Abstract
The study of the impact of anthropogenic and natural pollution on living organisms has become a major social issue. In this context, the objective of this work is to assess the use of the polychaete annelid Hediste diversicolor as a bioindicator organism for the quality of the marine environment. The concentration of four heavy metals (lead, copper, zinc, and cadmium) was determined in natural populations of H. diversicolor captured from four locations along the Tunisian coast using atomic absorption spectroscopy. Concentration ranges (µg/g dry weight) across all sites were as follows: Cd (0.12–0.43), Cu (3.80–6.45), Zn (18.35–42.78), and Pb (22.64–63.91). Statistical analysis confirmed significant spatial variation (Pb: F = 12.15, p < 0.001; Zn: F = 3.32, p = 0.04; Cd: F = 48.66, p < 0.001; Cu: F = 9.08, p < 0.001), with peak Pb in Bizerte and Cu in Sfax. These results highlight the influence of local environmental factors, such as industrial and urban pollution on metal accumulation in Hediste diversicolor. In this study, the accumulation of the analyzed elements in the tissues of H. diversicolor follows an increasing order as follows: Cd < Cu < Zn < Pb. Additionally, lead metal concentrations were higher than those of cadmium, zinc, and copper for all four studied locations. To our knowledge, this is the first study in Tunisia to assess heavy metal accumulation in H. diversicolor. The recorded levels were similar to, or lower than, those reported in other studies worldwide. These findings underscore the potential of H. diversicolor as a sensitive and effective bioindicator for monitoring coastal contamination and guiding environmental management strategies in Tunisia.
1. Introduction
The planet’s ecosystems are currently being significantly impacted by human development and the resulting pollution [1]. This environmental threat also extends to aquatic ecosystems in Tunisia, from the Mediterranean Sea to lakes, rivers, and inland wetlands. These diverse environments are home to a rich biodiversity, providing essential ecosystem services such as biodiversity, fishing and aquaculture [2,3,4,5,6,7]. However, the sustainability of these ecosystems is threatened by a variety of factors, including pollution, overfishing, global change. and habitat destruction [2,8].
In this context, we focus on metal pollution, which arises when trace-metal concentrations (e.g., Zn, Cu, Pb, Hg, Cd, Cr) deviate from their natural background levels. These micropollutants persist in water, sediments, and groundwater and exhibit high bioavailability, enabling uptake by aquatic organisms. Through bioaccumulation, metals are absorbed faster than they are eliminated, and via trophic transfer, they undergo biomagnification, leading to progressively higher tissue burdens at successive food-web levels. Consequently, metal concentrations in the tissues of marine organisms can exceed ambient environmental levels by several orders of magnitude, posing significant risks to biodiversity and ecosystem health [9]. In general, the two main sources of environmental contamination by trace elements are natural soil erosion processes linked to the nature of the parent rock, which directly influences the metallic composition of the soil [10], and human activity (air pollution, use of pesticides, fertilizers, urban and industrial waste), also contributing to the enrichment of ecosystems in heavy metals. In specific contexts and under certain conditions, the presence of metals in toxic concentrations can cause significant ecological damage [11,12]. Lead (Pb) and cadmium (Cd) are non-biodegradable and toxic, even at low concentration levels. It should be noted that even zinc (Zn), although essential to the proper functioning of the ecosystem, can become toxic at high doses. This form of pollution is of major concern due to its devastating effects on aquatic ecosystems, posing a threat to marine life and biodiversity. In certain environments, the introduction of chemicals into the marine environment can lead to the extinction of certain animal and/or plant species, causing the trophic chain to malfunction. The harmful effects of metal pollution are not limited exclusively to aquatic flora and fauna but also affect human beings. Contaminated water poses a direct threat to human health and can cause serious illness. A case in point was the situation in Japan in the 1950s–1960s, where cadmium contamination triggered multiple kidney and bone disorders, leading to significant mortality among populations in the affected areas [13].
In the northwestern coastal region of Tunisia, research on heavy metal contamination and bioaccumulation in marine ecosystems remains limited, despite the significant ecological importance and economic value of these coastal habitats. This study aims to assess the level of contamination of this ecosystem by measuring concentrations of metals (Cd, Pb, Cu, and Zn) in the tissues of the marine polychaete worm H. diversicolor. This species was chosen due to its widespread presence, ecological importance, and recognized role as a bioindicator of metal pollution. Although these aquatic organisms are recognized as excellent bioindicators due to their ability to accumulate metals, as well as their sessile and euryhaline lifestyle [14], marine worms are of great commercial importance and have been proposed as a very interesting novel feed ingredient for aquafeeds. The choice of inanimate (sediment and water) and living (polychaetes) substrates is justified by their ability to bind and accumulate various mineral and organic toxins [15]. This marine polychaete worm, belonging to the Nereididae family, is highly tolerant of extreme variations in temperature, salinity, and oxygen levels in its environment. It adapts to various types of sediment, from muddy to sandy, and survives in harsh and relatively polluted environments. As well as being an important food source for other animals, this Hedistus, often called Nereis in ancient literature, plays a crucial role in the mixing, aeration, and cycling of carbon and nitrogen in sandy and muddy sediment layers, through the phenomenon of bioturbation, similar to that of earthworms in soils.
In this context, this study focuses on a specific form of metal pollution, namely the bioaccumulation of heavy metals, by highlighting the marine worm species H. diversicolor. This benthic polychaete plays an essential role in aquatic ecosystems and is recognized as a significant indicator of the ecological and environmental consequences of metal contamination. Over time, the gradual accumulation of metals is becoming an increasing threat both to this species and to the entire aquatic food chain.
Various scientific studies have highlighted the risks associated with the accumulation of heavy metals in aquatic ecosystems, underscoring the imperative of in-depth analysis to understand bioaccumulation mechanisms and assess potential risks to biodiversity and human health. The variety of chemical compounds present in polluted marine ecosystems intensifies the phenomenon of bioaccumulation in the tissues and organs of marine fauna. Thus, polychaete marine worms become agents of this contamination, while providing indications of the degree of pollution of their natural environment. In this study, we examined the bioaccumulation of four trace metals (cadmium, lead, copper, and zinc) in worms (H. diversicolor) at four sites in different Tunisian aquatic ecosystems (Bizerte Lagoon, Sfax beach, El HichaGabes, and Djerba Island).
Accordingly, this study engages in an in-depth exploration of heavy metal bioaccumulation in H. diversicolor within various aquatic ecosystems in Tunisia. It aims to quantify metal concentrations, assess spatial variation, and compare the results with values reported in the literature, in order to provide essential baseline data for sustainable ecosystem management and to confirm the usefulness of H. diversicolor as a reliable bioindicator species for monitoring metal contamination in coastal environments.
2. Materials and Methods
2.1. Studied Sites
A studied sample was carried out in 4 different areas: Bizerte, Sfax, Gabes, and Djerba as shown in Figure 1.
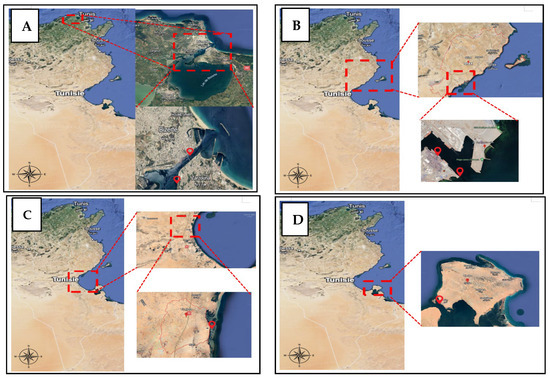
Figure 1.
Studied sites (A) Bizerte; (B) Sfax; (C) Gulf of Gabes; (D) Djerba.
2.1.1. Bizerte Lagoon
The Bizerte Lagoon, located in northern Tunisia (Figure 1), covers an area of approximately 128 km2 and holds significant ecological and geostrategic importance. It connects to the Mediterranean Sea via a navigable channel (1500 m long, 300 m wide, and 12 m deep) and to Lake Ichkeul through OuedTinja, a narrow channel approximately 5 km in length [6,7,8,9,10,11,12,13,14,15,16,17,18]. Historically, the lagoon benefited from a natural balance between freshwater inputs and seawater exchange, supporting diverse biological communities. However, in recent decades, rapid urbanization and the establishment of major industrial facilities along the shoreline have altered its ecological equilibrium. The construction of dams upstream, particularly in the catchment areas of Lake Ichkeulhas, significantly modified freshwater inflows and increased salinity levels within the lagoon [19]. The region’s Mediterranean climate is characterized by hot, dry summers and mild, rainy winters, with dominant northwesterly winds that further influence local hydrodynamics [20].
