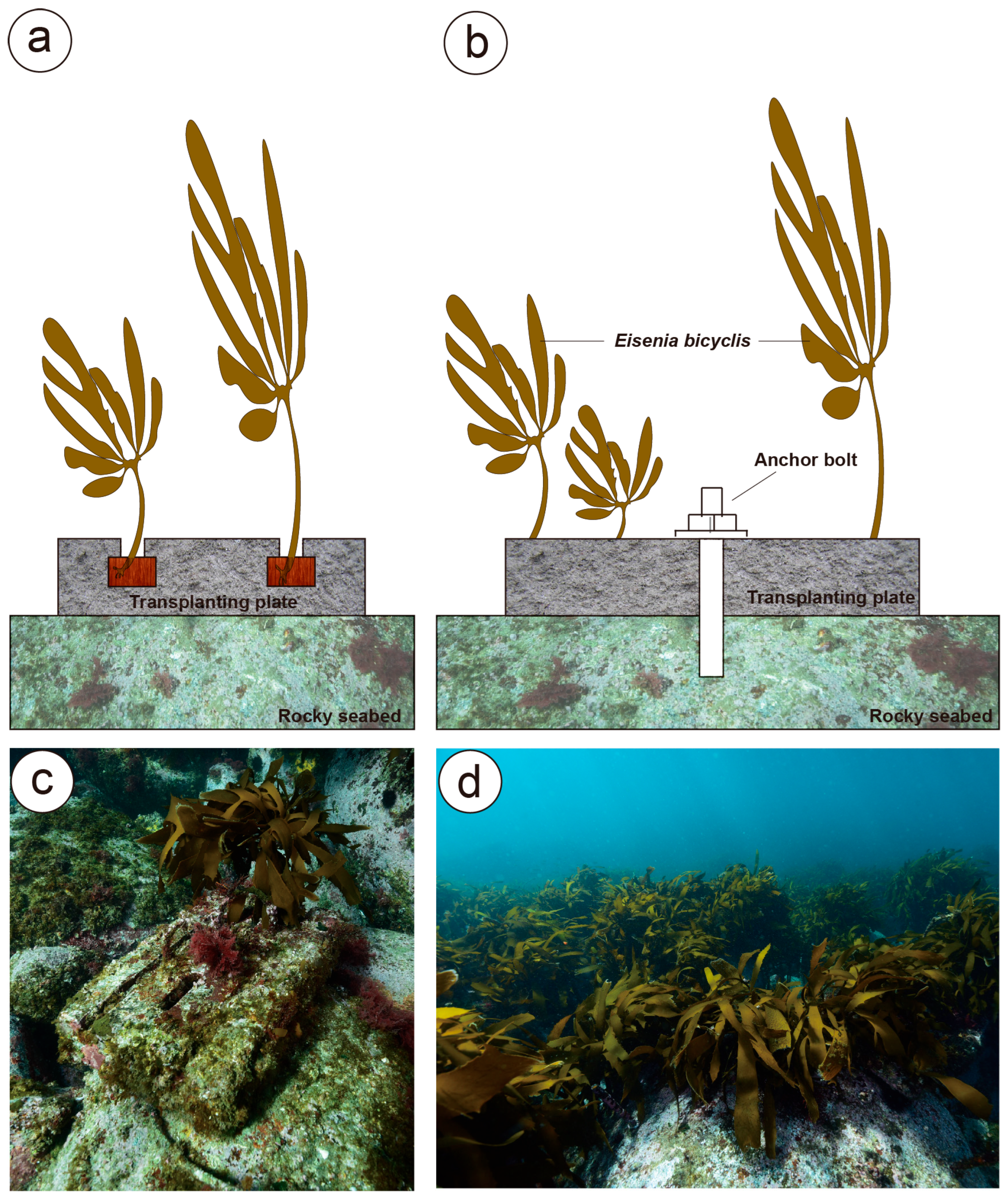
2.1. Experimental Sites and Transplantation Design
2.2. Sample Collection and Treatment
2.3. Stable Isotope Analysis
2.4. Data Analysis
3. Results
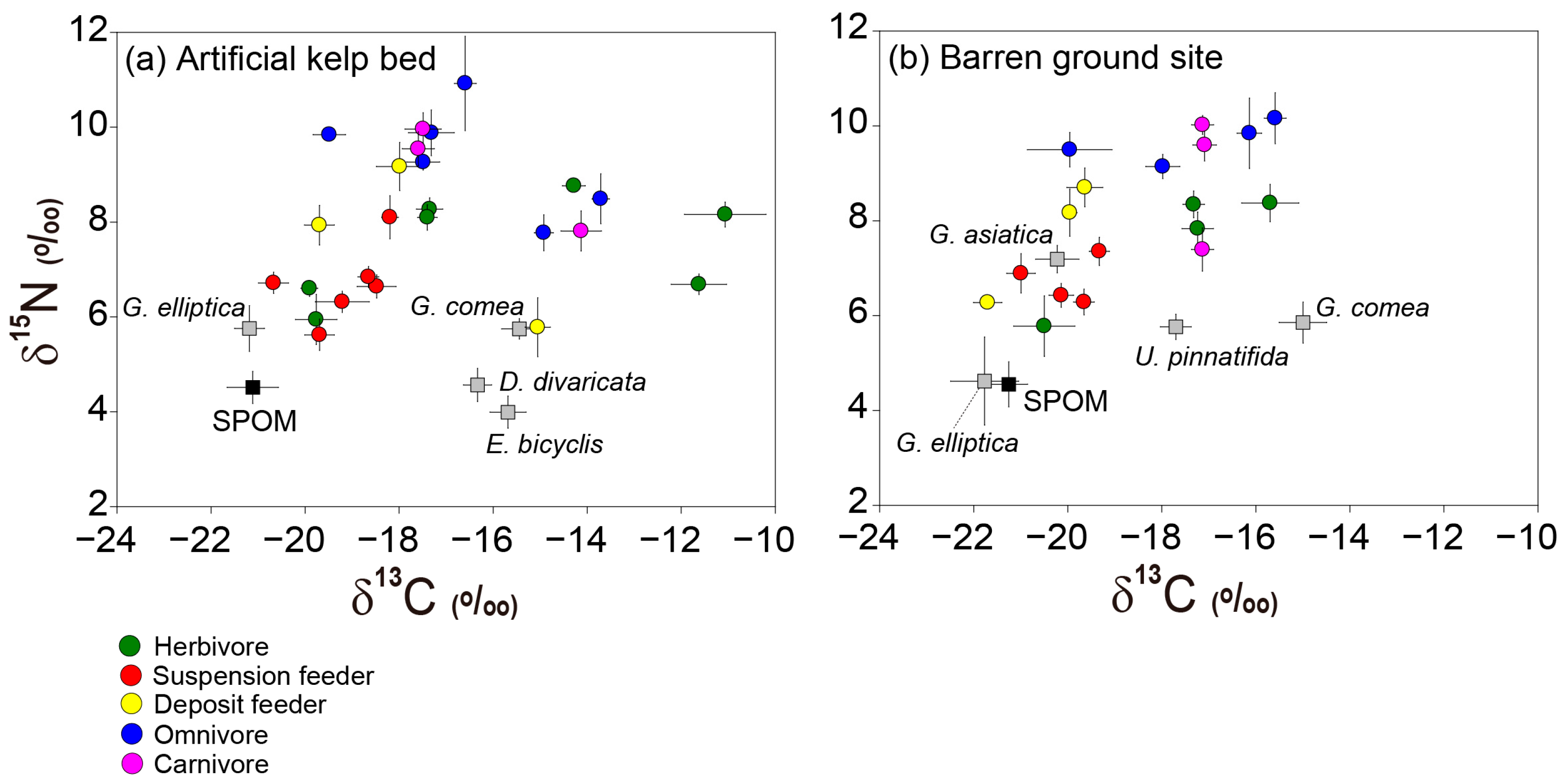
3.1. Isotopic Ratios of Organic Matter Sources
3.2. Isotopic Ratios of Macrobenthic Consumers
3.3. Isotopic Niche Indices of Macrobenthic Consumers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Jarvis, J.; Trevathan-Tackett, S.M.; Bellgrove, A. Seagrasses and macroalgae: Importance, vulnerability and impacts. Clim. Change Impacts Fish. Aquac. A Glob. Anal. 2017, 2, 729–770. [Google Scholar] [CrossRef]
- Fulton, C.J.; Berkström, C.; Wilson, S.K.; Abesamis, R.A.; Bradley, M.; Åkerlund, C.; Barrett, L.T.; Bucol, A.A.; Chacin, D.H.; Chong-Seng, K.M.; et al. Macroalgal meadow habitats support fish and fisheries in diverse tropical seascapes. Fish Fish. 2020, 21, 700–717. [Google Scholar] [CrossRef]
- Gedan, K.B.; Silliman, B.R.; Bertness, M.D. Centuries of human-driven change in salt marsh ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.E.; Benedetti-Cecchi, L.; Trinanes, J.; Muller-Karger, F.E.; Ambo-Rappe, R.; Boström, C.; Buschmann, A.H.; Byrnes, J.; Coles, R.G.; Creed, J.; et al. Toward a coordinated global observing system for seagrasses and marine macroalgae. Front. Mar. Sci. 2019, 6, 317. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Ballesteros, E.; Francour, P.; Guidetti, P.; Meinesz, A.; Thibaut, T.; Mangialajo, L. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of Marine Protected Areas. Adv. Oceanogr. Limnol. 2013, 4, 83–101. [Google Scholar] [CrossRef]
- Eger, A.M.; Marzinelli, E.M.; Christie, H.; Fagerli, C.W.; Fujita, D.; Gonzalez, A.P.; Hong, S.W.; Kim, J.H.; Lee, L.C.; McHugh, T.A.; et al. Global kelp forest restoration: Past lessons, present status, and future directions. Biol. Rev. 2022, 97, 1449–1475. [Google Scholar] [CrossRef]
- Terawaki, T.; Yoshikawa, K.; Yoshida, G.; Uchimura, M.; Iseki, K. Ecology and restoration techniques for Sargassum beds in the Seto Inland Sea, Japan. Mar. Pollut. Bull. 2003, 47, 198–201. [Google Scholar] [CrossRef]
