Intra-Seasonal Acoustic Variation in Humpback Whale Songs in the North Colombian Pacific
Abstract
1. Introduction
2. Methods
2.1. Study Area
2.2. Boat Surveys and Acoustic Recordings
2.3. Repertoire Analysis
2.4. Acoustic Analysis
- Peak Frequency (Hz) and its contour (PFC), which include maximum and minimum frequencies as well as maximum, minimum, and average slopes, giving insights into the dominant frequencies and their temporal evolution within each unit.
- Delta Frequency, Center Frequency, and Delta Time, which capture variations in the acoustic structure of the units.
- Bandwidth 90% (Hz) and Duration 90% (s), focusing on the core energy of the signal to ensure consistency across recordings.
2.5. Statistical Analysis
3. Results
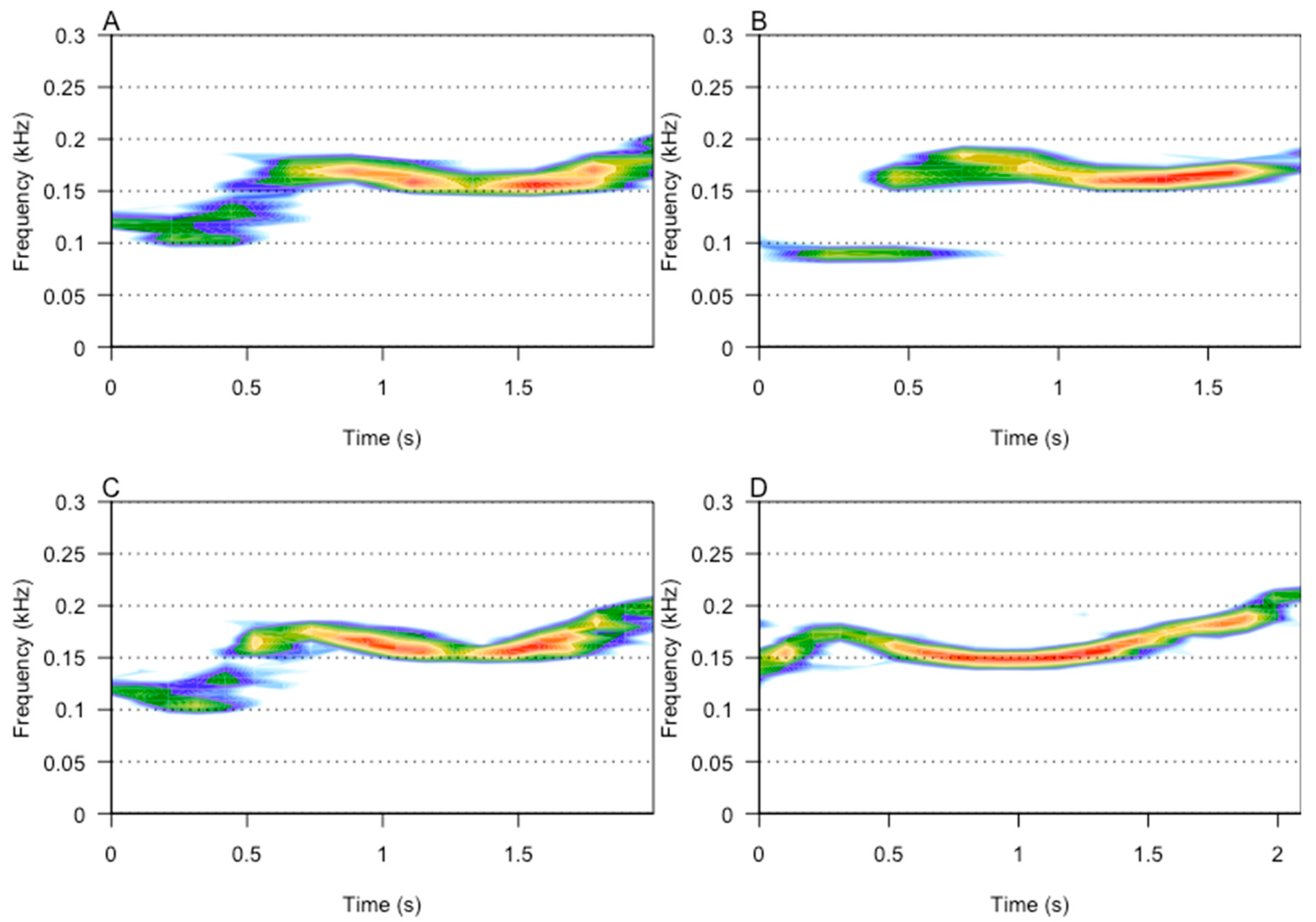
3.1. Repertoire Analysis
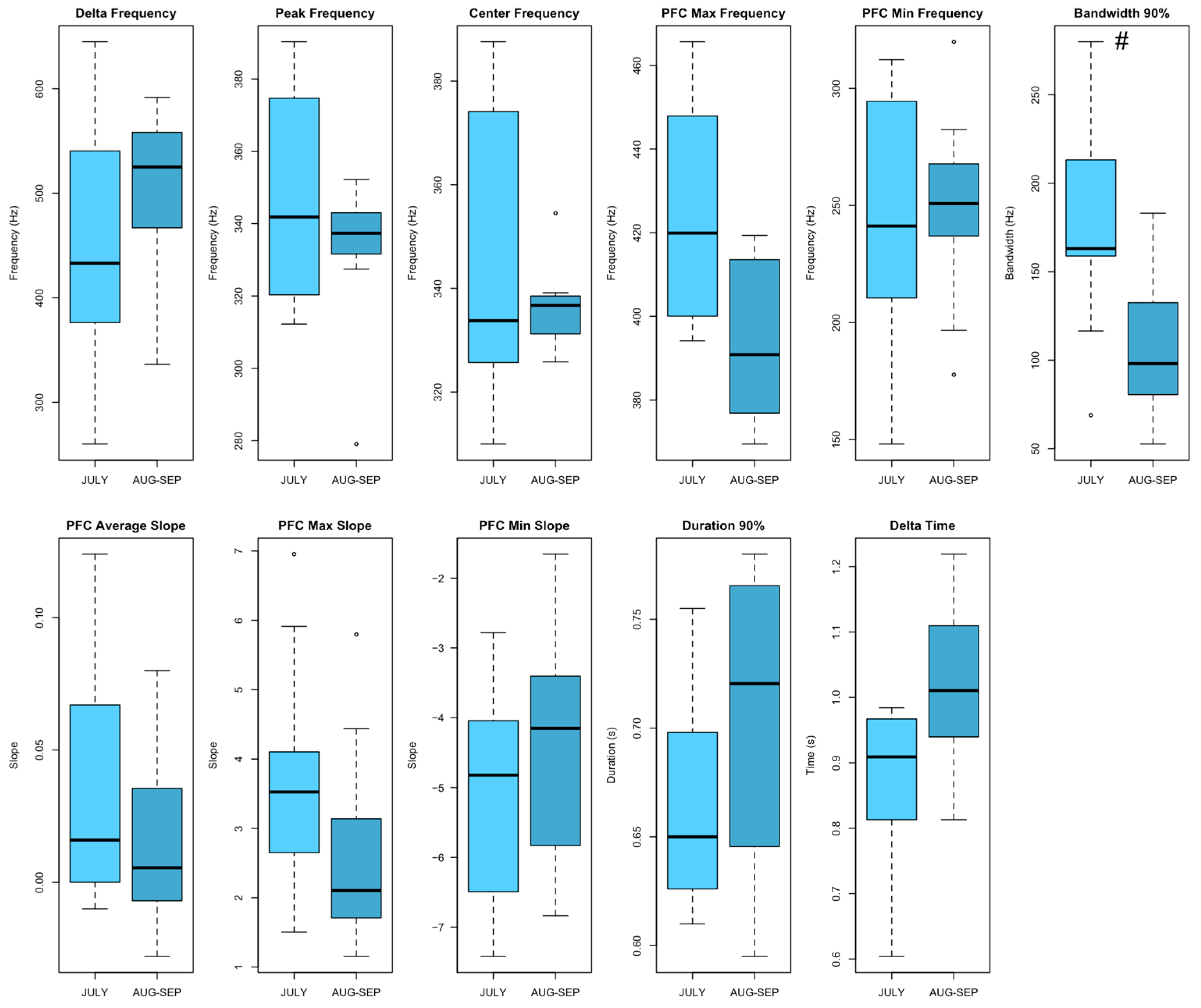
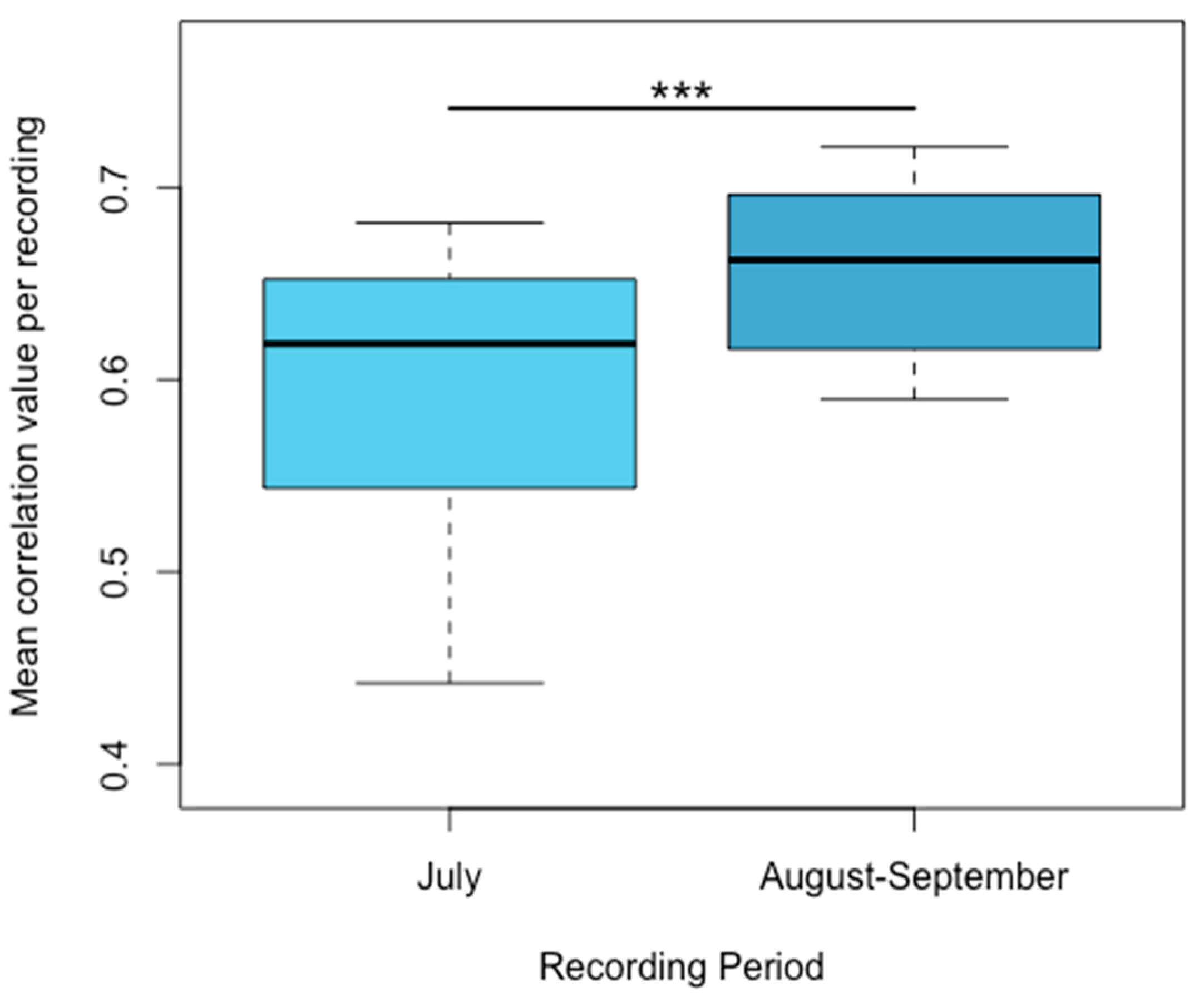
3.2. Acoustic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradbury, J.W.; Vehrencamp, S.L. Principles of Animal Communication, 2nd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2011; p. 697. [Google Scholar]
- Tyack, P.L.; Clark, C.W. Communication and acoustic behavior of dolphins and whales. In Hearing by Whales and Dolphins; Springer: Berlin/Heidelberg, Germany, 2000; pp. 156–224. [Google Scholar]
