Abstract
The environmental drivers of fin whale (Balaenoptera physalus) acoustic presence in Eastern Antarctic waters were investigated based on passive acoustic recordings from four sites, 2013–2019. Fin whale 20 Hz pulses were detected from late austral summer to early winter. Daily values of sea-ice concentration (SIC) were compared with the number of days with fin whale 20 Hz acoustic presence using a generalized additive model approach. At the Southern Kerguelen Plateau, Casey, and Dumont d’Urville sites, SIC correlated with fin whale calling activity, but less so at the Prydz site. Changes in SIC between sites resulted in variation in acoustic presence: Earlier sea-ice formation at Dumont d’Urville resulted in less acoustic presence in comparison to the Southern Kerguelen Plateau, where sea ice formed later in the season. Interannual variability in SIC impacted yearly acoustic presence, with a later onset of high SIC resulting in greater acoustic presence and later departure (migration timing) of the animals. Identifying the environmental drivers of fin whale presence is key to informing how this migratory species may be affected by environmental variability resulting from climate change.
1. Introduction
The fin whale (Balaenoptera physalus) is listed as ‘vulnerable’ on the IUCN red list [1] after a severe population decline during the industrial whaling era [2]. The species faces an ongoing threat from climate change [3]. Similar to other baleen whales, the fin whale is thought to utilize high-latitude, polar waters during the summer for feeding before migrating to low-latitude waters during the winter for calving and breeding [4,5]. Analysis of fin whale vocalizations from passive acoustic monitoring programs has revealed trends in their acoustic presence throughout the world’s oceans [6,7,8,9,10,11].
The 20 Hz pulse is the most widely identified and commonly reported vocalization of the fin whale. It is a short 1 s pulse that sweeps from 42 to 18 Hz [12,13]. The 20 Hz pulse is thought to be associated with a range of behaviors of the animals. Highly repetitive, stereotyped sequences (songs) of 20 Hz pulses are likely a reproductive display [12] only produced by males [14], while single, irregular 20 Hz pulses are suggested to be emitted by all demographic cohorts, in association with social behavior [6]. Production of 20 Hz pulses might also be indirectly associated with foraging, as the animals call more when not diving for prey [15,16].
More recently, studies have begun investigating environmental variables affecting fin whale acoustic presence [7,9,17,18]. These studies identify a range of regional-specific environmental drivers. For example, in the North Atlantic, off the Azores, fin whale acoustic activity was affected by water depth and temperature [17]. In the Chukchi Sea, fin whale acoustic presence was affected by wind, current velocity, and sea-surface temperature (SST) [7]. In the North Pacific, only SST affected fin whale acoustic presence [8]. In the Southern Hemisphere, off South Africa, fin whale acoustic presence was affected by chlorophyll-a (Chl-a) concentration, SST, and wind speed [9,18]. In polar regions, such as in Western Antarctica, the distance to the sea-ice edge was reported as an important driver of fin whale presence [19].
The Antarctic marine environment is affected by the Antarctic Circumpolar Current and seasonal sea-ice formation. This current generates nutrient upwelling and acts as a thermal barrier, isolating Antarctica from warmer northern waters [20,21]. This results in consistently cold water temperatures, ranging from 2 °C to −2 °C [20]. These cold waters promote perennial sea ice, along with seasonal sea-ice formation. The dynamics of seasonal sea ice have an important effect on the ecosystem, both during the growth and melting stages. In Eastern Antarctic waters, seasonal sea ice extends from a minimum of 800,000 km2 in summer to a maximum of 6.4 million km2 in winter [22]. This extended sea-ice coverage effects changes in water temperature and salinity [22] and provides shelter and access to food sources for krill species [23]. Seasonal sea-ice melts from October to the austral summer, releasing nutrients that enhance Chl-a concentration [24,25]. Chl-a is considered a proxy for primary productivity in Antarctic waters [24] as it has been reported to drive zooplankton and Antarctic krill (Euphausia superba) distribution, which require high Chl-a concentrations [26,27]. The Antarctic marine ecosystem is inhabited by many whale species taking advantage of this high productivity resulting from these seasonal fluctuations [28].
In Eastern Antarctic waters, seasonal fin whale acoustic presence has been identified across four sites (Prydz, Southern Kerguelen Plateau, Casey, and Dumont d’Urville) from late austral summer to early winter [11]. However, acoustic presence varied between years at each site and between the four sites. High acoustic presence of fin whales was observed at the Southern Kerguelen Plateau as opposed to low acoustic presence at Casey until 2019 when acoustic presence increased [11]. The animals are thought to utilize these areas for feeding before migrating north to Australian waters where they are present from austral autumn to mid-spring [10,11]. No studies are available on environmental variables affecting the acoustic presence and migration of Eastern Antarctic fin whale populations. In this study, we test the hypothesis that yearly acoustic presence of the fin whale 20 Hz pulse in Eastern Antarctic waters is influenced by sea-ice concentration (SIC). Identification of any environmental drivers of fin whale presence is key to understanding how this migratory species may be affected by environmental variability resulting from climate change and help inform management of this vulnerable species.