- Loke, L.H.; Ladle, R.J.; Bouma, T.J.; Todd, P.A. Creating complex habitats for restoration and reconciliation. Ecol. Eng. 2015, 77, 307–313. [Google Scholar] [CrossRef]
- Jung, S.; Chau, T.V.; Kim, M.; Na, W.B. Artificial seaweed reefs that support the establishment of submerged aquatic vegetation beds and facilitate ocean macroalgal afforestation: A review. J. Mar. Sci. Eng. 2022, 10, 1184. [Google Scholar] [CrossRef]
- Choi, C.G.; Serisawa, Y.; Ohno, M.; Sohn, C.H. Construction of artificial seaweed beds; Using the spore bag method. Algae 2000, 15, 179–182. [Google Scholar]
- Yu, Y.Q.; Zhang, Q.S.; Tang, Y.Z.; Zhang, S.B.; Lu, Z.C.; Chu, S.H.; Tang, X.X. Establishment of intertidal seaweed beds of Sargassum thunbergii through habitat creation and germling seeding. Ecol. Eng. 2012, 44, 10–17. [Google Scholar] [CrossRef]
- Chung, I.K.; Oak, J.H.; Lee, J.A.; Shin, J.A.; Kim, J.G.; Park, K.S. Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES J. Mar. Sci. 2013, 70, 1038–1044. [Google Scholar] [CrossRef]
- Choi, C.G.; Takeuchi, Y.; Terawaki, T.; Serisawa, Y.; Ohno, M.; Sohn, C.H. Ecology of seaweed beds on two types of artificial reef. J. Appl. Phycol. 2002, 14, 343–349. [Google Scholar] [CrossRef]
- Jung, S.M.; Lee, J.H.; Han, S.H.; Jeon, W.B.; Kim, G.Y.; Kim, S.; Lee, H.-R.; Hwang, D.S.; Jung, S.; Lee, J.; et al. A new approach to the restoration of seaweed beds using Sargassum fulvellum. J. Appl. Phycol. 2020, 32, 2575–2581. [Google Scholar] [CrossRef]
- Udy, J.A.; Wing, S.R.; O’Connell-Milne, S.A.; Durante, L.M.; McMullin, R.M.; Kolodzey, S.; Frew, R.D. Regional differences in supply of organic matter from kelp forests drive trophodynamics of temperate reef fish. Mar. Ecol. Prog. Ser. 2019, 621, 19–32. [Google Scholar] [CrossRef]
- Wing, S.R.; Durante, L.M.; Connolly, A.J.; Sabadel, A.J.M.; Wing, L.C. Overexploitation and decline in kelp forests inflate the bioenergetics costs of fisheries. Glob. Ecol. Biogeogr. 2022, 31, 621–635. [Google Scholar] [CrossRef]