2.1.2. Sfax Coast
Sfax, located on the southeastern coast of Tunisia, is one of the country’s most industrialized urban centers. Since the 1950s, rapid industrial growth, urban expansion, and population increase have contributed to significant discharges of both industrial and domestic wastewater. These inputs have resulted in heavy metal contamination, particularly affecting marine sediments along the coastline [21,22,23]. The sampling sites in this study are situated between latitudes 34°43′ and 34°46′ N and longitudes 10°46′ to 10°49′ E.
2.1.3. Gulf of Gabes
The study area is situated in southern Tunisia, approximately 35 km north of Gabes and just a few kilometers inland from the Mediterranean Sea. Spanning roughly 300 km2, it is bordered by the Gulf of Gabes to the east, El Hamma to the west, and Skhira to the north [24,25,26]. Although it is recognized for its high marine productivity and plays a central role as Tunisia’s main fishing ground also ranking among the most important in the Mediterranean, the Gulf of Gabes has suffered continuous ecological degradation over the past decades. This deterioration is largely due to rapid, unregulated industrial development and the discharge of both industrial and domestic waste into the marine environment.
2.1.4. Djerba Island
Located in south-eastern Tunisia, the island of Djerba is geographically close to the mainland, with two outposts on either side: Jorf and Ajim to the west, Zarzis and El Kantara to the east (Figure 1). Ajim is separated from Jorf by a 2 km strait, crossed by shuttles, while on the Zarzisside, a 7.5 km bridge links the island to the mainland. Although Jerba is increasingly perceived as a peninsula thanks to these land links, the majority of tourists reach it by air.
2.2. Sampling
The samples (N = 15 for each site) were taken at low tide, in the intertidal zone, more precisely in the lower mediolittoral of the Gulf. Individuals of H. diversicolor were carefully extracted from their sandy tubes and placed in clean 0.5 L plastic bottles of mineral water, thus creating an environment conducive to their preservation, protecting them from external factors. After a period of 15 to 30 min, the samples were stored in the refrigerator at a temperature of 6 °C to inhibit crystallization within the annelid tissues and promote elimination of their digestive tract contents. Before being used for analysis, samples were left to thaw at room temperature, preparing them for subsequent analysis.
2.3. Chemical Analysis
As a first step, tissue samples were dried in a G-Therm115 oven at 65 °C for 24 h to obtain a constant dry weight. After dehydration, the dry weight of each sample was recorded, and the samples were then prepared for mineralization. The concentrations of Cd, Zn, Pb, and Cu were determined following the procedure described by [27]. During the mineralization step, organic matter was removed using an acid digestion method. Dried samples (10 mg) were taken and digested with concentrated nitric acid (3 mL) at 120 °C. After dilution to 30 mL with ultrapure water, the digested solution was stored at 4 °C until analysis by flame atomic absorption spectrometry (Avanta GBC spectrometer, A6600 model, Melbourne, Australia). A hollow cathode lamp was utilized as a light source for Zn, Cd, Pb, and Cu at wavelengths of 213.9, 228.8, 283.3, and 248.3 nm, respectively, for the determination of each respective metal.
The equipment underwent calibration using NIST-traceable atomic absorption standards for metals to establish a calibration curve. Linear calibration curves were established with linear regression values exceeding R2 > 0.989 ± 0.10. The limit of detection (LOD) for each metal was determined as follows: Cu 0.0045 mg/L, Zn 0.0033 mg/L, Cd 0.0020 mg/L, and Pb 0.013 mg/L. Heavy metal concentrations are expressed in µg/g dry weight. The precision of the analytical procedure was assessed through triplicate analysis, and the relative standard deviation (%RSD) for each metal was calculated. The %RSD values obtained were found to be less than 10%.
To assess repeatability and verify the analytical procedure, samples were spiked with known concentrations of heavy metals. Each test was performed in triplicate. The spiked samples were digested and analyzed using the same protocol as for the original specimens. Recoveries for the analyzed metals ranged from 77% to 95%, and recovery correction was applied to each sample accordingly.
All reagent and standard solutions were prepared using ultrapure water. Only analytical-grade chemicals were used throughout the analysis. To minimize contamination, all glassware was soaked in 3% nitric acid for 24 h, rinsed thoroughly with ultrapure water, and dried in an oven prior to use.
The BSAF (bio-sediment accumulation factor) was also calculated. It is the ratio of the contaminant concentration in tissue to the contaminant concentration in sediment.
2.4. Statistical Analysis
Metal concentrations are expressed as means ± standard deviations (SD). Prior to statistical analysis, data normality was assessed. One-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test was performed using StatView software (version 5.0, by SAS Institute Inc., Cary, NC, USA). Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Trace Metal Accumulation in H. diversicolor Tissues
Figure 2 shows the results of assays for the metals studied bioaccumulated in the organs and tissues of H. diversicolor. The analyses revealed the presence of these xenobiotics in the tissues of the target worm, with heterogeneous levels. The highest levels of Pb were observed at all study sites. From the results obtained, we noted that in the four study sites, heavy metal levels follow a concentration scale in the following ascending order: Cd < Cu < Zn < Pb.
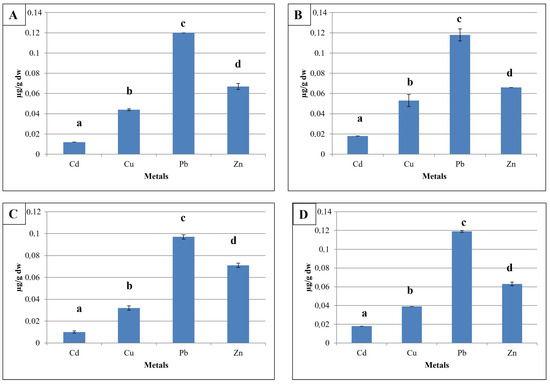
Figure 2.
Trace elements levels (mean ± SE; µg/g dry weight) in the studied station (A) Bizerte; (B) Sfax; (C) Gabes; (D) Djerba. Different letters indicate statistically significant differences.
3.1.1. Bizerte
Figure 2A shows the levels of trace metals (Cd, Cu, Pb, and Zn) bioaccumulated in H. diversicolor tissues (in µgg−1 dry weight) in the Bizerte region. According to the graph, Pb concentrations were the highest (0.12 ± 2.54 × 10−4 µgg−1 dry weight), compared with Zn (0.067 ± 0.003 µgg−1 dry weight), Cu (0.044 ± 0.001 µgg−1 dry weight), and Cd (0.012 ± 2.01 × 10−5 µgg−1 dry weight). Statistical analysis revealed a significant difference in the levels of these elements (F = 882.21; p < 0.001).
3.1.2. Sfax
Figure 2B shows the concentrations of trace metals (Cd, Cu, Pb, and Zn) bioaccumulated in the tissues of H. diversicolor caught in the Sfax region. Pb levels at this site were significantly higher (F = 103.761; p < 0.001) at 0.118 ± 0.006 µgg−1 dry weight, compared with Zn (0.066 ± 1.54 × 10−4 µgg−1 dry weight), Cu (0.053 ± 0.006 µgg−1 dry weight), and Cd (0.018 ± 2.75 × 10−4 µgg−1 dry weight).
3.1.3. Gabes
Results for trace metal content in samples of H. diversicolor caught in the Gabes region, presented in Figure 2C, show that Pb was the most important element, with levels in the order of 0.097 ± 0.002 µgg−1 dry weight, followed by Zn (0.071 ± 0.002 µgg−1 dry weight), Cu (0.032 ± 0.005 µgg−1 dry weight), and Cd (0.01 ± 0.001 µgg−1 dry weight). Statistical analysis revealed significant differences between these levels (F = 512; p < 0.001).
