Amphipathic Alpha-Helical Peptides AH1 and AH3 Facilitate Immunogenicity of Enhanced Green Fluorescence Protein in Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish, Rearing Conditions, and Animal Ethics
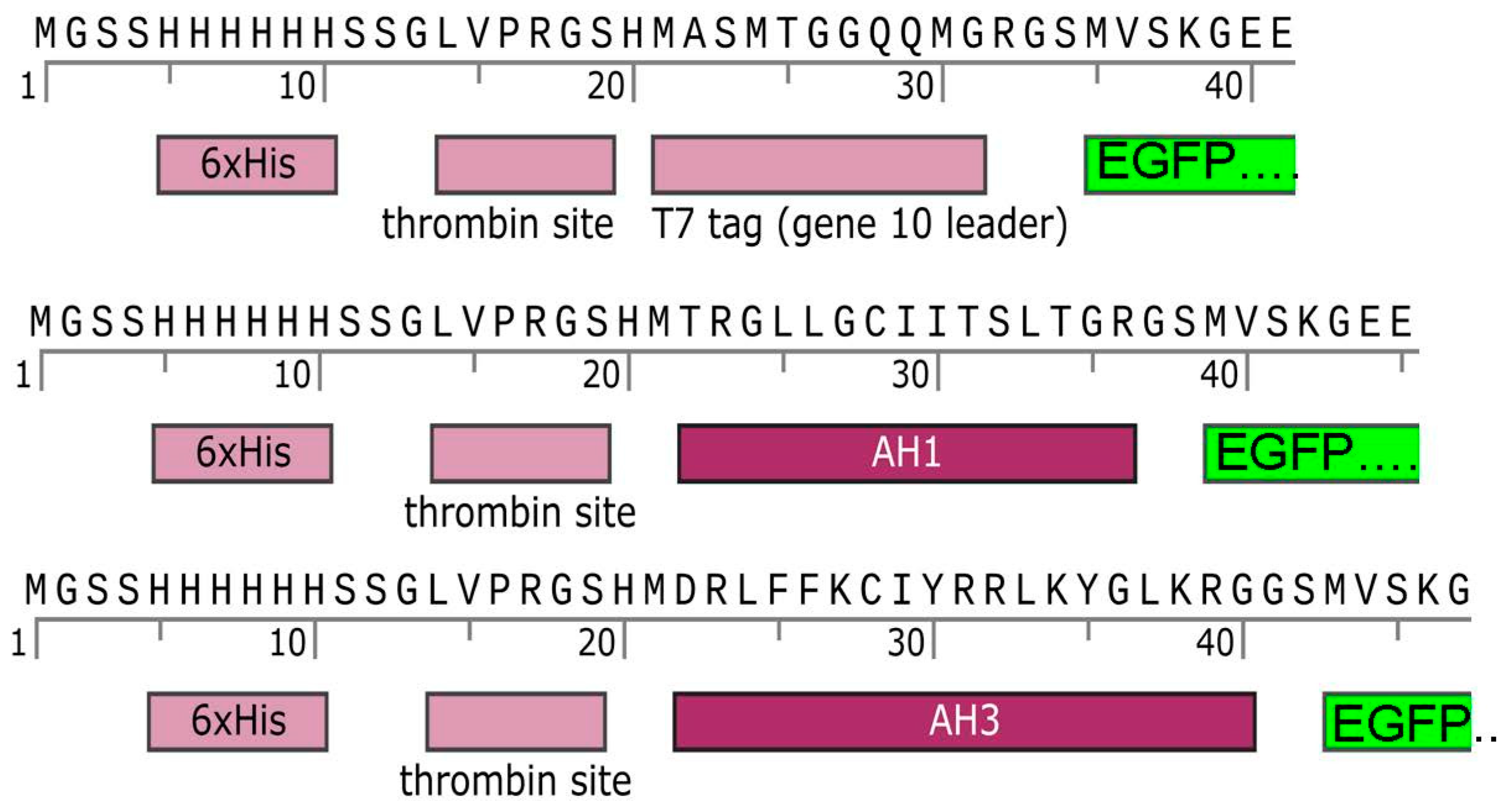
2.2. Recombinant Protein Expression and Purification
2.3. Cell Binding of EGFP, AH1-EGFP, and AH3-EGFP
2.4. Recombinant Protein Immunization, Blood Collection, and ELISA Analysis
2.5. Statistical Analysis
3. Result
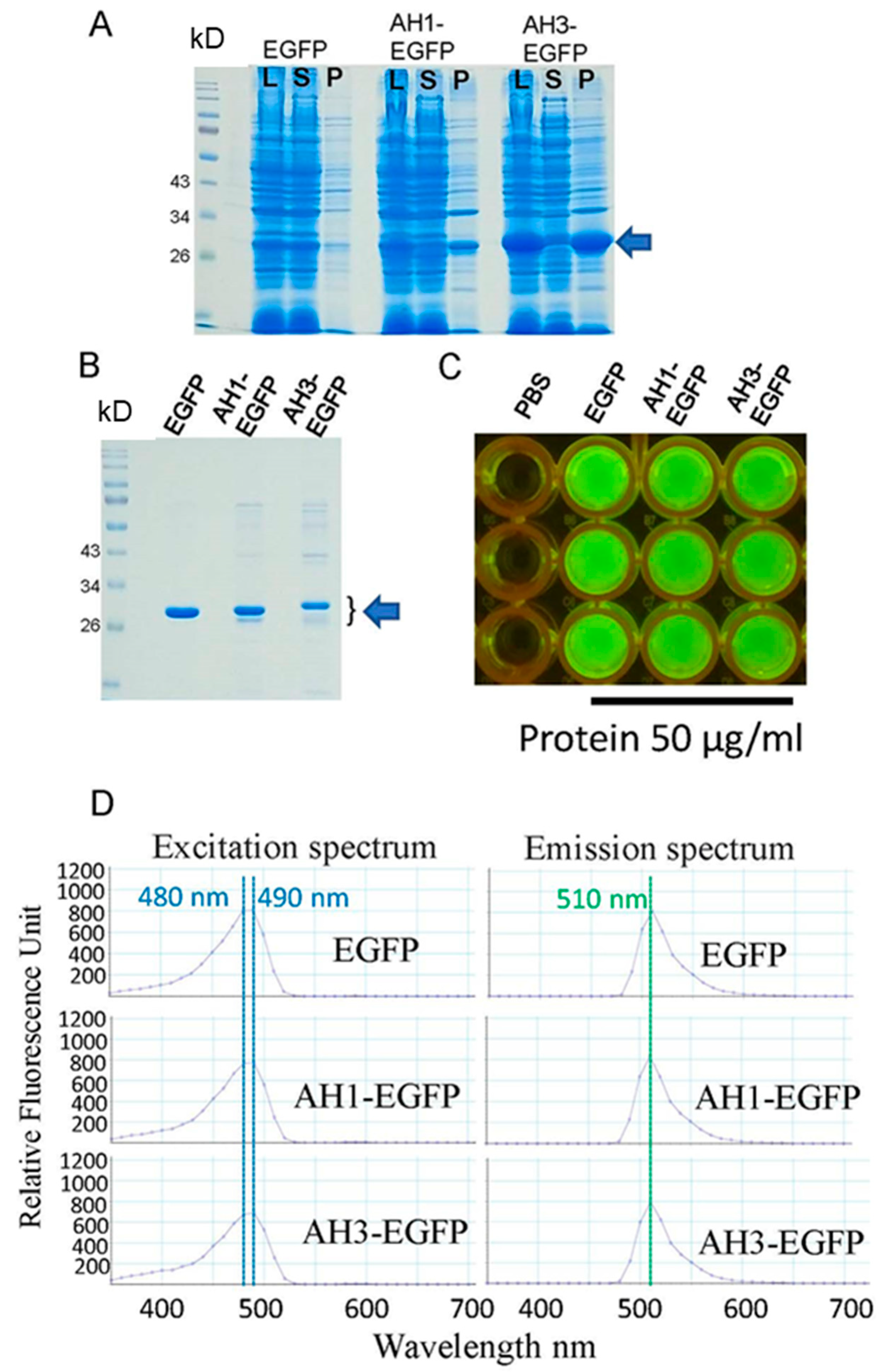
3.1. The Addition of AH1 or AH3 Peptide to the N-Terminal of EGFP Changed the Solubilities of Fusion Proteins but Did Not Affect Their Green Fluorescence
3.2. AH1 and AH3 Peptides Enhance the Binding of Their Fusion EGFPs to HEK 293 Cells
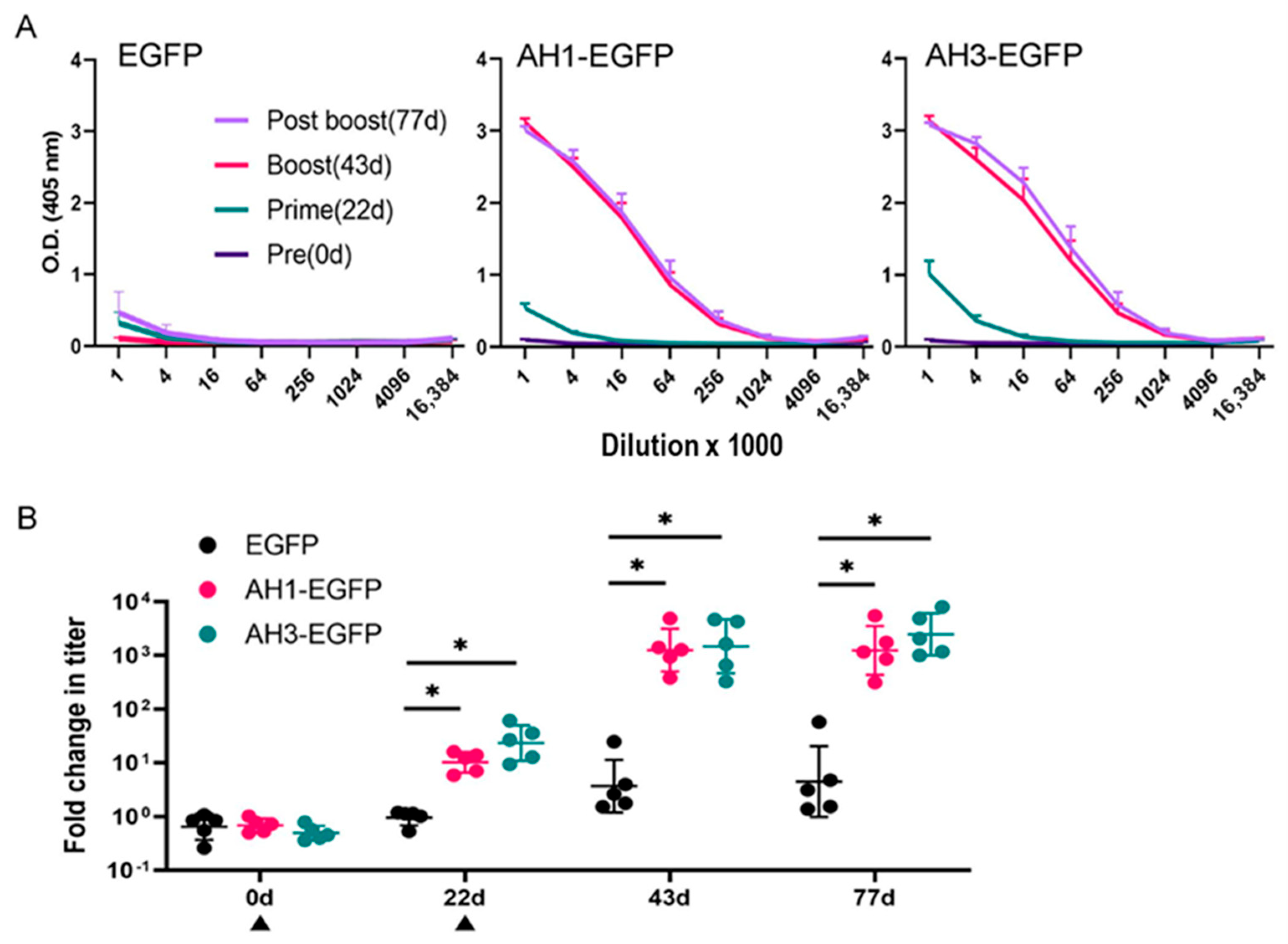
3.3. AH1 or AH3 Peptide Enhances the Immunogenicity of EGFP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-Year Retrospective Review of Global Aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Føre, M.; Frank, K.; Norton, T.; Svendsen, E.; Alfredsen, J.A.; Dempster, T.; Eguiraun, H.; Watson, W.; Stahl, A.; Sunde, L.M.; et al. Precision Fish Farming: A New Framework to Improve Production in Aquaculture. Biosyst. Eng. 2018, 173, 176–193. [Google Scholar] [CrossRef]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.-E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and Future Perspectives of Vaccines for Industrialised Finfish Farming. Fish. Shellfish. Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Horzinek, M.C.; Schijns, V.E.C.J.; Denis, M.; Desmettre, P.; Babiuk, L.A. General Description of Vaccines. In Veterinary Vaccinology; Pastoret, P.P., Blancou, J., Vannier, P., Verschueren, C., Eds.; Elsevier Press: Amsterdam, The Netherlands, 1997; pp. 132–152. [Google Scholar]