- Au, W.W.; Pack, A.A.; Lammers, M.O.; Herman, L.M.; Deakos, M.H.; Andrews, K. Acoustic properties of humpback whale songs. J. Acoust. Soc. Am. 2006, 120, 1103–1110. [Google Scholar] [CrossRef]
- Payne, R.S.; McVay, S. Songs of Humpback Whales: Humpbacks emit sounds in long, predictable patterns ranging over frequencies audible to humans. Science 1971, 173, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Payne, K. Progressive changes in the songs of humpback whales Megaptera novaeangliae: A detailed analysis of two seasons in Hawaii. Commun. Behav. Whales 1983, 9–57. [Google Scholar]
- Payne, K.; Payne, R. Large scale changes over 19 years in songs of humpback whales in Bermuda. Z. Tierpsychol. 1985, 68, 89–114. [Google Scholar] [CrossRef]
- Guinee, L.N.; Payne, K.B. Rhyme-like repetitions in songs of humpback whales. Ethology 1988, 79, 295–306. [Google Scholar] [CrossRef]
- Winn, H.; Winn, L. The song of the humpback whale Megaptera novaeangliae in the West Indies. Mar. Biol. 1978, 47, 97–114. [Google Scholar] [CrossRef]
- Cerchio, S.; Jacobsen, J.K.; Norris, T.F. Temporal and geographical variation in songs of humpback whales, Megaptera novaeangliae: Synchronous change in Hawaiian and Mexican breeding assemblages. Anim. Behav. 2001, 62, 313–329. [Google Scholar] [CrossRef]