3.1.4. Djerba
Analyses of samples collected in the Djerba region (Figure 2D) show that cadmium is the element with the lowest concentration (0.018 ± 3.28 × 10−4 µgg−1 dry weight), compared with lead, which has the highest concentration (0.119 ± 0.001 µgg−1 dry weight). Zn and Cu contents were of the order of 0.063 ± 0.002 µgg−1 dry weight and 0.039 ± 2.21 × 10−4, respectively. Statistical analyses showed significant differences between these different grades (F = 1387.194; p < 0.001).
3.2. Site-Specific Variations in Average Heavy Metal Concentrations
3.2.1. Lead
Pb concentrations at the four study stations are shown in Figure 3A. The results show that Pb levels were lowest in samples collected in the Gabes region (0.097 ± 0.002 µgg−1 dry weight), compared with levels in the other three study stations (Table 1). Statistical analysis revealed a statistically significant difference between samples from the Gabes site and the other three stations (F = 12.15; p < 0.001).
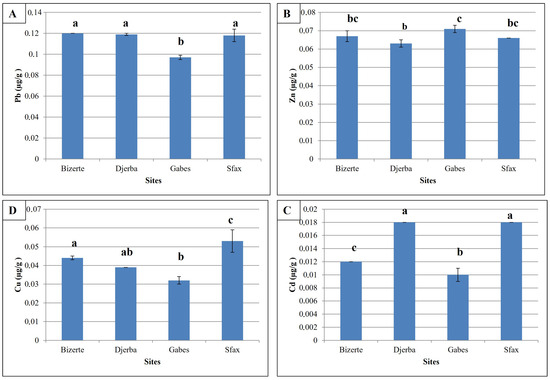
Figure 3.
Trace elements levels (mean ± SD; µgg−1 dry weight) between study stations. (A) Pb; (B) Zn; (C) Cd; (D) Cu. Different letters indicate statistically significant differences.

Table 1.
Results of statistical analyzes between study sites for Pb, Zn, Cd, and Cu contents (µgg−1).
3.2.2. Zinc
The results for Zn levels at the study stations showed that samples from the Djerba region had the lowest values (0.063 ± 0.002 µgg−1 dry weight), while the highest levels were observed in samples from Gabes (Figure 3B; 0.071 ± 0.002 µgg−1 dry weight) (Table 1). Statistical analyses showed a significant difference between these two study stations, while there were no statistically significant differences between concentrations at the Bizerte and Sfax stations.
3.2.3. Cadmium
Cadmium levels in the samples studied showed that the Djerba and Sfax regions had the highest values, 0.018 ± 3.28−4 and 0.018 ± 2.75−4 µgg−1 dry weight, respectively (Figure 3C; Table 1), while the lowest concentrations were reported in the Bizerte and Gabes regions (in the order of 0.012 ± 2.01−5 and 0.1 ± 0.001 µgg−1 dry weight). Analysis of Cd concentrations at the various study stations revealed statistically significant differences between samples from Bizerte and those from Djerba, Gabes and Sfax (p < 0.001). Also, levels in the Gabes region were significantly lower than levels in the Djerba and Sfax regions (p < 0.001).
3.2.4. Copper
Figure 3D revealed the levels of lead, zinc, cadmium and copper in tissues from the studied sites. The lowest levels of these four heavy metals were found in samples collected from the two stations, Gabes and Djerba, while the other two stations, Bizerte and Sfax, contained the highest heavy metal values (Table 1). Significant differences were obtained between levels in the Sfax region with Djerba, Bizerte and Gabes, while there was no significant difference with levels in Bizerte. The grades of samples from Bizerte show statistically significant differences with grades from the Gabes region. There were no differences between Djerba and Gabes/Bizerte.
4. Discussion
This study confirmed the effective presence of these xenobiotics in the tissues of the target annelid, with highly variable levels. It is important to note that cadmium was identified as the least bioaccumulated metal among samples from all four sites studied, while lead was detected as the most bioaccumulated metal in samples from all four sites studied. The presence of copper and zinc varied from site to site.
The results revealed that lead levels were the highest among the four stations sampled, with maximum concentrations recorded in organisms sampled in the Bizerte Lagoon. The average for this pollution was 0.12 ± 2.54 × 10−4 µgg−1 dry weight. On the other hand, the lowest lead concentration was observed in Gabes, with an average of 0.097 ± 0.002 µgg−1 dry weight. Lead can have a natural origin, but its main sources of emission are the lead industries and especially road traffic. It is used in construction, mechanical engineering, batteries, cables, and pigments. This substance tends to accumulate in fish and mammals, presenting potential risks for fertility, neurotoxicity, and immune responses [28].
Similarly to lead, mean zinc concentrations recorded in H. diversicolor samples showed a diversity of variations. Significant values were observed in worms collected in the El Hicha region of Gabes. Indeed, the average for this pollutant was 0.071 ± 0.002 and 0.063 ± 0.002 µgg−1 dry weight, respectively, in annelids from Gabes and Djerba. These higher zinc values, like those of lead, could originate from common anthropogenic sources such as industrial effluents from phosphate and chemical processing plants. Moreover, the lack of sufficient water dilution and circulation in the Gabes coastal zone may limit the dispersion of contaminants, enhancing their retention in sediments and increasing their bioavailability to benthic organisms like H. diversicolor.
Zinc, a relatively common metal, is found in metalliferous seams, coal, bitumen, and oil, with frequent occurrence in mining areas. It can also be of anthropogenic origin. Industrial applications for zinc and its compounds are numerous, ranging from metal coatings to the manufacture of paint pigments, plastics, rubber, pharmaceuticals, and insecticides. Water-soluble zinc salts tend to accumulate in organisms, and zinc is an essential metal for all living organisms [28]. Zinc is mainly used in corrosion protection coatings, alloy manufacture (brass, bronze, light alloys), building construction, automotive equipment, railroads, and the production of rolled or formed products. It plays an intermediary role in the manufacture of other compounds, acting as a reducing agent in organic chemistry and as a reagent in analytical chemistry [29]. Although zinc is not generally considered a toxic metal, high concentrations can cause physiological disturbances in the body [30]. Although its toxicity to aquatic organisms does not classify it as a priority contaminant, effects on oyster reproduction and larval growth can be observed at high concentrations [29]. Zinc has the capacity to accumulate in aquatic organisms, with bioconcentration factors reported at 1000 for freshwater fish and 2000 for marine fish [31].
Lead is frequently found in association with zinc in ores, along with various other elements such as iron (Fe), copper (Cu), cadmium (Cd), bismuth (Bi), antimony (Sb), germanium (Ge), arsenic (As), silver (Ag), and gold (Au). These elements, with the exception of iron, are generally recovered during metallurgical operations. Mixed lead and zinc ores account for around 70% of lead mine production, while pure lead ores account for around 20%. Around 10% of lead production comes from co-production during the processing of copper, zinc, or other metal ores. Galena (PbS) is the main lead ore, often associated with sphalerite (zinc) and pyrite [32].
The different cadmium concentrations obtained show that the cadmium concentration varies according to the sampling site. Cadmium was detected in 100% of samples from the Djerba and Sfax sites. Maximum mean cadmium levels were recorded in H. diversicolor tissues, reaching 0.018 ± 3.28 × 10−4 and 0.018 ± 2.75 × 10−4 µgg−1 dry weight, respectively, at the Djerba and Sfax sites. Cadmium (Cd) is a silvery-white or blue metal, often associated with lead, copper, or zinc deposits. It is generally found as cadmium oxide, chloride, or sulfate/sulfide. In zinc deposits, cadmium sulfide is the most common form. Cadmium production depends on zinc demand, as it is often a by-product of zinc concentrate processing [33]. Cadmium metal is used in the anti-corrosion coating of metals (cadmium plating by electrolysis or dipping and spraying), in the manufacture of negative electrodes for rechargeable nickel-cadmium silver-cadmium batteries, and in the production of stabilizers for plastics (chloride, nitrate). Once across the biological barrier, the Cd2+ form is captured by numerous intracellular ligands, including metallothioneins (MTs), proteins that regulate cellular concentrations of essential free metals such as zinc (Zn2+) and copper (Cu2+). Cadmium’s high affinity for MTs leads to displacement of zinc (initially bound to the MT), thus disrupting zinc uptake and transport. Target tissues include biological barriers such as gills and the digestive tract, as well as detoxification organs such as the kidneys and liver. It should be noted that muscle does not appear to be a preferred storage site of this element [34].