- Kang, C.K.; Choy, E.J.; Son, Y.; Lee, J.Y.; Kim, J.K.; Kim, Y.; Lee, K.S. Food web structure of a restored macroalgal bed in the eastern Korean peninsula determined by C and N stable isotope analyses. Mar. Biol. 2008, 153, 1181–1198. [Google Scholar] [CrossRef]
- Kim, M.J.; Yun, H.Y.; Shin, K.H.; Kim, J.H. Evaluation of food web structure and complexity in the process of Kelp bed recovery using stable isotope analysis. Front. Mar. Sci. 2022, 9, 885676. [Google Scholar] [CrossRef]
- Park, T.H.; Jang, J.B.; Chun, C.K.; Lee, Y.; Han, K.S.; Yang, S.J.; Park, H.J. Trophic assessment of an artificial kelp Eisenia bicyclis bed off the eastern coast of Korea based on Stable isotope analyses. Water 2023, 15, 1099. [Google Scholar] [CrossRef]
- Boecklen, W.J.; Yarnes, C.T.; Cook, B.A.; James, A.C. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 411–440. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib. Mar. Sci. 1984, 27, 13–47. [Google Scholar]
- Vander Zanden, M.J.; Rasmussen, J.B. Variation in δ15N and δ13C trophic fractionation: Implications for aquatic food web studies. Limnol. Oceanogr. 2001, 46, 2061–2066. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Park, H.J.; Park, T.H.; Kang, H.Y.; Lee, K.S.; Kim, Y.K.; Kang, C.K. Assessment of restoration success in a transplanted seagrass bed based on isotopic niche metrics. Ecol. Eng. 2021, 166, 106239. [Google Scholar] [CrossRef]
- Anderson, M. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; Primer-E Limited: Auckland, New Zealand, 2008. [Google Scholar]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
- Catry, T.; Lourenço, P.M.; Lopes, R.J.; Carneiro, C.; Alves, J.A.; Costa, J.; Rguibi-Idrissi, H.; Bearhop, S.; Piersma, T.; Granadeiro, J.P. Structure and functioning of intertidal food webs along an avian flyway: A comparative approach using stable isotopes. Funct. Ecol. 2016, 30, 468–478. [Google Scholar] [CrossRef]
- West, T.L.; Clough, L.M.; Ambrose, W.G., Jr. Assessment of function in an oligohaline environment: Lessons learned by comparing created and natural habitats. Ecol. Eng. 2000, 15, 303–321. [Google Scholar] [CrossRef]