- Smith, G.T.; Brenowitz, E.A.; Beecher, M.D.; Wingfield, J.C. Seasonal Changes in Testosterone, Neural Attributes of Song Control Nuclei, and Song Structure in Wild Songbirds. J. Neurosci. 1997, 17, 6001–6010. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.D.; Amiot, C.; Ludbrook, M.R.; Ji, W. Seasonal variation in the song structure of tui (Prosthemadera novaeseelandiae). N. Z. J. Ecol. 2015, 39, 110–115. [Google Scholar]
- Van Duyse, E.; Pinxten, R.; Eens, M. Seasonal fluctuations in plasma testosterone levels and diurnal song activity in free-living male great tits. Gen. Comp. Endocrinol. 2003, 134, 1–9. [Google Scholar] [CrossRef]
- Nottebohm, F. Plasticity in Adult Avian Central Nervous System: Possible Relation Between Hormones, Learning, and Brain Repair. Compr. Physiol. 1987, 85–108. [Google Scholar] [CrossRef]
- Gahr, M. Seasonal hormone fluctuations and song structure of birds. In Coding Strategies in Vertebrate Acoustic Communication; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 163–201. [Google Scholar]
- Alward, B.A.; Cornil, C.A.; Balthazart, J.; Ball, G.F. The regulation of birdsong by testosterone: Multiple time-scales and multiple sites of action. Horm. Behav. 2018, 104, 32–40. [Google Scholar] [CrossRef]
