Abstract
The alga Chondracanthus chamissoi, commonly known as “yuyo” or “mococho” is found along the coasts of Peru and Chile. Due to its multiple applications in industrial, health, pharmaceutical, and productive sectors, its demand has increased, leading to the uncontrolled exploitation of natural banks and negatively impacting marine ecosystems. This experimental study evaluated the viability of propagating C. chamissoi propagules using the foliar fertilizer Bayfolan® from Bayer, as well as its continuous, non-seasonal cultivation in La Ramada. This initiative aims to establish a productive area in La Libertad to meet the needs of national and international markets, reducing the indiscriminate exploitation of seaweed in natural banks. The results indicated that continuous cultivation is feasible, with growth rates of 0.0369 and 0.0388 g.day−1 (0% Bayfolan) and 0.0397 and 0.0399 g.day−1 (1% Bayfolan) during propagule propagation. Slight statistically significant differences were observed in final biomass between 0% and 1% Bayfolan treatments, and Bayfolan use reduced healing time by seven days. Nutritional and microbiological assays confirmed that fresh “yuyo” is suitable for human consumption; hence, La Ramada provides suitable physical–chemical and microbiological conditions for extracting and cultivating hydrobiological species, offering a viable alternative to the seasonal overexploitation of the algae and potential economic benefits for coastal families.
1. Introduction
Globally, seaweed has played a crucial role in human nutrition and the economy of coastal communities in the Pacific and Asia. Historically, this product has been used in food, traditional medicine, and agriculture [1,2,3]. With technological advances, its potential use in solving human health problems and its application in the food, pharmaceutical, and agricultural industries have been recognized [4,5]. Asian countries like China, Japan, and South Korea were the first to develop techniques for industrial seaweed cultivation to commercialize seaweed and its derived products [6,7].
Algae farming has become an attractive venture for many countries due to the substantial global market growth [8]. Notably, worldwide algae production surged from 34.7 thousand tons to more than 34.7 million tons between 1950 and 2019. Industrial cultivation accounts for 97% of this production, while wild harvesting and cultivation contribute only 3% [9,10].
The research by Brown et al. and Choudhary et al. highlights the health benefits of seaweed, aiming to interrelate its nutritional value with the potential benefits for human health [11,12]. Seaweed contains active metabolites, vitamins (A, D, E, C, and B), and essential minerals such as calcium, potassium, magnesium, and iron. Additionally, it provides antioxidants and omega-3 fatty acids that support the immune system and human health [7]. At an industrial level, researchers have investigated seaweed’s applications in agriculture [4,13] and the food and pharmaceutical industries, where it is used as a thickener, stabilizer, and gelling agent in a variety of food products [14], as well as in the production of biofuel [15]. With all this potential that is already known in algae, research is being carried out that seeks to develop and improve cultivation techniques, taking into consideration external factors such as the selection of cultivation sites and the evolution of growth conditions to maximize their production [16,17,18].
The red alga Chondracanthus chamissoi, commonly known as “yuyo” or “mococho” is found along the coasts of Peru and Chile. In Peru, it is mainly located on the coasts of Trujillo (La Libertad), Marcona (Ica), and Ilo (Moquegua). This species belongs to the family Gigartinaceae, order Gigartinales, and grows in low intertidal to subtidal zones, attached to rocky and calcareous substrates by a basal disc. The propagation of this macroalga occurs both naturally and through controlled cultivation techniques, including vegetative propagation (from algae fragments) and sporulation (via spores) [19,20]. This alga is used both fresh and dehydrated in human food and in the hydrocolloid industry [21]. Due to its popularity in gastronomy, there has been an increase in demand for this raw material in both local and Asian markets, leading to uncontrolled exploitation of its natural habitats and negatively impacting marine ecosystems [22]. In order to avoid the overexploitation and/or predation of C. chamissoi, as well as the destruction of its habitat, strategies have been implemented to improve the cultivation and long-term production of this alga [23,24].
The depletion of marine resources, such as algae, is a global problem that affects Chile and mainly Peru, which are the main producers of algae in South America, where macroalgae production is mainly based in the exploitation of natural populations [25,26]. Starting in 2000, Chile began to study and apply new methods for vegetative propagation and/or reproductive phases for the in vitro and at-sea cultivation of C. chamissoi (Rhodophyta) and Lessonia trabeculata (Phaeophyceae) [27,28,29]. In Peru, these studies began in 2019 on the south coast, in the departments of Moquegua [30,31], Pisco [20,32,33], and Lima [32] and are currently being carried out on the north coast of Peru [34].