2. Materials and Methods
2.1. Acoustic Data and Pulse Detections
Passive acoustic data were collected north of Prydz Bay at the Southern Kerguelen Plateau, north of the Casey research station, and off the Dumont d’Urville research station in Eastern Antarctic waters between 2013 and 2019, using moored acoustic recorders of the Australian Antarctic Division (Figure 1, Table 1) [29]. The receivers recorded continuously at a sampling frequency of 12 kHz; however, recording effort varied between these four sites (Table 1).

Figure 1.
Locations of underwater acoustic recording; equidistant conic projection.

Table 1.
Site and year, latitude, longitude, start and end dates, cumulative recording hours (effort), and deployment depth for Antarctic acoustic recordings.
A total of 575 d and 4549 h with fin whale 20 Hz acoustic presence was previously detected across all these sites and recording years (Table 2, Figure 2) [11]. The workflow combined automatic detection with manual verification of all detections, removal of false alarms, search for missed detections within 3 h of validated detections, and insertion of automatically missed detections (see details in [10,11]). A pattern of seasonal fin whale acoustic presence was identified across these four Eastern Antarctic sites: at Prydz from late January to May, at the Southern Kerguelen Plateau from February to June, at Casey from February to May, and at Dumont d’Urville from February to June (Table 2) [11].

Table 2.
Fin whale acoustic presence at all recording site years, including first and last 20 Hz pulse detected as well as total days and hours with acoustic presence.
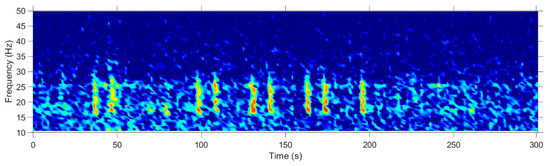
Figure 2.
Spectrogram example of a fin whale 20 Hz pulse sequence from Casey (28-Mar-2014, 11:00). Colors indicate relative acoustic power (low: blue; high: red), plotted versus time and frequency. Spectrogram was calculated in 2048-point Hann windows, 0.59 Hz frequency resolution, and sampling frequency was 12 kHz.
2.2. Detection Range Estimation
Detection ranges of fin whale 20 Hz pulses were estimated for each site in Eastern Antarctica to provide an area for environmental variable analysis. Propagation loss was modeled with RAMGeo software [30] in AcTUP [31]. RAMGeo required the following input parameters: The bathymetry at each site was extracted from the ETOPO2 v2 global relief data [32] along four bearings (north, south, east, and west) from the hydrophone location. Sound-speed profiles were extracted from the GLORYS12V1 product of the E.U. Copernicus Marine Service at each site for the months with peak fin whale acoustic presence [11,33]. Geoacoustic properties of a silt seafloor were used, as described by [34]. The depth of the calling whale was assumed to be 30 m, as fin whales vocalize between 10 m and 50 m [12,35]. The source level of a fin whale 20 Hz pulse was taken as 185 dB re 1 µPa2 m2 at 30 m depth [36], and the ambient noise level at 20 Hz was assumed to be 90 dB re 1 µPa2/Hz in Antarctic waters [37]. The principle of reciprocity was applied, equating the loss from the acoustic receiver to the whale to that from the calling whale to the receiver [34]. The propagation loss was smoothed over range and the average (over the four bearings) detection range for each site was calculated as 36 km at Prydz, 45 km at the Southern Kerguelen Plateau, 39 km at Casey, and 43 km at Dumont d’Urville.
2.3. Environmental Variables
Satellite-derived daily SIC (%) was obtained from the Advanced Microwave Scanning Radiometer-2 (AMSR-2) with a 6.25 km grid resolution [38], using the R package raadtools, version 0.6.0.9024 [39]. Daily measurements of SIC were then averaged over a 100 km 100 km grid (i.e., 50 km in each cardinal direction, rounded up to reduce zeros in environmental samples) around the average hydrophone deployment location at each site, providing daily mean values. The estimated detection ranges (36–45 km) support this 100 km 100 km grid choice around each hydrophone location.
Additional environmental variables were also initially considered as potential drivers of whale acoustic presence. Daily SST variation data were obtained from Optimum Interpolation Sea-Surface Temperature (OISST) products based on Advanced Very High Resolution Radiometer (AVHRR) infrared satellite SST data with a grid resolution of 0.25°. The OISST product also incorporates SST observations from ships and buoys, as well as proxy SSTs generated from SIC [40]. Although SST is ecologically relevant, we opted not to include it as a predictor in the models due to its strong physical and statistical dependence on SIC [41,42,43]. Including both variables risks redundancy and may obscure the distinct ecological signal provided by SIC, which is more directly relevant to the species and processes under study. However, we recognize the importance of SST in characterizing the Antarctic environment and therefore retain it in our descriptive analysis to contextualize spatial and temporal variability across regions. Other environmental variables, such as Chl-a concentration and sea-surface salinity, were also considered due to their potential ecological relevance. However, like SST, these variables may have confounding effects with SIC. Moreover, their spatial and temporal coverage was highly limited across study sites, preventing their consistent inclusion in the analysis.