- Rodney, W.S.; Paynter, K.T. Comparisons of macrofaunal assemblages on restored and non-restored oyster reefs in mesohaline regions of Chesapeake Bay in Maryland. J. Exp. Mar. Biol. Ecol. 2006, 335, 39–51. [Google Scholar] [CrossRef]
- Wortley, L.; Hero, J.M.; Howes, M. Evaluating ecological restoration success: A review of the literature. Restor. Ecol. 2013, 21, 537–543. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Olden, J.D.; Gratton, C. Food-Web Approaches in Restoration Ecology. In Foundations of Restoration Ecology; Falk, D.A., Palmer, M., Zedler, J., Eds.; Island Press: Washington, DC, USA, 2006; pp. 165–189. [Google Scholar]
- Kang, C.K.; Choy, E.J.; Hur, Y.B.; Myeong, J.I. Isotopic evidence of particle size-dependent food partitioning in cocultured sea squirt Halocynthia roretzi and Pacific oyster Crassostrea gigas. Aquat. Biol. 2009, 6, 289–302. [Google Scholar] [CrossRef][Green Version]
- Park, H.J.; Kwak, J.H.; Kang, H.Y.; Kwon, K.Y.; Lim, W.; Kang, C.K. Incorporation of Cochlodinium bloom-derived organic matter into a temperate subtidal macrobenthic food web as traced by stable isotopes. Mar. Pollut. Bull. 2020, 154, 111053. [Google Scholar] [CrossRef]
- James, W.R.; Lesser, J.S.; Litvin, S.Y.; Nelson, J.A. Assessment of food web recovery following restoration using resource niche metrics. Sci. Total Environ. 2020, 711, 134801. [Google Scholar] [CrossRef] [PubMed]
- Grall, J.; Le Loc’h, F.; Guyonnet, B.; Riera, P. Community structure and food web based on stable isotopes (δ15N and δ13C) analysis of a North Eastern Atlantic maerl bed. J. Exp. Mar. Biol. Ecol. 2006, 338, 1–15. [Google Scholar] [CrossRef]
- Marconi, M.; Giordano, M.; Raven, J.A. Impact of taxonomy, geography, and depth on δ 13C and δ15N variation in a large collection of macroalgae. J. Phycol. 2011, 47, 1023–1035. [Google Scholar] [CrossRef]
- Rezek, R.J.; Lebreton, B.; Roark, E.B.; Palmer, T.A.; Pollack, J.B. How does a restored oyster reef develop? An assessment based on stable isotopes and community metrics. Mar. Biol. 2017, 164, 54. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, C.; Kang, C.K. Recovery of macrobenthic food web on rocky shores following the Hebei Spirit oil spill as revealed by C and N stable isotopes. Water 2022, 14, 2335. [Google Scholar] [CrossRef]