- Caballero, S.; Hamilton, H.; Jaramillo, C.; Capella, J.; Flórez-González, L.; Olavarria, C.; Rosenbaum, H.; Guhl, F.; Baker, C.S. Genetic characterisation of the Colombian Pacific Coast humpback whale population using RAPD and mitochondrial DNA sequences. Mem.-Qld. Mus. 2001, 47, 459–464. [Google Scholar]
- Mercado, E.; Herman, L.M.; Pack, A.A. Song copying by humpback whales: Themes and variations. Anim. Cogn. 2005, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mercado, E. Song morphing by humpback whales: Cultural or epiphenomenal? Front. Psychol. 2021, 11, 574403. [Google Scholar] [CrossRef] [PubMed]
- Perazio, C.E.; Mercado, E. Singing humpback whales Megaptera novaeangliae favor specific frequency bands. In Proceedings of the Meetings on Acoustics, Minneapolis, MN, USA, 7–11 May 2018. [Google Scholar]
- Perazio, C.E.; Zapetis, M.E.; Roberson, D.; Botero, N.; Kuczaj, S. Humpback whale, Megaptera novaeangliae, song during the breeding season in the Gulf of Tribugá, Colombian Pacific. Madag. Conserv. Dev. 2018, 13, 83–90. [Google Scholar] [CrossRef]