2.4. Statistical Analysis
To explore the seasonal pattern in whale acoustic presence, we determined the days with fin whale 20 Hz pulses detected in at least one hour of each 24 h period (value of 1 for presence and 0 for absence), representing a daily binomial response variable. The models were constructed separately for each site because sampling was conducted unevenly in different years. The SIC, Julian day, and Year variables were considered as the main predictors affecting the acoustic presence of whales. Variance inflation factor (VIF) analysis was implemented using the R package car, version 3.1-3 [44], to assess potential collinearity between predictors. Low to moderate collinearity was observed between predictors across each site (range 1.02–4.72) and therefore all predictors considered in the models. Since the influence of SIC and Julian day on fin whale acoustic presence may be non-linear, we used a generalized additive model (GAM) with binomial distributions (link function) for each site with the R package mgcv, version 1.9-3 [45,46]. We estimated smoothed parameters with the restricted maximum likelihood method (REML) for the best fit of data with thin plate regression splines. Interaction effects between SIC and Year were also tested in candidate models by including interaction terms. Year was included as a fixed factor in models for sites that had multiple recording years (i.e., Southern Kerguelen Plateau, Casey, and Dumont d’Urville). Finally, the significance of a random effect of Year on the response was further tested using mixed-effects GAMs (i.e., GAMM). The inclusion of each explanatory variable and the best model selection were assessed with Akaike’s information criterion. Model fits were visually inspected for inconsistencies using diagnostic Q-Q plots. The resulting best models for each site were illustrated as yearly predictions of the probability of acoustic presence and by plotting the smoothed estimates, when appropriate.
3. Results
3.1. Acoustic Whale Presence and Environmental Dynamics
At Prydz, located in the Indian sector of Antarctica, data availability was limited to a single year, during which very few fin whale acoustic presence hours (FWPH) were recorded, particularly under high SIC (Figure 3). In contrast, at the Southern Kerguelen Plateau, SIC tended to rise later in the season (May–June), with a maximum SIC of 26.8% recorded alongside 3 FWPH in mid-June 2019 (Figure 3). During the fin whale presence period at this site, SST generally declined from 2.19 °C (February 2017) to −1.61 °C (June 2016). At Casey, 2019 showed notably higher FWPH compared to other years, coinciding with much lower SIC (Figure 3). An anomalous FWPH event was observed in late May 2019 under 64.9% SIC and −1.48 °C SST, while all other detections that year occurred when SIC remained at 0% and SST was at −1.26 °C. At Dumont d’Urville, FWPH was consistently high from late February to mid-March in both years, then declined sharply as SIC increased (Figure 3). Maximum SIC during this transition reached 42.4% in 2018 and 8.6% in 2019, with late-season detections occurring under much higher SICs (up to 92.9%). The highest and lowest SST values associated with FWPH at Dumont d’Urville were 1.78 °C (February 2019) and −1.79 °C (June 2018), respectively.

Figure 3.
Daily values of sea-surface temperature (SST), sea-ice concentration (SIC), and total fin whale presence hours (FWPH) across acoustic recording years at each site. X-axis outlines the fin whale seasonal presence period for each site.
3.2. Drivers of Fin Whale Acoustic Presence
Model selection using GAMs/GAMMs identified different best-fitting models for each site, prioritizing simpler models that sufficiently captured the data patterns (Table 3). At the Prydz site, high concurvity (0.87) between smooth terms indicated poor separation of effects, so the smooth term for SIC was replaced with a fixed linear effect. At the Southern Kerguelen Plateau and Casey sites, models including Year as a random effect or as an interaction term with SIC did not improve model fit or resulted in non-significant effects. Similarly, at Dumont d’Urville, including Year did not significantly improve model performance, and a simpler model was therefore selected. Additionally, the estimated degrees of freedom for smoothed SIC were consistently close to the basis dimension (k′), suggesting that SIC had a near-linear effect in all cases. This pattern was robust to changes in k′, justifying the use of a fixed linear term for SIC in all final models.

Table 3.
Ranked models identifying factors influencing the probability of fin whale presence across four sites in Eastern Antarctic waters. Models are ranked by Akaike’s information criterion (AIC), with the best-supported, most parsimonious models shown in bold. d.f. = degrees of freedom; Dev. = explained deviance (%); SIC = sea-ice concentration (%).
At Prydz, the smallest dataset with only one year of recording showed no significant effect of SIC on fin whale acoustic presence (Table 4). In contrast, a general pattern of increased fin whale acoustic presence under lower SIC conditions was evident across the Southern Kerguelen Plateau, Casey, and Dumont d’Urville sites (Table 4, Figure 4), suggesting that increasing sea ice may consistently limit fin whale presence across multiple regions. Although a general pattern is evident, yearly predictions for the Southern Kerguelen Plateau and Casey sites reveal some degree of interannual variation in the relationship between the probability of acoustic whale detection and SIC. Regarding the timing of acoustic whale presence, the smooth effects of Julian day indicate strong variation between sites, with a positive association that shifts across study periods (Figure 5), suggesting that whales arrive at different times depending on the location.

Table 4.
Best generalized additive models (GAMs) describing the probability of fin whale presence across four sites in Eastern Antarctic waters. edf = estimated degrees of freedom; s = smooth function. Significant effects are shown in bold.