- France, R.L. Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnol. Oceanogr. 1995, 40, 1310–1313. [Google Scholar] [CrossRef]
- Newsome, S.D.; Martinez del Rio, C.; Bearhop, S.; Phillips, D.L. A niche for isotopic ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; Macleod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef]
- Rigolet, C.; Thiebaut, E.; Brind’Amour, A.; Dubois, S.F. Investigating isotopic functional indices to reveal changes in the structure and functioning of benthic communities. Funct. Ecol. 2015, 29, 1350–1360. [Google Scholar] [CrossRef]
- Starko, S.; Demes, K.W.; Neufeld, C.J.; Martone, P.T. Convergent evolution of niche structure in Northeast Pacific kelp forests. Funct. Ecol. 2020, 34, 2131–2146. [Google Scholar] [CrossRef]
- Kang, H.Y.; Lee, B.G.; Park, H.J.; Yun, S.G.; Kang, C.K. Trophic structures of artificial reef communities off the southern coast of the Korean peninsula as determined using stable isotope analyses. Mar. Pollut. Bull. 2021, 69, 112474. [Google Scholar] [CrossRef] [PubMed]
- Oyamada, K.; Tsukidate, M.; Watanabe, K.; Takahashi, T.; Isoo, T.; Terawaki, T. A Field Test of Porous Carbonated Blocks Used as Artificial Reef in Seaweed Beds of Ecklonia Cava. In Nineteenth International Seaweed Symposium. Developments in Applied Phycology; Borowitzka, M.A., Critchley, A.T., Kraan, S., Peters, A., Sjøtun, K., Notoya, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 2. [Google Scholar] [CrossRef]
- Fredriksen, S.; Filbee-Dexter, K.; Norderhaug, K.M.; Steen, H.; Bodvin, T.; Coleman, M.A.; Moy, F.; Wernberg, T. Green gravel: A novel restoration tool to combat kelp forest decline. Sci. Rep. 2020, 10, 3983. [Google Scholar] [CrossRef]
- Campbell, A.H.; Marzinelli, E.M.; Vergés, A.; Coleman, M.A.; Steinberg, P.D. Towards restoration of missing underwater forests. PLoS ONE 2014, 9, e84106. [Google Scholar] [CrossRef] [PubMed]