The main techniques and/or methods used for the propagation and cultivation of C. chamissoi are spore cultivation and vegetative propagation. The first is based on cultivation via spores, but this option is not very viable as it has a high mortality rate and high production costs [33,35]. Vegetative propagation is an alternative method, which consists of placing the algae on a natural or artificial substrate. This latter process is convenient and advantageous due to its lower mortality rate and operation cost [20,31]. The vegetative propagation studies conducted by Colque (2017) [36] and Arbaiza et al. (2018, 2023) [20,37] included the use of Bayfolan®, a foliar fertilizer from Bayer, which is used as a stimulant for plant development. Its composition includes macronutrients (N = 110 g/L, P2O5 = 80 g/L, K2O = 60 g/L) and micronutrients (Fe = 190 mg/L, Mn = 162 mg/L, B = 102 mg/L, Cu = 81 mg/L, Zn = 61 mg/L, Mb = 9 mg/L, Co = 3.5 mg/L), as well as vitamin B1 and growth hormones (4 ppm) [20,36,37]. The study by Abayza et al. (2023) showed that the use of Bayfolan reduced biomass loss, making vegetative cultivation viable through the formation of secondary attachment discs (SAD) [20]. The study by Huaman-Fernández et al. (2024) on vegetative propagation using holdfast discs indicates the necessity of performing this procedure under controlled conditions to prevent the growth of diatoms and other microorganisms [38], while the study by Ruiz-Ipanaque et al. (2024) conducted in the sea of Paracas, department of Ica, showed that 4–5 cm propagules can regenerate during their cultivation [39].
In Chile, vegetative propagation has proven to be successful. This method of asexual reproduction involves the growth of new algae from fragments of the parent algae and is based on the ability of macroalgae to regenerate, allowing them to develop new vegetative structures (fronds, stipes, or thalli) from a portion of algal tissue [29,40]. In Peru, there are few published studies where vegetative propagation is used for cultivation and industrial production purposes; they simply continue with the exploitation of natural algae banks that occur seasonally [31]. The seasonal extraction of C. chamissoi directly influences its commercial value, which varies between 0.5 and 5.0 dollars per kg of fresh seaweed [19].
The increasing demand for C. chamissoi has sparked interest in the food industry and scientific research due to its numerous health benefits and applications in industrial and productive sectors. In our country, this resource is directly harvested from natural populations by artisanal fishermen. However, high demand, price variability, and seasonal production contribute to overexploitation, affecting marine ecosystems. Acknowledging that macroalgae play a fundamental role in aquatic ecosystems by providing a wide range of economic, ecological, and nutritional benefits, it is crucial to understand the mechanisms for their propagation, as this knowledge requires special attention in the context of scientific research and aquaculture. In particular, the propagation of C. chamissoi (Rodophyta) has gained relevance in various fields due to its multiple benefits and applications, highlighting its ecological and economic importance for its rapid and uniform production capacity, which allows for consistent quality maintenance in cultivation, providing an advantage in aquaculture. It is important to highlight that this type of research is novel in northern Peru, especially due to the scarcity of studies in our country. Most of the research has been conducted in the southern region, where suitable environmental conditions for the cultivation of C. chamissoi have been found. In comparison with the studies carried out in the departments of Ica and Arequipa, where the water temperature is 2 or 3 °C lower than in the north, this study aims to propagate propagules under laboratory conditions and subsequently cultivate them in the sea under specific conditions where the sea temperature is slightly higher.
This study aims to evaluate the viability of propagating C. chamissoi propagules using 1% Bayfolan and its possible continuous cultivation in La Ramada, La Libertad. For this, the growth rate of C. chamissoi under laboratory conditions, the microbiological and physicochemical quality of the fresh algae obtained in suspended cultivation, and the nutritional quality of the fresh algae obtained from suspended cultivation and from the natural bank in La Ramada have been analyzed. This study is an initiative to implement the industrial cultivation of C. chamissoi in the future, aiming to reduce the depletion of natural habitats and meet the demands of national and international markets. Likewise, this study aims to raise awareness and provide practical solutions to the overexploitation of C. chamissoi, including promoting cultivation and encouraging sustainable practices. It also calls on the responsible authorities to manage the monitoring of natural populations and the extraction quota of C. chamissoi during the harvesting seasons.