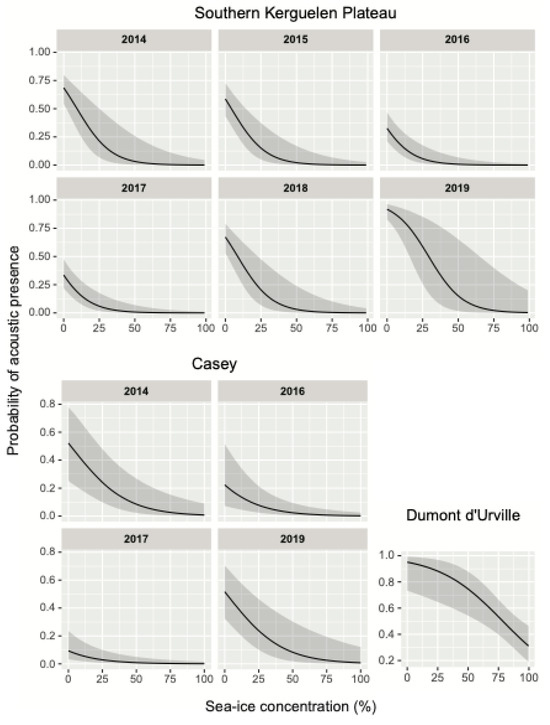
Figure 4.
Generalized additive model predictions for sea-ice concentration (%) obtained for fin whale acoustic presence in each recording year at the Southern Kerguelen Plateau, Casey, and Dumont d’Urville sites. The approximate 95% confidence intervals based on 2 standard deviations are indicated (gray shading).
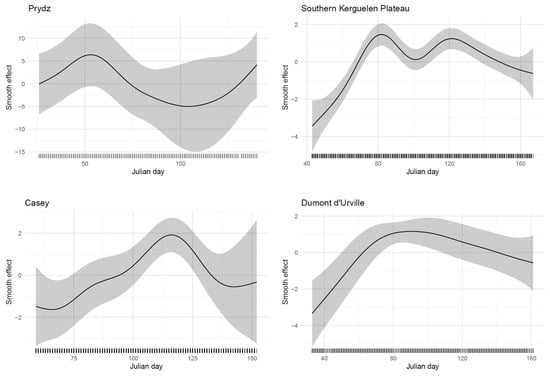
Figure 5.
Generalized additive model smooth effects for Julian day obtained for fin whale acoustic presence per site. The approximate 95% confidence intervals are indicated (gray shading).
4. Discussion
This study confirms a reduction in fin whale acoustic presence in Eastern Antarctic waters when SIC increases. SIC particularly drove fin whale acoustic presence at the Southern Kerguelen Plateau, Casey, and Dumont d’Urville—the richest sites in data. This resulted in interannual variation in the presence and migration timing of the animals as they likely sought to avoid sea-ice cover and disperse out of the area.
For example, at the Southern Kerguelen Plateau, when high SIC occurred earlier in the year (2015), the last detection of a fin whale pulse occurred the earliest across all years. In contrast, in 2019, high SIC occurred later in the season and fin whale acoustic presence was greater, with the latest detection date and the greatest presence days and hours across all years. This pattern of seasonal SIC driving the acoustic presence of fin whales was equally easily seen at Casey, where in 2019, high SIC occurred later in the season, driving high acoustic presence and a later cessation of acoustic presence. From 2014 to 2017, high SIC occurred earlier in the season at Casey, leading to very limited (mostly absent) fin whale acoustic presence. At Dumont d’Urville, the earlier onset of high SIC in 2018 resulted in fewer acoustic presence days and hours. Prydz had only one year of recording and thus, low statistical support in the model; however, the general pattern was observed in that the majority of fin whale acoustic presence had ceased prior to any sea-ice formation at this site. In the future, additional acoustic recording at the Prydz site could be informative.
Comparing spatially across sites, Prydz, Casey, and Dumont d’Urville are closer to the Antarctic continent than the Southern Kerguelen Plateau is, and therefore, these three sites ice up earlier in the year than the Southern Kerguelen Plateau does. The observed effect of SIC on fin whale acoustic presence highlights the impact of site-specific sea-ice coverage, resulting in varying spatial and temporal fin whale acoustic presence between these regions of Eastern Antarctica.
The relationship between fin whale presence and the ice edge is confirmed by non-acoustic data. Data from 20th century whaling [47,48] and circum-Antarctic visual surveys [49] provide evidence that fin whales were encountered and taken throughout the high latitudes of the Southern Hemisphere all the way to the edge of the sea ice in summer and autumn. El-Gabbas et al. [50] compiled archival sightings data to build a mysticete species distribution model for the Southern Ocean, which identified SIC as the main driver. Our contemporary acoustic results are in accord with those from archival visual datasets. Darkness, ice cover, remoteness, and wind have historically presented barriers to accessing winter Antarctic waters and precluded collection of visual data on whale distribution at this time.
Though Antarctic baleen whales are generally believed to be migratory, there have been indications in other regions that blue, humpback, and minke whales can be found within winter and spring sea ice in Western Antarctic waters [51,52]. Long-term passive acoustics at high latitude provided a means to conduct such winter investigations, assuming that—like blue and humpback whales—overwintering fin whales remained vocal.
An inherent limitation of passive acoustic monitoring is that it can only provide information on vocal animals. At Elephant Island, Western Antarctica, fin whales were visually sighted earlier in the season than acoustically recorded, likely because they arrived for feeding, during which they do not produce their 20 Hz song [53]. Therefore, our study provides no means to rule out or disprove the presence of silent animals. However, Aulich et al. [16] demonstrated that fin whales vocalize at other latitudes adjacent to Eastern Antarctic waters in winter. Their acoustic presence in winter at ice-free latitudes, and absence in ice-covered latitudes makes it seem likely that few, if any, individuals are present within winter sea ice in this region. Our absence of fin whale vocalization in sea ice off East Antarctica are in accord with those of Širović et al. [51] from West Antarctica for this species.