- FIRA. Annual Report for Marine Forest Project. Report Number: FIRA-IR-24-003; Korean Fisheries Resource Agency: Busan, Republic of Korea, 2022; (In Korean). [Google Scholar] [CrossRef]
- Nordström, M.C.; Currin, C.A.; Talley, T.S.; Whitcraft, C.R.; Levin, L.A. Benthic food-web succession in a developing salt marsh. Mar. Ecol. Prog. Ser. 2014, 500, 43–55. [Google Scholar] [CrossRef]

| Artificial Kelp Bed | Barren Ground Site | PERMANOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organic Matter Source | δ13C | δ15N | δ13C | δ15N | ||||||||
| n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | Pseudo-F | p-Value | |
| Dictyopteris divaricata | 3 | −16.3 | 0.3 | 4.6 | 0.3 | 4.47 | 0.026 | |||||
| Eisenia bicyclis | 5 | −15.7 | 0.4 | 4.0 | 0.3 | |||||||
| Grateloupia asiatica | 3 | −20.2 | 0.5 | 7.2 | 0.3 | |||||||
| Gelidium comea | 3 | −15.4 | 0.4 | 5.7 | 0.2 | 3 | −15.0 | 0.5 | 5.9 | 0.4 | ||
| Gelidium elegans | ||||||||||||
| Grateloupia elliptica | 3 | −21.2 | 0.3 | 5.8 | 0.5 | 3 | −21.8 | 0.7 | 4.6 | 0.9 | ||
| Undaria pinnatifida | 3 | −17.7 | 0.3 | 5.8 | 0.3 | |||||||
| SPOM | 4 | −21.1 | 0.5 | 4.5 | 0.3 | 4 | −20.2 | 0.5 | 7.2 | 0.3 | 0.05 | 0.911 |
| No. | Species Name | Taxon | Artificial Kelp Bed | Barren Ground Site | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | δ13C | δ15N | n | δ13C | δ15N | |||||||
| Herbivore | ||||||||||||
| 1 | Acmaea pallida | Gas | 3 | −15.7 | 0.6 | 8.4 | 0.4 | 3 | −17.4 | 0.2 | 8.1 | 0.3 |
| 2 | Aplysia japonica | Gas | 2 | −20.5 | 0.7 | 5.8 | 0.6 | 2 | −19.9 | 0.2 | 6.6 | 0.2 |
| 3 | Cantharidus jessoensis | Gas | 2 | −14.3 | 0.2 | 8.8 | 0.1 | |||||
| 4 | Chlorostoma turbinata | Gas | 3 | −17.2 | 0.3 | 7.8 | 0.4 | 3 | −17.4 | 0.3 | 8.3 | 0.2 |
| 5 | Haliotis discus hannai | Gas | 2 | −19.8 | 0.4 | 5.9 | 0.5 | |||||
| 6 | Kelletia lischkei | Gas | 3 | −17.3 | 0.2 | 8.3 | 0.3 | |||||
| 7 | Lottia tenuisculptata | Gas | 3 | −11.1 | 0.9 | 8.2 | 0.3 | |||||
| 8 | Strongylocentrotus nudus | Ech | 2 | −11.6 | 0.6 | 6.7 | 0.2 | |||||
| Suspension feeder | ||||||||||||
| 9 | Arca boucardi | Biv | 3 | −19.3 | 0.2 | 7.4 | 0.3 | 2 | −18.5 | 0.4 | 6.6 | 0.2 |
| 10 | Crassostrea nipponica | Biv | 2 | −18.2 | 0.2 | 8.1 | 0.5 | |||||