2. Materials and Methods
2.1. Study Area
The study was conducted in two environments. The first was in a controlled greenhouse at the laboratories of the Institute of Research in Sciences and Technologies at César Vallejo University (UCV) in the district of Trujillo, La Libertad, aimed at propagating propagules of the macroalga C. chamissoi. The second environment was located on the La Ramada beach, in the district of Salaverry, La Libertad (8°17′3.8″ S, 78°57′30.9″ W), where samples were obtained from a natural seaweed bank and the propagule process for pilot trials of continuous cultivation under natural conditions was implemented (Figure 1).
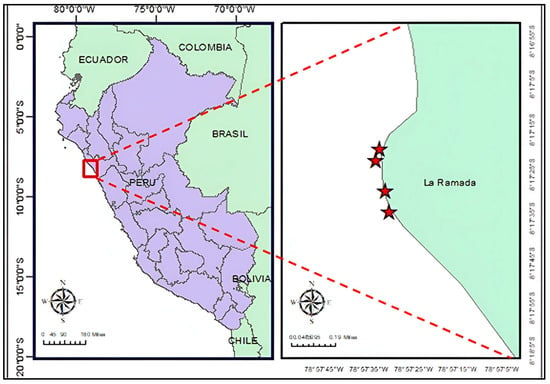
Figure 1.
Geographic location of La Ramada beach in the Salaverry district, La Libertad, Peru (image created by marine biologist Max Castañeda-Franco). The stars on the map indicate the area where there is a greater presence of the macroalga C. chamissoi.
2.2. Sample Collection
Seawater and C. chamissoi samples were collected from La Ramada beach (8°17′3.8″ S, 78°57′30.9″ W) every two months, between June 2022 and April 2023, in order to evaluate the physicochemical and microbiological quality. In addition, samples of the macroalgae C. chamissoi were obtained in order to obtain raw material and use it in the propagule propagation process and for the physical, chemical, and microbiological analyses carried out in an accredited external laboratory. The seaweed collection process was carried out by manual extraction in the rocky–sandy intertidal zone at a depth of less than 1.50 m in the meadow of La Ramada beach. Personnel experienced in this type of activity collected the seaweed by free diving. The collected samples of C. chamissoi were evenly distributed in 3 first-use polyethylene bags: one for microbiological processing, another for physicochemical analysis, and the last one for the process of obtaining gametophytes to be used in the propagule propagation stage. The collected samples were identified and stored in isothermal containers (4–8 °C) for transport to the laboratories of the Institute for Research in Sciences and Technologies of the Cesar Vallejo University (UCV)—Trujillo, where the first stage of propagule propagation took place.
2.3. Propagule Propagation
The propagation process of C. chamissoi propagules began with the manual cleaning of the algae. Then, successive washes were carried out with filtered seawater (0.45 µm) to remove excess sand adhered to the algae. The cystocarpic and tetrasporic regions of the algal biomass were removed. The algae were uniformly fragmented into 5 cm portions and 8 to 10 pieces of the sheets (10 g) were placed inside a plastic mesh to prevent their dispersion in the propagation systems. Each mesh that was introduced into the propagation system contained 10 sections with 10 g of the algae (Figure 2a). The propagation systems were composed of a borosilicate glass vessel with a capacity of 5 L, with 4 L of filtered seawater (0.45 µm) into which air was injected (Figure 2). Two propagation systems were prepared, and in one of them, the sterile seawater exchange was supplemented with 1% Bayfolan (10 mL of Bayfolan was added for each liter of filtered seawater). Ten replicas of each system were conducted in order to obtain sufficient data for the growth rate analysis. In both systems, temperature, pH, dissolved oxygen, and total dissolved solids were measured for 60 days; readings of these parameters were recorded daily using the HANNA HI9829 multiparameter meter (Hanna Instruments Ltd., Leighton Buzzard, UK). At this stage, the filtered seawater was replaced every 6 days, avoiding the deterioration and contamination of the propagation system. After 60 days, the nets were removed from the propagation systems and were transferred to the sea, specifically to the meadow of La Ramada beach, in the district of Salaverry.