While SIC was an important predictor of fin whale acoustic presence, it is correlated with SST, and likely to be correlated with other environmental and biological processes not accounted for in this study (such as Chl-a and salinity, for which data in our region was limited). Santora et al. [54] built a GAM for fin whale sightings in West Antarctica, identifying a relationship with SST, eddy kinetic energy, and krill biomass. Antarctic krill are highly sensitive to environmental conditions [55], with their abundance and growth directly influenced by SST and Chl-a [3]. Antarctic krill is a key prey source for many cetacean species in Antarctic waters, including the fin whale [4], and aggregations of krill have been identified throughout the Indian sector of Antarctica [56,57]. Further investigation into the primary productivity and krill density at our four recording sites may provide additional insight into drivers of fin whale presence at these locations.
Changes in the environmental conditions in Antarctic waters due to climate change pose a threat to the Antarctic marine ecosystem, including fin whales, which time their arrival to coincide with great prey biomass [53,54]. At the Western Antarctic Peninsula, summer SST has reportedly risen by >1 °C since the 1950s [55], and forecast models predict SST increasing by a further ~0.5–1.0 °C by the end of this century [20]. This increase in SST has resulted in a decline in sea-ice extent and duration in this region of Antarctica [20,58,59]. Whilst a trend of yearly increasing sea-ice extent and concentration was reported in regions of the Indian and Pacific sectors of Antarctica [58,60], strong interannual and regional variability was also identified [60,61]. Yearly positive and negative trends in sea-ice extent around Prydz Bay and strong regional differences between this location and waters off the Casey and Dumont d’Urville research stations have been reported [61].
Rising SST and a decline in sea-ice coverage have impacted the distribution and migration timing of fin whale populations in the Northern Hemisphere [62]. In the Gulf of St. Lawrence, Canada, fin whales were arriving earlier each year to their summer feeding grounds by 1 day and exhibited an extended seasonal presence of 16 d/y [63]. Around the Svalbard Archipelago, a change in fin whale distribution was reported, with animals shifting further north each year by 1° [64].
5. Conclusions
As fin whale acoustic presence was affected by SIC in Eastern Antarctic waters, any future short-term (e.g., interannual variability) or long-term (e.g., climate change) changes in this environmental condition will likely affect how fin whales utilize their seasonal Antarctic habitat. A long-term decrease in SIC might temporarily increase fin whale acoustic presence, but the critical question is whether such habitat changes can support fin whale (feeding) ecology in the long term. Ongoing passive acoustic monitoring might be an efficient way of tracking fin whale presence in Antarctic waters and inform future management of this vulnerable species.
Author Contributions
Conceptualization, M.G.A., B.S.M., R.D.M., B.J.S. and C.E.; data curation, B.S.M. and F.S.; methodology, M.G.A., B.S.M., B.J.S., A.M.D.W. and C.D.S.T.; formal analysis, M.G.A. and A.M.D.W.; writing—original draft preparation, M.G.A.; writing—review and editing, C.E., B.S.M., B.J.S., A.M.D.W., R.D.M. and C.D.S.T.; supervision, B.S.M., R.D.M., B.J.S. and C.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The passive acoustic data that support the findings of this study are available from the Australian Antarctic Division: https://data.aad.gov.au/metadata/records/AAS_4102_longTermAcousticRecordings (accessed on 1 March 2021). The SST and SIC data are available from the RStudio Package raadtools: https://github.com/AustralianAntarcticDivision/raadtools (accessed on 1 March 2021).
Acknowledgments
The Australian Antarctic Division (AAD) provided underwater acoustic recordings made under the Australian Antarctic Science Projects 4101, 4102, and 4600 and the International Whaling Commission’s Southern Ocean Research Partnership (IWC-SORP) Southern Ocean Hydrophone Network (SOHN). The Dumont d’Urville deployments were possible with the support of the Institut Polaire Français Paul Emile Victor under the program SOHN, AREA V (http://dx.doi.org/10.18142/313).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cooke, J.G. Balaenoptera physalus. The IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Cambridge UK, 2018; p. e.T2478A50349982. Available online: https://www.iucnredlist.org/species/2478/50349982 (accessed on 20 April 2025).