- Gudding, R.; Goodrich, T. The History of Fish Vaccination. Fish. Vaccin. 2014, 1, 1–11. [Google Scholar]
- Dhar, A.K.; Manna, S.K.; Thomas Allnutt, F.C. Viral Vaccines for Farmed Finfish. Virusdisease 2014, 25, 1–17. [Google Scholar] [CrossRef]
- Rathor, G.S.; Swain, B. Advancements in Fish Vaccination: Current Innovations and Future Horizons in Aquaculture Health Management. Appl. Sci. 2024, 14, 5672. [Google Scholar] [CrossRef]
- Du, Y.; Hu, X.; Miao, L.; Chen, J. Current status and development prospects of aquatic vaccines. Front. Immunol. 2022, 13, 1040336. [Google Scholar] [CrossRef]
- Kibenge, F.S.B.; Godoy, M.G.; Fast, M.; Workenhe, S.; Kibenge, M.J.T. Countermeasures Against Viral Diseases of Farmed Fish. Antivir. Res. 2012, 95, 257–281. [Google Scholar] [CrossRef]
- Valero, Y.; Cuesta, A. Reassortant viruses threatening fish aquaculture. Rev. Aquac. 2023, 15, 1720–1731. [Google Scholar] [CrossRef]
- Plant, K.P.; Lapatra, S.E. Advances in Fish Vaccine Delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef]
- Ebensen, T.; Link, C.; Guzmán, C.A. Classical and Novel Vaccination Strategies: A Comparison. In Novel Vaccination Strategies; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Goldman, M.; Lambert, P.H.; Kaufmann, S.H. Novel Vaccination Strategies; Blackwell Pub: New York, NY, USA, 2004. [Google Scholar]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. An Overview of Challenges Limiting the Design of Protective Mucosal Vaccines for Finfish. Front. Immunol. 2015, 6, 542. [Google Scholar] [CrossRef]
- Loessner, H.; Schwantes, A.; Hamdorf, M.; Komor, U.; Leschner, S.; Weiss, S. Employing Live Microbes for Vaccine Delivery. In Development of Novel Vaccines: Skills, Knowledge and Translational Technologies; Springer: Berlin/Heidelberg, Germany, 2012; pp. 87–124. [Google Scholar] [CrossRef]