Results concerning Cu content showed that the latter was particularly detected in high quantities in samples from the Sfax beach, with maximum mean values recorded in H. diversicolor tissues reaching 0.053 ± 0.006 µgg−1 dry weight at Sfax. Copper is a metal that occurs quite frequently in nature. It is of major importance as an essential microelement for the respiration of many organisms and other enzymatic functions. In humans, copper is stored in the digestive gland (hepatopancreas) and gills of molluscan invertebrates. Excess copper in humans can damage liver tissue and affect blood pressure [28,35]. Similarly, in aquatic organisms, high concentrations of this metal can cause oxidative damage to lipids and proteins, as well as DNA alterations.
The accumulation of metals in organs is a very important parameter to study, since unlike other pollutants, metals can accumulate in organs to concentrations well above those present in the environment and can reach toxic thresholds. Our results on metal accumulation show inter-population variation in the levels of the elements measured. Generally speaking, polluted sediments indicate environmental degradation and pose significant risks to organisms that inhabit or interact with them, particularly because such areas often serve as critical nurseries and spawning grounds for benthic species [36]. An increase in metal accumulation in the tissues of the polychaete annelid H. diversicolor is, therefore, probably highly favored, given the ecobiological nature of this species. This partly explains the higher metal levels in the tissues of the Gabes population, since this site also has high sediment values. These observations corroborate earlier studies by Machreki-Ajmi and Hamza-Chaffai [37], who confirmed that the northern part of the Gulf of Gabes is the most polluted zone in this geographical area. This part of the gulf is subject to large-scale industrial and urban effluent discharges, in addition to a stock of phosphogypsum, leading to contamination of the environment mainly by metals. The contamination of the golf course by these elements is essentially attributed to urban and industrial liquid discharges, as well as to pollution generated by public landfills. Characterization of these discharges has shown them to be highly polluting, particularly in terms of Zn.
The lead levels obtained in H. diversicolor tissues collected from some Tunisian aquatic ecosystems are closer to those reported in samples from: Venice, Italy, for the species H. diversicolor [38], and Oualidia lagoon, Morocco, for the species H. diversicolor [39] (Table 2). However, the concentration of this element at the tissue level is much lower than that reported in samples from Bouregreg Estuary, Morocco, for the species H. diversicolor [40], Buleji Karachi, Pakistan, for the species Eurytho ecomplanata [41] (Table 2).
Mean zinc values obtained in H. diversicolor tissues collected from a few Tunisian regions are very low compared to those reported in samples from (Table 2): JorfLasfar, Morocco, for the species Sabellaria alveolata [42]; Bouregreg Estuary, Morocco, for the species H. diversicolor [43]; and Mersey Estuary, UK, for the species H. diversicolor [44].
The average cadmium levels obtained in H. diversicolor tissues collected from several Tunisian aquatic ecosystems are closer to those reported in samples from: Homa Lagoon Izmir, Turkey, for the species H. diversicolor [45], and Oualidia lagoon, Morocco, for H. diversicolor [39]. In contrast, our results seem only slightly different from those of JorfLasfar, Morocco, for the species Sabellaria alveolata [42], and the Ross Sea, Antarctica, for the species Perkinsiana littoralis [46] (Table 2).
The toxicity and environmental behavior of metals in aquatic systems—such as their mobility and bioavailability—are largely influenced by their speciation, which refers to the distribution of an element among its various chemical forms or phases (both soluble and insoluble) [47]. These effects also depend on the characteristics of the exposed organism, including species, sex, age, developmental stage, and the metal concentration in specific organs [48,49]. Even if metal analyses in samples from different localities do not reveal situations of real concern, questions remain due, on the one hand, to the large volume of discharges and, on the other, to the bioaccumulation of heavy metals in aquatic species.
Numerous studies have shown that biological variability plays a key role in shaping organism responses to a broad spectrum of contaminants, often influenced by spatial and seasonal environmental fluctuations [42,50,51,52,53,54,55,56]. Moreover, metal bioaccumulation is governed by a complex interplay of factors. These include physical parameters such as temperature, salinity, pH, organic carbon content, food availability, dissolved oxygen levels, sediment granulometry, and the system’s hydrological characteristics; chemical aspects like metal concentration, speciation, and bioavailability; and physiological traits of the organism itself, including growth rate, weight fluctuation, sexual maturity, reproductive stage, metal uptake efficiency, and internal accumulation capacity [53,55,56,57,58,59,60,61].

Table 2.
A compilation of Cd, Cu, Zn, and Pb (µgg−1dwt) contents in different polychaete species from the literature.
Table 2.
A compilation of Cd, Cu, Zn, and Pb (µgg−1dwt) contents in different polychaete species from the literature.
| Locality | Species | Metals | References | |||
|---|---|---|---|---|---|---|
| Cd | Cu | Zn | Pb | |||
| Homa Lagoon–Izmir–Turkey | Hediste diversicolor | 1.5–4.5 | 14.0–26.5 | - | - | [62] |
| Homa Lagoon–Izmir–Turkey | Hediste diversicolor | 1.6–2.7 | 24.2–28.5 | - | - | [63] |
| Homa Lagoon–Izmir–Turkey | Hediste diversicolor | 1.6–2.7 | 14.2–18.5 | - | - | [64] |
| Homa Lagoon–Izmir–Turkey | Hediste diversicolor | 0.03–0.43 | 17.2–41.0 | - | 6.5–19.1 | [44] |
| Essaouira. Morocco | Sabellariaalveolata | 4.14 ± 1.08 | 6.73 ± 4.48 | 168.39 ± 34.88 | 2.55–2.00 | |
| JorfLasfar. Morocco | Sabellariaalveolata | 38.44 ± 8.83 | 16.06–35.10 | 522.39 ± 140.26 | - | [42] |
| JorfLasfar. Morocco | Arenicolagrubii | 10.29 ± 7.18 | 6.62–50.43 | 86.97 ± 55.27 | - | |
| Oualidialagoo. Morocco | Hediste diversicolor | 0.09 ± 0.1 | 6.8 ± 2.5 | 115 ± 29.5 | 1.0 ± 0.5 | [39] |
| Khnifss lagoon. Morocco | Hediste diversicolor | 1.4 ± 0.2 | 8.8 ± 3.8 | 94 ± 44.3 | 3.0 ± 1.1 | |
| Bouregreg Estuary. Morocco | Hediste diversicolor | - | 53.00 | 555–654 | - | [43] |
| Bouregreg Estuary. Morocco | Hediste diversicolor | - | 22.11 | 102.27 | 33.17 | [40] |
| Mersey Estuary. United Kingdom | Hediste diversicolor | 0.7 ± 1.0 | 46 ± 22 | 196 ± 45 | 9.5 ± 4.2 | [44] |
| Buleji Karachi. Pakistan | Eurythoe complanata | 2.11 ± 0.38 | 3.67 ± 1.26 | 14.86 ± 1.64 | 11.12 ± 1.18 | [41] |
| Venice. Italy | Hediste diversicolor | 0.15 ± 0.09 | 24.91 ± 9.89 | - | 1.12 ± 0.4 | [38] |
| Todosos Santos Bay. Brazil | Chaetopterus variopedatus | - | 1.8–39 | 40.6–125 | - | [65] |
| ZolotoiRog Bay. Russia | Ophryotrocha sp. | 0.30 ± 0.02 | 3.1 ± 0.10 | 24.4 ± 0.73 | - | [66] |
| Nereis vexillosa | 0.33 ± 0.03 | 1.3 ± 0.25 | 44.5 ± 1.30 | - | ||
| Alitta brandti | 0.26 ± 0.02 | 1.8 ± 0.04 | 28.2 ± 0.80 | - | ||
| Capitella capitata | 0.30 ± 0.02 | 1.4 ± 0.03 | 30.3 ± 0.90 | - | ||
| Schistomeringos japonica | 0.49 ± 0.04 | 3.5 ± 0.07 | 32.7 ± 0.96 | - | ||
| Bengal Bay. India | Perenereis cultifera | - | 36.5–52 | 36.5–52 | - | [67] |
| Mastobranchus indicus | - | 25–44.5 | 150–320 | - | ||
| Namalycastis fauveli | - | 31.00 | 85.00 | - | ||
| Dendronerides arborifera | - | 33.00 | 140.00 | - | ||
| UK Estuaries | Arenicola marina | - | 2.9–125 | 43.3–141 | - | [68,69] |
| Estuaries SW England | Hediste diversicolor | 0.2–0.6 | 44–3900 | 150–170 | - | [70] |
| Atlantic Coast. France | Hediste diversicolor | 0.1–0.3 | 11–26 | 110–170 | - | [70] |
| Ross Sea. Antarctica | Perkinsiana littoralis | 23–33 | 4–9 | 140–200 | - | [46] |
The bio-sediment accumulation factor (BSAF) is a key index used to relate metal concentrations in organisms to those in sediments [71]. Accordingly, we used BSAF to evaluate the ability of H. diversicolor to accumulate metals from sediments. The BSAF for Cd, Cu, Zn, and Pb was calculated following the methods described by [71,72]. The resulting values are presented in Table 3.