- Rocha, R.C.; Clapham, P.J.; Ivashchenko, Y.V. Emptying the oceans: A summary of industrial whaling catches in the 20th century. Mar. Fish. Rev. 2014, 76, 37–48. [Google Scholar] [CrossRef]
- Tulloch, V.J.D.; Plagányi, É.E.; Brown, C.; Richardson, A.J.; Matear, R. Future recovery of baleen whales is imperiled by climate change. Glob. Chang. Biol. 2019, 25, 1263–1281. [Google Scholar] [CrossRef] [PubMed]
- Mizroch, S.A.; Rice, D.W.; Breiwick, J.M. The fin whale, Balaenoptera physalus. Mar. Fish. Rev. 1984, 46, 20–24. [Google Scholar]
- Aguilar, A.; García-Vernet, R. Fin whale: Balaenoptera physalus. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 368–371. [Google Scholar]
- McDonald, M.A.; Hildebrand, J.A.; Webb, S.C. Blue and fin whales observed on a seafloor array in the Northeast Pacific. J. Acoust. Soc. Am. 1995, 98, 712–721. [Google Scholar] [CrossRef]
- Escajeda, E.; Stafford, K.M.; Woodgate, R.A.; Laidre, K.L. Variability in fin whale (Balaenoptera physalus) occurrence in the Bering Strait and southern Chukchi Sea in relation to environmental factors. Deep-Sea Res. II Top. Stud. Oceanogr. 2020, 177, 104782. [Google Scholar] [CrossRef]
- Stafford, K.M.; Citta, J.J.; Moore, S.E.; Daher, M.A.; George, J.E. Environmental correlates of blue and fin whale call detections in the North Pacific Ocean from 1997 to 2002. Mar. Ecol. Prog. Ser. 2009, 395, 37–53. [Google Scholar] [CrossRef]
- Shabangu, F.W.; Findlay, K.P.; Yemane, D.; Stafford, K.M.; Van den Berg, M.; Blows, B.; Andrew, R.K. Seasonal occurrence and diel calling behaviour of Antarctic blue whales and fin whales in relation to environmental conditions off the west coast of South Africa. J. Mar. Syst. 2019, 190, 25–39. [Google Scholar] [CrossRef]
- Aulich, M.G.; McCauley, R.D.; Saunders, B.J.; Parsons, M.J.G. Fin whale (Balaenoptera physalus) migration in Australian waters using passive acoustic monitoring. Sci. Rep. 2019, 9, 8840. [Google Scholar] [CrossRef]
- Aulich, M.G.; McCauley, R.D.; Miller, B.S.; Samaran, F.; Giorli, G.; Saunders, B.J.; Erbe, C. Seasonal distribution of the fin whale (Balaenoptera physalus) in Antarctic and Australian waters based on passive acoustics. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Watkins, W.A.; Tyack, P.; Moore, K.E.; Bird, J.E. The 20-Hz signals of finback whales (Balaenoptera physalus). J. Acoust. Soc. Am. 1987, 82, 1901–1912. [Google Scholar] [CrossRef]
- Thompson, P.O.; Findley, L.T.; Vidal, O. 20-Hz pulses and other vocalizations of fin whales, Balaenoptera physalus, in the Gulf of California, Mexico. J. Acoust. Soc. Am. 1992, 92, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Croll, D.A.; Clark, C.W.; Acevedo, A.; Tershy, B.; Flores, S.; Gedamke, J.; Urban, J. Only male fin whales sing loud songs. Nature 2002, 417, 809. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, J.F.; Stredulinsky, E.; Abernethy, R.M.; Ford, J.K. Patterns of Fin Whale (Balaenoptera physalus) Seasonality and Relative Distribution in Canadian Pacific Waters Inferred from Passive Acoustic Monitoring; Canadian Science Advisory Secretariat, Ottawa, Canada. 2018. Available online: https://publications.gc.ca/collections/collection_2018/mpo-dfo/fs70-5/Fs70-5-2018-032-eng.pdf (accessed on 20 April 2025).
- Aulich, M.G.; Miller, B.S.; Samaran, F.; McCauley, R.D.; Saunders, B.J.; Erbe, C. Diel patterns of fin whale 20 Hz acoustic presence in Eastern Antarctic waters. R. Soc. Open Sci. 2023, 10, 220499. [Google Scholar] [CrossRef]
- Pérez-Jorge, S.; Tobeña, M.; Prieto, R.; Vandeperre, F.; Calmettes, B.; Lehodey, P.; Silva, M.A. Environmental drivers of large-scale movements of baleen whales in the mid-North Atlantic Ocean. Divers. Distrib. 2020, 26, 683–698. [Google Scholar] [CrossRef]
- Letsheleha, I.S.; Shabangu, F.W.; Farrell, D.; Andrew, R.K.; la Grange, P.L.; Findlay, K.P. Year-round acoustic monitoring of Antarctic blue and fin whales in relation to environmental conditions off the west coast of South Africa. Mar. Biol. 2022, 169, 41. [Google Scholar] [CrossRef]
- Shabangu, F.W.; Andrew, R.K.; Yemane, D.; Findlay, K.P. Acoustic seasonality, behaviour and detection ranges of Antarctic blue and fin whales under different sea ice conditions off Antarctica. Endang. Species Res. 2020, 43, 21–37. [Google Scholar] [CrossRef]