- Clark, T.G.; Cassidy-Hanley, D. Recombinant Subunit Vaccines: Potentials and Constraints. Dev. Biol. 2005, 121, 153–163. [Google Scholar]
- Pardoll, D.M. Spinning Molecular Immunology into Successful Immunotherapy. Nat. Rev. Immunol. 2002, 2, 227–238. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Tammas, I.; Bitchava, K.; Gelasakis, A.I. Advances in Vaccine Adjuvants for Teleost Fish: Implications for Aquatic Welfare and the Potential of Nanoparticle-Based Formulations. Vaccines 2024, 12, 1347. [Google Scholar] [CrossRef] [PubMed]
- Schödel, F.; Peterson, D.; Hughes, J.; Wirtz, R.; Milich, D. Hybrid Hepatitis B Virus Core Antigen as a Vaccine Carrier Moiety: I. Presentation of Foreign Epitopes. J. Biotechnol. 1996, 44, 91–96. [Google Scholar] [CrossRef]
- Chenal, A.; Ladant, D. Bioengineering of Bordetella Pertussis Adenylate Cyclase Toxin for Antigen-Delivery and Immunotherapy. Toxins 2018, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Andrés, M.; Čopič, A.; Antonny, B. The Many Faces of Amphipathic Helices. Biomolecules 2018, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-T.; Liou, G.-G.; Kan, M.-C. A Thermal-Stable Protein Nanoparticle that Stimulates Long Lasting Humoral Immune Response. Vaccines 2023, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.-H.; Liou, G.-G.; Wang, M.; Kan, M.-C.M. The Adjuvant Effects of Amphipathic Helical Peptides. bioRxiv 2023. [Google Scholar] [CrossRef]
- He, Y.; Weng, L.; Li, R.; Li, L.; Toyoda, T.; Zhong, J. The N-Terminal Helix α(0) of Hepatitis C Virus NS3 Protein Dictates the Subcellular Localization and Stability of NS3/NS4A Complex. Virology 2012, 422, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Horner, S.M.; Park, H.S.; Gale, M., Jr. Control of Innate Immune Signaling and Membrane Targeting by the Hepatitis C Virus NS3/4A Protease Are Governed by the NS3 Helix A0. J. Virol. 