Table 3.
Bio-sediment accumulation factors (BSAF) of Cd, Cu, Zn, and Pb in H. diversicolor tissues from Tunisian coastal sites.
At Bizerte Lagoon, Cd and Cu showed the highest BSAF values (0.01), indicating similar bioaccumulation potential. Zn showed very limited bioaccumulation, while Pb was moderately accumulated. This suggests that Cd and Cu are more bioavailable, whereas Zn is least accumulated despite relatively high sediment concentrations. At Sfax Coast, Cd exhibited the highest bioaccumulation (0.03), followed by Cu and Pb, while Zn was the least accumulated but still higher than at other sites. This indicates a generally high bioavailability of all metals, with Cd being the most accumulated. In the Gulf of Gabes, all metals showed very low BSAFs, with Pb slightly higher than the others. This suggests that, despite high sediment contamination, metals are likely present in non-bioavailable forms or are not readily taken up by organisms.
At Djerba Island, Pb showed the highest BSAF, followed by Cd. Cu showed no measurable accumulation. This indicates selective bioaccumulation, where Pb and Cd are more bioavailable, and Cu is largely unavailable to organisms.
The inter-site comparison of BSAF values revealed distinct patterns of metal bioaccumulation across the four coastal sites. Sfax Coast stands out with the highest BSAFs for all metals, especially for Cd (0.03) and Cu (0.02), indicating higher bioavailability and uptake efficiency of these metals by biota, likely due to favorable environmental conditions or more labile metal forms. In contrast, the Gulf of Gabes, despite showing the highest sediment concentrations, exhibited extremely low BSAFs for all metals, suggesting that metals are mostly in non-bioavailable or strongly bound forms, limiting their transfer to organisms. Bizerte Lagoon showed moderate BSAFs for Cd and Cu (0.01), but much lower values for Zn and Pb, indicating a selective accumulation pattern possibly influenced by metal speciation or sediment characteristics. Djerba Island displayed a similar trend, with Pb (0.0054) and Cd (0.01) showing the highest accumulation, while Cu is not accumulated at all, suggesting localized factors affecting metal mobility and organism exposure. Overall, while Sfax Coast poses the greatest ecotoxicological risk due to high bioaccumulation, the Gulf of Gabes may represent a contamination hotspot with limited biological impact, emphasizing the importance of assessing both sediment contamination levels and bioaccumulation potential for accurate ecological risk evaluation.
The differences in BSAF values between sites and metals reflect the combined influence of metal speciation, sediment characteristics, and local environmental conditions affecting metal bioavailability and organism uptake. Sfax Coast consistently showed the highest BSAF values for all metals, especially Cd and Cu, suggesting that metals there are present in more bioavailable forms, possibly due to factors such as finer sediment grain size, higher organic matter content, or more active benthic communities facilitating uptake. In contrast, the Gulf of Gabes, despite high total metal concentrations in sediments, showed extremely low BSAF values, indicating that metals are likely bound to stable mineral phases or complexed with substances that reduce their mobility and uptake by organisms. Bizerte Lagoon and Djerba Island show intermediate patterns, with Cd and Pb being more bioaccumulated than Cu and Zn, suggesting that metal-specific properties, such as ionic radius, affinity for organic matter, and redox sensitivity, influence their accumulation. Additionally, biological factors such as species metabolism, feeding strategy, and detoxification mechanisms may further explain the variability in BSAF values across both sites and elements.
According to [71], snail tissues can be categorized as macroconcentrators (BSAF > 2), microconcentrators (1 < BSAF < 2), or deconcentrators (BSAF < 1). In this study, the biota-sediment accumulation factors (BSAF) indicate that the soft tissues of H. diversicolor functioned as deconcentrators at all sampled sites. This suggests a limited capacity for metal uptake from sediments. Similarly, all calculated bioaccumulation factor (BAF) values were below 1000, indicating a low potential for significant bioaccumulation of heavy metals in this species. Based on the classification proposed by [77], BAF values are interpreted as follows: BAF < 1000 reflects negligible accumulation potential; values between 1000 and 5000 indicate moderate bioaccumulation; and BAF > 5000 denotes a high bioaccumulation risk. Generally, BSAF values greater than 1 imply that a metal or metalloid may accumulate in an organism’s soft tissues, whereas values below 1 suggest minimal accumulation and weak association with sediment concentrations [78]. Thus, the presence of metals in the tissues of H. diversicolor likely reflects limited environmental uptake rather than direct accumulation from sediment.
Based on the observed heavy metal contamination, particularly the elevated levels of lead and the presence of cadmium, it is imperative to implement effective environmental management and protection strategies to safeguard Tunisia’s aquatic ecosystems. Establishing regular biomonitoring programs using reliable bioindicator species such as Hediste diversicolor will be essential for tracking pollutant trends and identifying emerging risks. Strengthening regulations on industrial discharges and urban wastewater treatment is crucial to reducing the input of toxic metals into coastal waters. Furthermore, raising public awareness about the ecological and health risks associated with heavy metal contamination can enhance community engagement and support pollution prevention efforts. Sustainable coastal development practices, including habitat restoration and pollution source control, will be vital to preserving biodiversity and ensuring the long-term health of marine environments and the human populations that rely on them.
5. Conclusions
This study is the first to focus on the use of polychaete H. diversicolor as a sentinel species in Tunisia for the biomonitoring of aquatic ecosystems. There are few studies available in the literature regarding the use of H. diversicolor as a bioindicator species for monitoring heavy metals. Indeed, most of the studies carried out mainly concern the monitoring of organic pollutants. The different heavy metal levels recorded at the sites investigated during this work confirm the usefulness of using this polychaete species as a sentinel species in biomonitoring programs. As perspectives for this preliminary study, we intend to use this species as a sentinel for the monitoring of organic pollutants along the Gulf of Gabes and for the monitoring of metallic and organic pollutants in other Tunisian aquatic ecosystems.