| 11 | Crepidula onyx | Gas | 3 | −19.7 | 0.2 | 6.3 | 0.3 | 3 | −19.2 | 0.6 | 6.3 | 0.2 |
| 12 | Halocynthia roretzi | Cho | 3 | −21.0 | 0.3 | 6.9 | 0.4 | 3 | −20.7 | 0.3 | 6.7 | 0.2 |
| 13 | Modiolus agripetus lredale | Biv | 2 | −20.1 | 0.3 | 6.4 | 0.2 | |||||
| 14 | Mytilisepta keenae | Biv | 3 | −19.7 | 0.3 | 5.6 | 0.3 | |||||
| 15 | Mytilus unguiculatus | Biv | 3 | −18.7 | 0.2 | 6.8 | 0.2 | |||||
| Deposit feeder | ||||||||||||
| 16 | Acanthochitona achates | Ppl | 2 | −20.0 | 0.2 | 8.2 | 0.5 | 2 | −19.7 | 0.3 | 7.9 | 0.4 |
| 17 | Ampithoe lacertosa | Cru | 3 | −15.1 | 0.3 | 5.8 | 0.6 | |||||
| 18 | Apostichopus japonicus | Ech | 3 | −21.7 | 0.3 | 6.3 | 0.1 | |||||
| 19 | Pachycheles stevensii | Cru | 3 | −18.0 | 0.5 | 9.2 | 0.5 | |||||
| 20 | Pugettia quadridens | Cru | 3 | −19.6 | 0.4 | 8.7 | 0.4 | |||||
| Omnivore | ||||||||||||
| 21 | Asterina pectinifera | Ech | 2 | −13.7 | 0.2 | 8.5 | 0.5 | |||||
| 22 | Eunice sp. | Pol | 2 | −20.0 | 0.9 | 9.5 | 0.4 | 2 | −19.5 | 0.3 | 9.8 | 0.1 |
| 23 | Halosydna brevisetosa | Pol | 2 | −18.0 | 0.4 | 9.1 | 0.3 | 2 | −17.5 | 0.3 | 9.3 | 0.2 |
| 24 | Henricia leviuscula | Ech | 2 | −14.9 | 0.2 | 7.8 | 0.4 | |||||
| 25 | Paguristes ortmanni | Cru | 2 | −16.1 | 0.3 | 9.8 | 0.7 | 2 | −16.6 | 0.2 | 10.9 | 1.0 |
| 26 | Pagurus samuelis | Cru | 2 | −17.3 | 0.5 | 9.9 | 0.5 | |||||
| 27 | Pagurus proximus | Cru | 2 | −15.6 | 0.2 | 10.2 | 0.5 | |||||
| Carnivore | ||||||||||||
| 28 | Doris odhneri | Gas | 3 | −17.1 | 0.2 | 7.4 | 0.5 | |||||
| 29 | Mitrella bicincta | Gas | 3 | −17.1 | 0.3 | 9.6 | 0.3 | 3 | −17.5 | 0.4 | 10.0 | 0.3 |
| 30 | Reishia bronni | Gas | 3 | −17.1 | 0.2 | 10.0 | 0.2 | 3 | −17.6 | 0.3 | 9.5 | 0.4 |
| 31 | Triopha modesta | Gas | 3 | −14.1 | 0.4 | 7.8 | 0.4 | |||||
| Artificial Kelp Bed | Barren Ground Site | PERMANOVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feeding Groups | δ13C | δ15N | δ13C | δ15N | |||||||||
| n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | Pseudo-F | p-Value | ||
| Herbivore | 17 | −15.8 | 3.4 | 7.6 | 1.0 | 11 | −17.4 | 1.7 | 7.7 | 1.1 | 1.83 | 0.160 | |