2012, 86, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Rossman, J.S.; Jing, X.; Leser, G.P.; Lamb, R.A. Influenza Virus M2 Protein Mediates ESCRT-Independent Membrane Scission. Cell 2010, 142, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.L.; Leser, G.P.; Ma, C.; Lamb, R.A. The Amphipathic Helix of Influenza A Virus M2 Protein Is Required for Filamentous Bud Formation and Scission of Filamentous and Spherical Particles. J. Virol. 2013, 87, 9973–9982. [Google Scholar] [CrossRef]
- Waldo, G.S.; Standish, B.M.; Berendzen, J.; Terwilliger, T.C. Rapid Protein-Folding Assay Using Green Fluorescent Protein. Nat. Biotechnol. 1999, 17, 691–695. [Google Scholar] [CrossRef]
- Waldo, G.S. Genetic Screens and Directed Evolution for Protein Solubility. Curr. Opin. Chem. Biol. 2003, 7, 33–38. [Google Scholar] [CrossRef]
- Pédelacq, J.-D.; Piltch, E.; Liong, E.C.; Berendzen, J.; Kim, C.-Y.; Rho, B.-S.; Park, M.S.; Terwilliger, T.C.; Waldo, G.S. Engineering Soluble Proteins for Structural Genomics. Nat. Biotechnol. 2002, 20, 927–932. [Google Scholar] [CrossRef]
- Pédelacq, J.-D.; Cabantous, S.; Tran, T.; Terwilliger, T.C.; Waldo, G.S. Engineering and Characterization of a Superfolder Green Fluorescent Protein. Nat. Biotechnol. 2006, 24, 79–88. [Google Scholar] [CrossRef]
- Vlassov, V.V.; Balakireva, L.A.; Yakubov, L.A. Transport of Oligonucleotides Across Natural and Model Membranes. Biochim. Biophys. Acta 1994, 1197, 95–108. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The Third Helix of the Antennapedia Homeodomain Translocates Through Biological Membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Fawell, S.; Seery, J.; Daikh, Y.; Moore, C.; Chen, L.L.; Pepinsky, B.; Barsoum, J. Tat-Mediated Delivery of Heterologous Proteins into Cells. Proc. Natl. Acad. Sci. USA 1994, 91, 664–668. [Google Scholar] [CrossRef]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, α-Helical Antimicrobial Peptides. Pept. Sci. 