Author Contributions
Formal analysis, W.B.A., M.M. and A.A.; Methodology, N.A., W.B.A., M.M. and A.A.; Resources, A.A.; Supervision, A.A.; Validation, M.M. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank all members of the Life Sciences Department of the Faculty of Sciences of Gabes, University of Gabes, for providing reagents and scientific technical assistance. They would like also to thank the Ministry of Higher Education and Scientific Research of Tunisia for supporting this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Edo, G.I.; Itoje-akpokiniovo, L.O.; Obasohan, P.; Ikpekoro, V.O.; Samuel, P.O.; Jikah, A.N.; Nosu, L.C.; Ekokotu, H.A.; Ugbune, U.; Oghroro, E.E.A.; et al. Impact of Environmental Pollution from Human Activities on Water, Air Quality and Climate Change. Ecol. Front. 2024, 44, 874–889. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Ben Alaya, M.; Daghbouj, N.; Castet, S.; Besson, P.; Michel, S.; Bejaoui, N.; Courjault-Radé, P. Seawater Quality Assessment and Identification of Pollution Sources along the Central Coastal Area of Gabes Gulf (SE Tunisia): Evidence of Industrial Impact and Implications for Marine Environment Protection. Mar. Pollut. Bull. 2018, 127, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, B.; Ennouri, R.; Fatnassi, M.; Zarrouk, H.; Romdhane, N.; Chalghaf, M.; Mili, S. Review of the Situation of Tunisian Lagoon of Bizerta Using Marine Spatial Planning as a Key to Sustainable Blue Growth. J. Biomed. Res. Environ. Sci. 2022, 3, 149–162. [Google Scholar] [CrossRef]
- Duplay, J.; Khedhiri, S.; Semhi, K.; Darragi, F. Water Quality in a Protected Natural Wetland: El Kelbia Sebkhet, Tunisia. Int. J. Environ. Stud. 2013, 70, 33–48. [Google Scholar] [CrossRef]
- Dghim, A.; Ben Ameur, W.; Annabi, A. Assessment of the Accumulation of Trace Metals and Oxidative Stress Response Biomarkers in the Portunid Portunus segnis. Appl. Sci. 2023, 13, 7197. [Google Scholar] [CrossRef]
- Ouchir, N.; Amri, I.; Boughdiri, M. Morphological Responses of Ostracods to Heavy Metal Contamination: Freshwater Ostracods as Bioindicators of Pollution in Ichkeul Lake and Rivers Ecosystem, Northern Tunisia. Euro-Mediterr. J. Env. Integr. 2025, 10, 515–541. [Google Scholar] [CrossRef]
- Ben Ameur, W.; El Megdiche, Y.; Ennaceur, S.; Mhadhbi, T.; Ben Hassine, S.; Annabi, A.; De Lapuente, J.; Driss, M.R.; Borràs, M.; Eljarrat, E. Biomarkers Responses and Polybrominated Diphenyl Ethers and Their Methoxylated Analogs Measured in Sparus aurata from the Lagoon of Bizerte, Tunisia. Environ. Sci. Pollut. Res. 2022, 29, 38618–38632. [Google Scholar] [CrossRef]
- Ameur, W.B.; Annabi, A.; Mhadhbi, T.; Hassine, S.B.; Safouen, G.; El Megdiche, Y.; Khadija, M.; Ennaceur, S.; Trabelsi, S.; Hammami, B.; et al. Polycyclic Aromatic Hydrocarbons in Mullet (Chelon auratus) from Two Lagoons of Great Ecological and Economic Importance in Tunisia: Levels, Sources and Human Health Risk Implications. J. Sea Res. 2023, 192, 102325. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, C.; Luo, Y.; Liu, C.; Kyzas, G.Z.; Luo, Y.; Zhao, D.; An, S.; Zhu, H. Heavy Metals in surface sediments of the jialu river, china: Their relations to environmental factors. J. Hazard. Mater. 2014, 270, 102–109. [Google Scholar] [CrossRef]
- Shallari, S.; Schwartz, C.; Hasko, A.; Morel, J.L. Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci. Total Environ. 1998, 209, 133–142. [Google Scholar] [CrossRef]
- Jefferies, D.J.; Freestone, P. Chemical Analysis of Some Coarse Fish from a Suffolk River Carried Out as Part of the Preparation for the First Release of Captive-Bred Otters; Springer: New York, NY, USA, 1984. [Google Scholar]
- Güven, K.; Özbay, C.; ÜNLÜ, E.; Satar, A. Acute lethal toxicity and accumulation of copper in Gammarus pulex (L.) (Amphipoda). Turk. J. Biol. 1999, 23, 513–522. [Google Scholar]
- Boucheseiche, C.; Cremille, E.; Pelte, T.; Pojer, K. Pollution Toxique et Ecotoxicologie: Notion de Base. Guide Technique n°7. Agence de l’Eau Rhône-Méditerranée Corse. Montpellier (France). 2002. Available online: https://www.corse.eaufrance.fr/sites/siecorse/files/content/migrate_documents/guide-technique-sdage-7.pdf (accessed on 1 April 2024).
- Jer’onimo, D.; Lillebø, A.I.; Rey, F.; Ii, H.K.; Domingues, M.R.M.; Calado, R. Optimizing the timeframe to produce polychaetes (Hediste diversicolor) enriched with essential fatty acids under different combinations of temperature and salinity. Front. Mar. Sci. 2021, 8, 671545. [Google Scholar] [CrossRef]
- Geffard, O. Toxicité Potentielle des Sédiments Marins et Estuariens Contaminent: Évaluation Chimique et Biologique, Biodisponibilité des Contaminants Sédimentaire. Ph.D. Thesis, Université Bordeaux 1, Bordeaux, France, 2001. [Google Scholar]
- ANPE. Étudepréliminaire de L’écologiedulac de Bizert; Rapport; Agence Nationale de Protection de l’Environnement: Tunis, Tunisie, 1990; p. 100. [Google Scholar]
- Ouakad, M. Évolution Sédimentologique et Caractères Géochimiques des Dépôts Récents de la GaraâtIchkeul (Tunisie septentrionale). Ph.D. Thesis, Université de Perpignan, Pézenay, France, 1982; p. 178. [Google Scholar]
- Ouakad, M. Caractères sédimentologiques et géochimiques des dépôts superficiels de la lagune de Bizerte (Tunisie septentrionale). In Circulation des Eaux et Pollution des Côtes Méditérranéennes des Pays du Maghreb; Edition INOCI: Izmir, Turquie, 1993; pp. 187–194. [Google Scholar]
- Harzallah, A. Transport de polluants dans la lagune de Bizerte simule par un modèle de circulation de l’eau, Pollutant advection in the Bizerte lagoonsimulated by a water circulation model. Bull. INSTM Salammbô 2003, 30, 121–133. [Google Scholar]
- Portuguesa, H. Etude Générale Pour la Protection du Littoraltunisien; Rapports, Ministère de l’Equipementet de l’Habitat; Agence de Protection et d’Aménagement du Littoral A.P.A.L: Tunis, Tunisie, 1995; Available online: http://www.apal.nat.tn/site_web/creation_mission.html (accessed on 23 May 2025).
- Aloulou, F.; EllEuch, B.; Kallel, M. Benthic Foraminiferal Assemblages as Pollution Proxies in the Northern Coast of Gabes Gulf, Tunisia. Environ. Monit. Assess. 2012, 184, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, D.; Azri, C.; Serbaji, M.M.; Jedoui, Y.; Montacer, M. Heavy Metal Concentrations in the Surface Marine Sediments of Sfax Coast, Tunisia. Environ. Monit. Assess. 2011, 175, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Gargouri-Ben, A.Z.; Souissi, R.; Soussi, M.; Abdeljaouad, S.; Zouari, K. Sedimentary Dynamics and Ecological State of Nakta Tidal Flat (Littoral), South of Sfax, Gulf of Gabès, Tunisia). Chin. J. Geochem. 2007, 26, 244–251. [Google Scholar] [CrossRef]
- Castany, G. Les Plissements Quaternaires En Tunisie; Compte Rendu Sommaire des Seances de la Societe Geologique de France: Paris, France, 1953; Volume 11–12, pp. 155–157. [Google Scholar]
- Burollet, P.F. Contribution à l’Etude Stratigraphique de la Tunisie Centrale. In Annales des Mines et de la Géologie Tunisie; 1965; Volume 18, p. 350. ISSN 0365-4397. [Google Scholar]
- Zebidi, H. Hydrogéologie de la Nappe Profonde de Sfax; Bibliothèque Nationale de Tunisie: Tunis, Tunisie, 1989; p. 27. [Google Scholar]
- Annabi, A.; Bardelli, R.; Vizzini, S.; Mancinelli, G. Baseline Assessment of Heavy Metals Content and Trophic Position of the Invasive Blue Swimming Crab Portunus segnis (Forskål, 1775) in the Gulf of Gabès (Tunisia). Mar. Pollut. Bull. 2018, 136, 454–463. [Google Scholar] [CrossRef]
- Dons, C.; Beckp, A. Priority hazardous substances in Norway. Nor. State Pollut. Control Rep. 1993, 93, 22–115. [Google Scholar]
- Casas, S. Modélisation de la Bioaccumulation de Métaux Traces (Hg, Cd, Pb, Cu et Zn) chez la Moule, Mytilus galloprovincialis en Milieu Méditerranéen. Ph.D. Thesis, Université du Sud Toulon Var, Toulon, France, 2005. [Google Scholar]
- Fairbrother, A.; Wenstel, R.; Sappington, K.; Wood, W. Framework for Metals Risk Assessment. Ecotoxicol. Environ. Saf. 2007, 68, 145–227. [Google Scholar] [CrossRef]
- United States, Environmental Protection Agency. Guidelines for Carcinogen Risk Assessment: Risk Assessment Forum EPA/630/P-03/001B; U.S. Environmental Protection Agency: Washington, DC, USA, 2005. Available online: https://www3.epa.gov/airtoxics/cancer_guidelines_final_3-25-05.pdf (accessed on 1 April 2024).