- Perea-Ardila, M.A.; Oviedo-Barrero, F. Batimetría derivada por satélite (sdb): Una aproximación a la cartografía batimétrica con imágenes multiespectrales en aguas poco profundas de Bahía Solano, Colombia. Rev. Cienc. Mar. Costeras 2020, 12, 117–134. [Google Scholar] [CrossRef]
- Rey-Baquero, M.P.; Huertas-Amaya, L.V.; Seger, K.D.; Botero-Acosta, N.; Luna-Acosta, A.; Perazio, C.E.; Boyle, J.K.; Rosenthal, S.; Vallejo, A.C. Understanding effects of whale-watching vessel noise on humpback whale song in the North Pacific coast of Colombia with propagation models of masking and acoustic data observations. Front. Mar. Sci. 2021, 8, 623724. [Google Scholar] [CrossRef]
- Avila, I.C.; Dormann, C.F.; García, C.; Payán, L.F.; Zorrilla, M.X. Humpback whales extend their stay in a breeding ground in the Tropical Eastern Pacific. ICES J. Mar. Sci. 2019, 77, 109–118. [Google Scholar] [CrossRef]
- Millien, V.; Stafiej, N.; Félix, F.; Guzman, H.M. Migratory behaviour of humpback whales in the southeastern Pacific under climate change. Sci. Rep. 2025, 15, 3989. [Google Scholar] [CrossRef]
- K. Lisa Yang Center for Conservation Bioacoustics at the Cornell Lab of Ornithology. Raven Pro: Interactive Sound Analysis Software, Version 1.6.5. [Computer software]. The Cornell Lab of Ornithology: Ithaca, NY, USA, 2024. Available online: https://www.ravensoundsoftware.com/ (accessed on 13 April 2025).