| Suspension feeder | 16 | −19.3 | 0.9 | 6.6 | 0.8 | 11 | −20.0 | 0.7 | 6.8 | 0.5 | 2.13 | 0.140 | |
| Deposit feeder | 8 | −17.3 | 2.0 | 7.6 | 1.6 | 8 | −20.5 | 1.0 | 7.7 | 1.2 | 4.52 | 0.020 | |
| Omnivore | 12 | −16.6 | 2.0 | 9.4 | 1.1 | 8 | −17.4 | 1.9 | 9.7 | 0.6 | 0.53 | 0.584 | |
| Carnivore | 9 | −16.4 | 1.7 | 9.1 | 1.0 | 9 | −17.1 | 0.2 | 9.0 | 1.3 | 0.64 | 0.511 | |
| Isotopic niche areas | Artificial kelp bed | Barren ground site | Percentage overlap (%) | ||||||||||
| TA | SEAc | TA | SEAc | ||||||||||
| Herbivore | 20.61 | 10.58 | 3.83 | 2.55 | 19.8 | ||||||||
| Suspension feeder | 4.19 | 2.11 | 2.27 | 1.30 | 20.5 | ||||||||
| Deposit feeder | 8.91 | 8.17 | 1.88 | 1.41 | 1.0 | ||||||||
| Omnivore | 11.88 | 6.25 | 4.43 | 3.36 | 34.5 | ||||||||
| Carnivore | 3.81 | 2.75 | 1.40 | 0.94 | 10.5 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-Y.; Kim, D.; Chun, C.-K.; Lee, Y.; Han, K.-S.; Kim, H.K.; Park, T.H.; Park, H.J. Evaluating the Trophic Structure of an Artificial Macroalgal Bed of Eisenia bicyclis Using C and N Stable Isotopes. J. Mar. Sci. Eng. 2025, 13, 1514. https://doi.org/10.3390/jmse13081514
Lee D-Y, Kim D, Chun C-K, Lee Y, Han K-S, Kim HK, Park TH, Park HJ. Evaluating the Trophic Structure of an Artificial Macroalgal Bed of Eisenia bicyclis Using C and N Stable Isotopes. Journal of Marine Science and Engineering. 2025; 13(8):1514. https://doi.org/10.3390/jmse13081514
Chicago/Turabian StyleLee, Dong-Young, Dongyoung Kim, Chan-Kil Chun, Youngkweon Lee, Kyu-Sam Han, Hyun Kyum Kim, Tae Hee Park, and Hyun Je Park. 2025. "Evaluating the Trophic Structure of an Artificial Macroalgal Bed of Eisenia bicyclis Using C and N Stable Isotopes" Journal of Marine Science and Engineering 13, no. 8: 1514. https://doi.org/10.3390/jmse13081514
APA StyleLee, D.-Y., Kim, D., Chun, C.-K., Lee, Y., Han, K.-S., Kim, H. K., Park, T. H., & Park, H. J. (2025). Evaluating the Trophic Structure of an Artificial Macroalgal Bed of Eisenia bicyclis Using C and N Stable Isotopes. Journal of Marine Science and Engineering, 13(8), 1514. https://doi.org/10.3390/jmse13081514