- Mintenbeck, K. Impacts of climate change on the Southern Ocean. In Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis; Phillips, B.F., Pérez-Ramírez, M., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 663–701. [Google Scholar] [CrossRef]
- Rintoul, S.; Steele, J.; Thorpe, S.; Turekian, K. Antarctic circumpolar current. In Ocean Currents; Elsevier: Amsterdam, The Netherlands, 2010; pp. 196–208. [Google Scholar]
- Heil, P.; Allison, I. The pattern and variability of Antarctic sea-ice drift in the Indian Ocean and western Pacific sectors. J. Geophys. Res. Oceans 1999, 104, 15789–15802. [Google Scholar] [CrossRef]
- Thorpe, S.E.; Murphy, E.J.; Watkins, J.L. Circumpolar connections between Antarctic krill (Euphausia superba Dana) populations: Investigating the roles of ocean and sea ice transport. Deep-Sea Res. I Oceanogr. Res. Pap. 2007, 54, 792–810. [Google Scholar] [CrossRef]
- Behera, N.; Swain, D.; Sil, S. Effect of Antarctic sea ice on chlorophyll concentration in the Southern Ocean. Deep-Sea Res. II Top. Stud. Oceanogr. 2020, 178, 104853. [Google Scholar] [CrossRef]
- Buesseler, K.O.; Barber, R.T.; Dickson, M.-L.; Hiscock, M.R.; Moore, J.K.; Sambrotto, R. The effect of marginal ice-edge dynamics on production and export in the Southern Ocean along 170°W. Deep-Sea Res. II Top. Stud. Oceanogr. 2003, 50, 579–603. [Google Scholar] [CrossRef]
- Matsuno, K.; Wallis, J.R.; Kawaguchi, S.; Bestley, S.; Swadling, K.M. Zooplankton community structure and dominant copepod population structure on the southern Kerguelen Plateau during summer 2016. Deep-Sea Res. II Top. Stud. Oceanogr. 2020, 174, 104788. [Google Scholar] [CrossRef]
- Nicol, S.; Pauly, T.; Bindoff, N.L.; Wright, S.; Thiele, D.; Hosie, G.W.; Strutton, P.G.; Woehler, E. Ocean circulation off east Antarctica affects ecosystem structure and sea-ice extent. Nature 2000, 406, 504–507. [Google Scholar] [CrossRef]
- Friedlaender, A.S.; Halpin, P.N.; Qian, S.S.; Lawson, G.L.; Wiebe, P.H.; Thiele, D.; Read, A.J. Whale distribution in relation to prey abundance and oceanographic processes in shelf waters of the Western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2006, 317, 297–310. [Google Scholar] [CrossRef]
- Miller, B.S.; Milnes, M.; Whiteside, S. Long-Term Underwater Acoustic Recordings 2013–2019, Ver. 4; Australian Antarctic Data Centre: Hobart, Australia, 2021. [Google Scholar]
- Collins, M.D. User’s Guide for RAM Version 1.0 and 1.0p. 2002. Available online: http://oalib.hlsresearch.com/AcousticsToolbox/ (accessed on 1 June 2023).
- Duncan, A. Acoustics Toolbox User Interface and Post Processor (AcTUP). 2005. Available online: http://cmst.curtin.edu.au/products/underwater/ (accessed on 12 June 2023).
- National Geophysical Data Center: 2-minute Gridded Global Relief Data (ETOPO2) v2. 2006. Available online: https://catalog.data.gov/dataset/2-minute-gridded-global-relief-data-etopo2-v21 (accessed on 3 July 2023).
- Copernicus Marine Service. Global Ocean Physics Reanalysis. Mercator Océan International. 2023. [CrossRef]
- Jensen, F.B.; Kuperman, W.A.; Porter, M.B.; Schmidt, H.; Tolstoy, A. Computational Ocean Acoustics; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Stimpert, A.K.; DeRuiter, S.L.; Falcone, E.A.; Joseph, J.; Douglas, A.B.; Moretti, D.J.; Friedlaender, A.S.; Calambokidis, J.; Gailey, G.; Tyack, P.L. Sound production and associated behavior of tagged fin whales (Balaenoptera physalus) in the Southern California Bight. Anim. Biotelemetry 2015, 3, 1–12. [Google Scholar] [CrossRef]
- Miller, B.S.; Calderan, S.; Leaper, R.; Miller, E.J.; Širović, A.; Stafford, K.M.; Bell, E.; Double, M.C. Source level of Antarctic blue and fin whale sounds recorded on sonobuoys deployed in the deep-ocean off Antarctica. Front. Mar. Sci. 2021, 8, 792651. [Google Scholar] [CrossRef]
- Menze, S.; Zitterbart, D.P.; van Opzeeland, I.; Boebel, O. The influence of sea ice, wind speed and marine mammals on Southern Ocean ambient sound. R. Soc. Open Sci. 2017, 4, 160370. [Google Scholar] [CrossRef]
- Melsheimer, C.; Spreen, G. AMSR2 ASI sea ice concentration data, Antarctic, version 5.4 (NetCDF) (July 2012–December 2019). PANGAEA. 2019. [Google Scholar] [CrossRef]
- Sumner, M. Raadtools: Tools for Synoptic Environmental Spatial Data. R Package. 2023. Available online: https://rdrr.io/github/AustralianAntarcticDivision/raadtools/man/raadtools-package.html (accessed on 20 April 2025).