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial peptides’ immune modulation role in intracellular bacterial infection. Front. Immunol. 2023, 14, 1119574. [Google Scholar] [CrossRef]
- Pavli, P.; Hume, D.A.; Van De Pol, E.; Doe, W.F. Dendritic Cells, the Major Antigen-Presenting Cells of the Human Colonic Lamina Propria. Immunology 1993, 78, 132–141. [Google Scholar]
- Unanue, E.R. Antigen-Presenting Function of the Macrophage. Annu. Rev. Immunol. 1984, 2, 395–428. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, K.C.; Wong, T.-T. Amphipathic Alpha-Helical Peptides AH1 and AH3 Facilitate Immunogenicity of Enhanced Green Fluorescence Protein in Rainbow Trout (Oncorhynchus mykiss). J. Mar. Sci. Eng. 2025, 13, 1497. https://doi.org/10.3390/jmse13081497
Peng KC, Wong T-T. Amphipathic Alpha-Helical Peptides AH1 and AH3 Facilitate Immunogenicity of Enhanced Green Fluorescence Protein in Rainbow Trout (Oncorhynchus mykiss). Journal of Marine Science and Engineering. 2025; 13(8):1497. https://doi.org/10.3390/jmse13081497
Chicago/Turabian StylePeng, Kuan Chieh, and Ten-Tsao Wong. 2025. "Amphipathic Alpha-Helical Peptides AH1 and AH3 Facilitate Immunogenicity of Enhanced Green Fluorescence Protein in Rainbow Trout (Oncorhynchus mykiss)" Journal of Marine Science and Engineering 13, no. 8: 1497. https://doi.org/10.3390/jmse13081497
APA StylePeng, K. C., & Wong, T.-T. (2025). Amphipathic Alpha-Helical Peptides AH1 and AH3 Facilitate Immunogenicity of Enhanced Green Fluorescence Protein in Rainbow Trout (Oncorhynchus mykiss). Journal of Marine Science and Engineering, 13(8), 1497. https://doi.org/10.3390/jmse13081497