- Chiffoleau, J.-F.; Auger, D.; Chartier, E.; Michel, P.; Truquet, I.; Ficht, A.; Gonzalez, J.-L.; Romana, L.-A. Spatiotemporal Changes in Cadmium Contamination in the Seine Estuary (France). Estuaries 2001, 24, 1029. [Google Scholar] [CrossRef]
- Environnement Canada; Santé Canada. Le Cadmium et ses Composés; Liste des substances d’intéret prioritaire, rapport d’évaluation; Environnement Canada: Ottawa, ON, Canada, 1994; ISBN 9780662998051.
- Nakib, L. Mise au Point D’une Technique D’extraction des Eléments Traces Métalliques Dans les Produits de la Mer et Leurs Dosages par Spectrophotométrie D’absorption Atomique. Master’s Thesis, Département des Sciences Vétérinaires, Université El Hadj Lakhdar Batna, Batna, Algeria, 2009; p. 118. [Google Scholar]
- Blomseth, L.H.; Hartmann-Pedersen, P. Grunnstoffene—Universets Byggesteiner; Universitetsforlaget AS Oslo: Oslo, Norway, 1995; p. 257. [Google Scholar]
- Machreki-Ajmi, M. Validation des Biomarqueurs de Pollution Chez le Mollusque Bivalve Cerastodermaglaucum Issu du Golfe de Gabès: Etude In Situ et Transplantation In Vivo. Ph.D. Thesis, Faculté des Sciences de Sfax, Sfax, Tunisie, 2009; p. 205. [Google Scholar]
- Machreki-Ajmi, M.; Hamza-Chaffai, A. Accumulation of Cadmium and Lead in Cerastodermagflaucum Originating from the Gulf of Gabès, Tunisia. Bull. Environ. Contam. Toxicol. 2006, 76, 529–537. [Google Scholar] [CrossRef]
- Frangipane, G.; Ghirardini, A.V.; Collavini, F.; Zaggia, L.; Pesce, A.; Tagliapietra, D. Heavy Metals in Hedistediversicolor (Polychaeta: Nereididae) and Salt Marsh Sediments from the Lagoon of Venice (Italy). Chem. Ecol. 2005, 21, 441–454. [Google Scholar] [CrossRef]
- Idardare, Z.; Moukrim, A.; Chiffoleau, J.F.; Ait Alla, A.; Auger, D.; Rozuel, E. Evaluation de la contamination métallique dans deux lagunes marocaines:Khnifiss et Oualidia. Rev. Marocaine Sci. Agron. Vétérinaires 2013, 2, 58–67. [Google Scholar]
- Khamar, M.; Cherkaoui, E.; Nounah, A. Bioaccumulation of Heavy Metals by the Flora and Benthic Macrofauna of the Bouregreg Estuary Wetland. MATEC Web Conf. 2018, 149, 02054. [Google Scholar] [CrossRef]
- Khan, M.U.; Kanwal, N.; Moinuddin, A. Metal bioavailability; Toxicity in sediments and accumulation in fire worm Eurythoecomplanata (pallas, 1766) (Polychaeta: Amphinomidae) from Buleji Karachi, Pakistan. FUUAST J. Boil. 2017, 7, 171–176. [Google Scholar][Green Version]
- Rouhi, A.; Sif, J.; Ferssiwi, A.; Chemaa, A. Bioaccumulation de quelques éléments métalliques par deux espèces d’Annélides Polychètes du littoral de JorfLasfar (Région d’El Jadida, Maroc). Bull. L’institutscientifique Sect. Sci. Vie 2007, 29, 81–87. [Google Scholar][Green Version]
- Cheggour, M.; Texier, H.; Moguedet, G.; Elkaïm, B. Metal Exchanges in the Fauna-Sediment System. The Case of Nereis Diversicolor and Scrobicularia Plana in the BouRegreg Estuary (Morocco). Hydrobiologia 1990, 207, 209–219. [Google Scholar] [CrossRef]
- Langston, W.J. Metals in Sediments and Benthic Organisms in the Mersey Estuary. Estuar. Coast. Shelf Sci. 1986, 23, 239–261. [Google Scholar] [CrossRef]
- Dora, E.Ç.; Sunlu, U.; Ergen, Z. Heavy Metal Concentrations In Hedistediversicolor (Polychaeta) and Sediments from Homa Lagoon (Izmir Bay-Turkey). Rapp. Comm. Int. Mer. Medit. 2007, 38, 253. [Google Scholar]
- Fattorini, D.; Notti, A.; Nigro, M.; Regoli, F. Hyperaccumulation of vanadium in the Antarctic polychaete Perkinsianalittoralis as a natural chemical defense against predation. Environ. Sci. Pollut. Res. 2010, 17, 220–228. [Google Scholar] [CrossRef]
- Boust, D.; Fischer, J.C.; Poulin, M.C.; Rozet, M.C.; Voyer, J.J.C.; Ouddane, B.C.; Petit, F.; Wartel, M.; Abarnou, A.; Ficht, A.; et al. Programme Scientifique Seine-Aval (9, Fer et Manganèse: Réactivités et Recyclages); 1999; p. 39. ISBN 978-2-84433-022-2. Available online: https://www.librairiedialogues.fr/livre/199930-programme-scientifique-seine-aval-9-9-fer-et-manganese-reactivites-et-recyclages-dominique-boust-quae (accessed on 23 May 2025).