- Reynolds, R.W.; Smith, T.M.; Liu, C.; Chelton, D.B.; Casey, K.S.; Schlax, M.G. Daily high-resolution-blended analyses for sea surface temperature. J. Clim. 2007, 20, 5473–5496. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Momin, I.M.; George, J.P.; Prasad, V.S. Variability of sea ice concentration over Antarctica during recent decade. J. Earth Syst. Sci. 2024, 134, 13. [Google Scholar] [CrossRef]
- Yu, L.-S.; He, H.; Leng, H.; Liu, H.; Lin, P. Interannual variation of summer sea surface temperature in the Amundsen Sea, Antarctica. Front. Mar. Sci. 2023, 10, 1050955. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, J.; Mohan, R. Seasonal sea-ice variability and its trend in the Weddell Sea sector of West Antarctica. Environ. Res. Lett. 2021, 16, 024046. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S.; An, R. Companion to Applied Regression, 3rd ed.; Sage: Thoasan Oaks, CA, USA, 2019. [Google Scholar]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- de la Mare, W.K. Abrupt mid-twentieth-century decline in Antarctic sea-ice extent from whaling records. Nature 1997, 389, 57–60. [Google Scholar] [CrossRef]
- de la Mare, W.K. Changes in Antarctic sea-ice extent from direct historical observations and whaling records. Clim. Change 2009, 92, 461–493. [Google Scholar] [CrossRef]
- Kasamatsu, F.; Matsuoka, K.; Hakamada, T. Interspecific relationships in density among the whale community in the Antarctic. Polar Biol. 2000, 23, 466–473. [Google Scholar] [CrossRef]
- El-Gabbas, A.; Van Opzeeland, I.; Burkhardt, E.; Boebel, O. Static species distribution models in the marine realm: The case of baleen whales in the Southern Ocean. Divers. Distrib. 2021, 27, 1536–1552. [Google Scholar] [CrossRef]
- Širović, A.; Hildebrand, J.A.; Wiggins, S.M.; McDonald, M.A.; Moore, S.E.; Thiele, D. Seasonality of blue and fin whale calls and the influence of sea ice in the Western Antarctic Peninsula. Deep-Sea Res. II Top. Stud. Oceanogr. 2004, 51, 2327–2344. [Google Scholar] [CrossRef]
- Van Opzeeland, I.; Van Parijs, S.; Kindermann, L.; Burkhardt, E.; Boebel, O. Calling in the cold: Pervasive acoustic presence of humpback whales (Megaptera novaeangliae) in Antarctic coastal waters. PLoS ONE 2013, 8, e73007. [Google Scholar] [CrossRef]
- Burkhardt, E.; Van Opzeeland, I.; Cisewski, B.; Mattmüller, R.; Meister, M.; Schall, E.; Spiesecke, S.; Thomisch, K.; Zwicker, S.; Boebel, O. Seasonal and diel cycles of fin whale acoustic occurrence near Elephant Island, Antarctica. R. Soc. Open Sci. 2021, 8, 201142. [Google Scholar] [CrossRef]
- Santora, J.A.; Schroeder, I.D.; Loeb, V.J. Spatial assessment of fin whale hotspots and their association with krill within an important Antarctic feeding and fishing ground. Mar. Biol. 2014, 161, 2293–2305. [Google Scholar] [CrossRef]
- Meredith, M.P.; King, J.C. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 2005, 32, L19604. [Google Scholar] [CrossRef]
- Nicol, S.; Kitchener, J.; King, R.; Hosie, G.; William, K. Population structure and condition of Antarctic krill (Euphausia superba) off East Antarctica (80–150 E) during the Austral summer of 1995/1996. Deep-Sea Res. II Top. Stud. Oceanogr. 2000, 47, 2489–2517. [Google Scholar] [CrossRef]
- Jarvis, T.; Kelly, N.; Kawaguchi, S.; van Wijk, E.; Nicol, S. Acoustic characterisation of the broad-scale distribution and abundance of Antarctic krill (Euphausia superba) off East Antarctica (30–80°E) in January-March 2006. Deep-Sea Res. II Top. Stud. Oceanogr. 2010, 57, 916–933. [Google Scholar] [CrossRef]
- Parkinson, C.L.; Cavalieri, D.J. Antarctic sea ice variability and trends, 1979–2010. Cryosphere 2012, 6, 871–880. [Google Scholar] [CrossRef]
- Jacobs, S.S.; Comiso, J.C. Climate variability in the Amundsen and Bellingshausen Seas. J. Clim. 1997, 10, 697–709. [Google Scholar] [CrossRef]
- Hobbs, W.R.; Massom, R.; Stammerjohn, S.; Reid, P.; Williams, G.; Meier, W. A review of recent changes in Southern Ocean sea ice, their drivers and forcings. Glob. Planet. Change 2016, 143, 228–250. [Google Scholar] [CrossRef]
- Massom, R.; Reid, P.; Stammerjohn, S.; Raymond, B.; Fraser, A.; Ushio, S. Change and variability in East Antarctic sea ice seasonality, 1979/80–2009/10. PLoS ONE 2013, 8, e64756. [Google Scholar] [CrossRef]
- van Weelden, C.; Towers, J.R.; Bosker, T. Impact of climate change on cetacean distribution, habitat and migration. Clim. Change Ecol. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Ramp, C.; Delarue, J.; Palsbøll, P.J.; Sears, R.; Hammond, P.S. Adapting to a Warmer Ocean—Seasonal Shift of Baleen Whale Movements over Three Decades. PLoS ONE 2015, 10, e0121374. [Google Scholar] [CrossRef]
- Storrie, L.; Lydersen, C.; Andersen, M.; Wynn, R.B.; Kovacs, K.M. Determining the species assemblage and habitat use of cetaceans in the Svalbard Archipelago, based on observations from 2002 to 2014. Polar Res. 2018, 37, 1463065. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).