- Clark, C.W.; Marler, P.; Beeman, K. Quantitative-Analysis of Animal Vocal Phonology—An Application to Swamp Sparrow Song. Ethology 1987, 76, 101–115. [Google Scholar] [CrossRef]
- Dalisio, A.C.; Jensen, W.E.; Parker, T.H. Divergence of vocal culture among isolated alpine habitats is inconsistent among three oscine species. J. Ornithol. 2015, 156, 165–178. [Google Scholar] [CrossRef]
- Cholewiak, D.M.; Sousa-Lima, R.S.; Cerchio, S. Humpback whale song hierarchical structure: Historical context and discussion of current classification issues. Mar. Mammal Sci. 2013, 29, E312–E332. [Google Scholar] [CrossRef]
- Kershenbaum, A.; Blumstein, D.T.; Roch, M.A.; Akçay, Ç.; Backus, G.; Bee, M.A.; Bohn, K.; Cao, Y.; Carter, G.; Cäsar, C.; et al. Acoustic sequences in non-human animals: A tutorial review and prospectus. Biol. Rev. 2016, 91, 13–52. [Google Scholar] [CrossRef]
- Sueur, J.; Aubin, T.; Simonis, C. Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics 2008, 18, 213–226. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Noble, W.S. How does multiple testing correction work? Nat. Biotechnol. 2009, 27, 1135–1137. [Google Scholar] [CrossRef]
- Garland, E.C.; Goldizen, A.W.; Rekdahl, M.L.; Constantine, R.; Garrigue, C.; Hauser, N.D.; Poole, M.M.; Robbins, J.; Noad, M.J. Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Curr. Biol. 2011, 21, 687–691. [Google Scholar] [CrossRef]
- Noad, M.J.; Cato, D.H.; Bryden, M.M.; Jenner, M.-N.; Jenner, K.C.S. Cultural revolution in whale songs. Nature 2000, 408, 537. [Google Scholar] [CrossRef] [PubMed]
- Van Duyse, E.; Pinxten, R.; Eens, M. Effects of testosterone on song, aggression, and nestling feeding behavior in male great tits, Parus major. Horm. Behav. 2002, 41, 178–186. [Google Scholar] [CrossRef]
- Kempenaers, B.; Peters, A.; Foerster, K. Sources of individual variation in plasma testosterone levels. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1711–1723. [Google Scholar] [CrossRef]
- Hau, M. Regulation of male traits by testosterone: Implications for the evolution of vertebrate life histories. BioEssays 2007, 29, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, J.C.; Ramenofsky, M. Testosterone and Aggressive Behaviour during the Reproductive Cycle of Male Birds; Springer: Berlin/Heidelberg, Germany, 1985; pp. 92–104. [Google Scholar]
- Cates, K.A.; Atkinson, S.; Gabriele, C.M.; Pack, A.A.; Straley, J.M.; Yin, S. Testosterone trends within and across seasons in male humpback whales (Megaptera novaeangliae) from Hawaii and Alaska. Gen. Comp. Endocrinol. 2019, 279, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, C.K.; Slater, P.J.B. Bird Song Biological Themes and Variations, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Rivera-Gutierrez, H.F.; Pinxten, R.; Eens, M. Songs differing in consistency elicit differential aggressive response in territorial birds. Biol. Lett. 2011, 7, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Mercado, E.; Perazio, C.E. Similarities in composition and transformations of songs by humpback whales (Megaptera novaeangliae) over time and space. J. Comp. Psychol. 2021, 135, 28–50. [Google Scholar] [CrossRef]

| Category | Unit Types Count |
|---|---|
| First recording period (unique) | 474 |
| Second recording period (unique) | 271 |
| Shared units | 74 |
| Total | 819 |
| Jaccard Index | 0.0904 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Marulanda, J.; Rivera-Gutierrez, H.F. Intra-Seasonal Acoustic Variation in Humpback Whale Songs in the North Colombian Pacific. J. Mar. Sci. Eng. 2025, 13, 1360. https://doi.org/10.3390/jmse13071360
López-Marulanda J, Rivera-Gutierrez HF. Intra-Seasonal Acoustic Variation in Humpback Whale Songs in the North Colombian Pacific. Journal of Marine Science and Engineering. 2025; 13(7):1360. https://doi.org/10.3390/jmse13071360
Chicago/Turabian StyleLópez-Marulanda, Juliana, and Hector Fabio Rivera-Gutierrez. 2025. "Intra-Seasonal Acoustic Variation in Humpback Whale Songs in the North Colombian Pacific" Journal of Marine Science and Engineering 13, no. 7: 1360. https://doi.org/10.3390/jmse13071360
APA StyleLópez-Marulanda, J., & Rivera-Gutierrez, H. F. (2025). Intra-Seasonal Acoustic Variation in Humpback Whale Songs in the North Colombian Pacific. Journal of Marine Science and Engineering, 13(7), 1360. https://doi.org/10.3390/jmse13071360