- Rand, G.M. Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment; CRC Press: Boca Raton, FL, USA, 1995; p. 1148. ISBN 9781003075363. [Google Scholar]
- Amiard-Triquet, C.; Rainbow, P.S. Environmental Assessment of Estuarine Ecosystems: A Case Study, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9780429142451. [Google Scholar]
- Kaimoussi, A.; Chafik, A.; Mouzdahir, A.; Bakkas, S. The Impact of Industrial Pollution on the JorfLasfarCoastal Zone (Morocco, Atlantic Ocean): The Mussel as an Indicator of Metal Contamination. Comptes Rendus L’académie Sci.-Ser. IIA-Earth Planet. Sci. 2001, 333, 337–341. [Google Scholar] [CrossRef]
- Bouthir, F.Z.; Chafik, A.; Benbrahim, S.; Souabi, S.; Mardhy, H.; Messoudi, A. Qualité physicochimique des eaux côtières du littoral de la Wilaya du grand Casablanca (océan Atlantique marocain) utilisant la moule Mytilus galloprovincialis comme indicateur de la contamination métallique. Mar. Life 2004, 14, 59–70. [Google Scholar]
- Benbrahim, S.; Chafik, A.; Chfiri, R.; Zohra Bouthir, F.; Siefeddine, M.; Makaoui, A. Etude des facteurs influençant la répartition géographique et temporelle de la contamination des côtes atlantiques marocaines par les métaux lourds: Cas du mercure, du plomb et du cadmium. Mar. Life. 2006, 16, 37–47. [Google Scholar]
- Fattorini, D.; Notti, A.; Di Mento, R.; Cicero, A.M.; Gabellini, M.; Russo, A.; Regoli, F. Seasonal, Spatial and Inter-annual variations of trace metals in mussels from the adriatic sea: A regional gradient for arsenic and implications for monitoring the impact of off-shore activities. Chemosphere 2008, 72, 1524–1533. [Google Scholar] [CrossRef]
- Rouane-Hacene, O.; Boutiba, Z.; Belhaouari, B.; Guibbolini-Sabatier, M.E.; Francour, P.; Risso-de Faverney, C. Seasonal assessment of biological indices, bioaccumulation and bioavailability of heavy metals in mussels Mytilus galloprovincialis from Algerian west coast, applied to environmental monitoring. Oceanologia 2015, 57, 362–374. [Google Scholar] [CrossRef]
- Langston, W.J.; Spence, S.K. Biological factors involved in metal concentrations observed in aquatic organisms. Metal. Speciat. Bioavailab. Aquat. Syst. 1995, 3, 407–478. [Google Scholar]
- Batten, S.D.; Bamber, R.N. The effects of acidified seawater on the polychaete Nereisvirenssars, 1835. Mar. Pollut. Bull. 1996, 32, 283–287. [Google Scholar] [CrossRef]
- Amiard, J.C.; Geffard, A.; Amiard-Triquet, C. La métallothionéine chez la moule Mytilusedulis comme biomarqueur de pollution métallique: Variabilité entre sites, saisons et organes. J. Rech. Océanogr. 1998, 23, 25–30. [Google Scholar]
- Boening, D.W. An Evaluation of bivalves as biomonitors of heavy metals pollution in marine waters. Environ. Monit. Assess 1999, 55, 459–470. [Google Scholar] [CrossRef]
- Casas, S.; Bacher, C. Modelling Trace metal (Hg and Pb) bioaccumulation in the mediterranean mussel, Mytilusgalloprovincialis, applied to environmental monitoring. J. Sea Res. 2006, 56, 168–181. [Google Scholar] [CrossRef]
- Stankovic, S.; Jovic, M.; Stankovic, A.R.; Katsikas, L. Heavy Metals in Seafood Mussels. Risks for Human Health. In Environmental Chemistry for a Sustainable World; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 311–373. ISBN 9789400724419. [Google Scholar]
- Perošević, A.; Pezo, L.; Joksimović, D.; Đurović, D.; Milašević, I.; Radomirović, M.; Stanković, S. The impacts of seawater physicochemical parameters and sediment metal contents on trace metal concentrations in mussels—A chemometric approach. Environ. Sci. Pollut. Res. 2018, 25, 28248–28263. [Google Scholar] [CrossRef] [PubMed]
- Yaramaz, O.; Mordogan, H.; Sunlu, U.; Onen, M. A comparative study on some heavy metal concentrations (Zn, Cu, Pb, Cd, Ni, Cr) in the sediments: From Homa (Izmir) and Karine (Aydin-Türkiye) Fisheries Lagoons. Rapp. Comm. Int. Mer Médit. 1992, 33, 87. [Google Scholar]
- Egemen, O.; Sunlu, U.; Kaymakci, A. Heavy metal concentrations in some molluscs and in surficial sediments from izmir bay/tùrkey. Rapp. Comm. Int. Mer Médit. 1998, 35, 250. [Google Scholar]
- Sunlu, U. Comparison of heavy metal levels in native and cultured mussel Mytilus galloprovincialis (L., 1758) from the Bay of Izmir (Aegean Sea/Turkey). Mediterranean Mussel Watch. CIESM Workshop Ser. 2002, 15, 101–103. Available online: https://ciesm.org/online/monographs/15/WM_15_101_103.pdf (accessed on 1 April 2024).
- Eça, G.F.; Pedreira, R.M.A.; Hatje, V. Trace and Major Elements Distribution and Transfer within a Benthic System: Polychaete Chaetopterus Variopedatus, Commensal Crab Polyonyx Gibbesi, Worm Tube, and Sediments. Mar. Pollut. Bull. 2013, 74, 32–41. [Google Scholar] [CrossRef]
- Davydkova, I.L.; Fadeeva, N.P.; Kovekovdova, L.T.; Fadeev, V.I. Heavy metal contents in tissues of dominant species of the benthos and in bottom sediments of zolotoirog bay, sea of Japan. Russ. J. Mar. Biol. 2005, 31, 176–180. [Google Scholar] [CrossRef]
- Alam, M.A.; Gomes, A.; Sarkar, S.K.; Shuvaeva, O.V.; Vishnevetskaya, N.S.; Gustaytis, M.A.; Bhattacharya, B.D.; Godhantaraman, N. Trace Metal Bioaccumulation by Soft-Bottom Polychaetes (Annelida) of Sundarban Mangrove Wetland, India and Their Potential Use as Contamination Indicator. Bull. Environ. Contam. Toxicol. 2010, 85, 492–496. [Google Scholar] [CrossRef]
- Casado-Martinez, M.C.; Smith, B.D.; DelValls, T.A.; Luoma, S.N.; Rainbow, P.S. Biodynamic Modelling and the Prediction of Accumulated Trace Metal Concentrations in the Polychaete Arenicola Marina. Environ. Pollut. 2009, 157, 2743–2750. [Google Scholar] [CrossRef]
- Casado-Martinez, M.C.; Smith, B.D.; Luoma, S.N.; Rainbow, P.S. Bioaccumulation of Arsenic from Water and Sediment by a Deposit-Feeding Polychaete (Arenicola marina): A Biodynamic Modelling Approach. Aquat. Toxicol. 2010, 98, 34–43. [Google Scholar] [CrossRef]
- Berthet, B.; Mouneyrac, C.; Amiard, J.C.; Amiard-Triquet, C.; Berthelot, Y.; Le Hen, A.; Mastain, O.; Rainbow, P.S.; Smith, B.D. Accumulation and Soluble Binding of Cadmium, Copper, and Zinc in the Polychaete Hediste diversicolor from Coastal Sites with Different Trace Metal Bioavailabilities. Arch. Environ. Con. Tox. 2003, 45, 468–478. [Google Scholar] [CrossRef]
- Orabi, O.; Khalifa, M.M. Biota Sediment Accumulation and Bioconcentration Factors of Trace Metals in the Snail Melanoides tuberculata Form the Agricultural Drains of the Manzala Lagoon, Egypt. Environ. Sci. Pollut. Res. 2020, 27, 17754–17761. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Pi, J.; Yu, J.; Zhang, T. Biota-sediment metal accumulation and human health risk assessment of freshwater bivalve Corbicula fluminea in Dongting Lake, China. Environ. Sci. Pollut. Res. 2019, 26, 14951–14961. [Google Scholar] [CrossRef] [PubMed]
- El Zrelli, R.; Yacoubi, L.; Wakkaf, T.; Castet, S.; Grégoire, M.; Mansour, L.; Courjault-Radé, P.; Rabaoui, L. Surface Sediment Enrichment with Trace Metals in a Heavily Human-Impacted Lagoon (Bizerte Lagoon, Southern Mediterranean Sea): Spatial Distribution, Ecological Risk Assessment, and Implications for Environmental Protection. Mar. Pollut. Bull. 2021, 169, 112512. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, Z.; Ayadi, H. Heavy Metal Accumulation in Diplodus annularis, Liza aurata, and Solea vulgaris Relevant to Their Concentration in Water and Sediment from the Southwestern Mediterranean (Coast of Sfax). Environ. Sci. Pollut. Res. 2016, 23, 13895–13906. [Google Scholar] [CrossRef] [PubMed]
- El Zrelli, R.; Courjault-Radé, P.; Rabaoui, L.; Castet, S.; Michel, S.; Bejaoui, N. Heavy Metal Contamination and Ecological Risk Assessment in the Surface Sediments of the Coastal Area Surrounding the Industrial Complex of Gabes City, Gulf of Gabes, SE Tunisia. Mar. Pollut. Bull. 2015, 101, 922–929. [Google Scholar] [CrossRef]
- Rabaoui, L.; El Zrelli, R.; Ben Mansour, M.; Balti, R.; Mansour, L.; Tlig-Zouari, S.; Guerfel, M. On the Relationship between the Diversity and Structure of Benthic Macroinvertebrate Communities and Sediment Enrichment with Heavy Metals in Gabes Gulf, Tunisia. J. Mar. Biol. Ass. 2015, 95, 233–245. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A. A Review of Bioconcentration Factor (BCF) and Bioaccumulation Factor (BAF) Assessments for Organic Chemicals in Aquatic Organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Melake, B.A.; Endalew, S.M.; Alamirew, T.S.; Temesegen, L.M. Bioaccumulation and Biota-Sediment Accumulation Factor of Metals and Metalloids in Edible Fish: A Systematic Review in Ethiopian Surface Waters. Environ. Health Insights 2023, 17, 11786302231159349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).