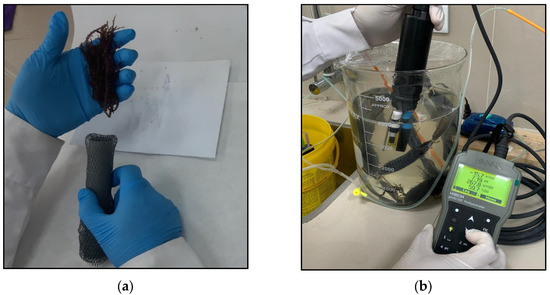
Figure 2.
Chondracanthus chamissoi propagule propagation system under laboratory conditions. (a) Introduction of the algae into the meshes; (b) measurement of parameters using the HANNA HI9829 multiparameter meter in the propagation system used in the laboratory.
2.4. Seaweed Cultivation in the Sea
This process was carried out using a semi-suspended tree-type cultivation system between the months of October 2022 and February 2023. During this period of time, the microbiological and physical–chemical quality of the seawater was analyzed. While the macroalgae were evaluated post-harvest, the microbiological and nutritional quality of the culture were compared with those obtained from the samples collected from the natural seaweed bank. This system consisted of a ¾ raffia with a buoy attached at one end and anchored to a 15 kg weight at the other end. Along the raffia, mesh structures containing the C. chamissoi propagules were positioned during the propagation stage (Figure 3 and Figure 4) [41].
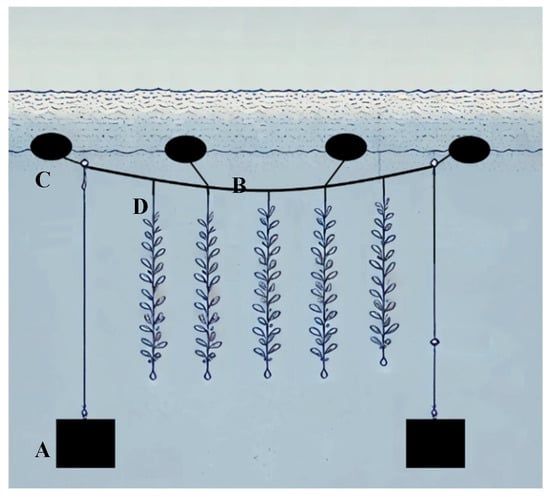
Figure 3.
Diagram of the semi-suspended tree-type cultivation system. (A) Anchor, (B) mother line, (C) buoys, and (D) net with the cultivation of C. chamissoi.

Figure 4.
C. chamissoi cultivation in La Ramada meadow. (a) Location of the semi-suspended tree system on La Ramada beach; (b) Evidence of algae growth on the cultivation nets during their evaluation at 30 days.
2.5. Collection and Statistical Analysis of Data
The data were collected in physical formats and recorded in Microsoft Excel spreadsheets until subsequent statistical analysis with SPSS v.26 software. Statistically, the distribution of the data was verified with the normality test and the t-student test was applied to compare independent samples.
To determine the growth rate of C. chamissoi, Equation (1) was used, as proposed by Yong et al. (2013) as the one that presents the lowest degree of error [42].
where Wo is the initial mass value, Wt is the final mass value, and t is the propagation time of C. chamissoi propagules (60 days).
3. Results
3.1. Propagule Propagation Stage
During this stage, temperature, pH, dissolved oxygen (DO), and total dissolved solids (ppt TDS) were measured, observing a variation between ±0.1 and ±1.5 for the evaluated parameters. This minimal variation would be explained by the fact that, during the propagation of the algae propagule, the filtered seawater was replaced every 6 days, avoiding the deterioration and contamination of the propagation medium (Table 1).

Table 1.
Abiotic factors evaluated during the propagule propagation stage under laboratory conditions.
3.2. Physical–Chemical and Microbiological Quality of Seawater for Cultivation Purposes
It was shown that the values of the parameters measured in the seawater are below the maximum permissible limit according to Peruvian legislation that was issued by the Ministry of the Environment MINAM [43] (Table 2).

Table 2.
Measurement of water quality standards required for the extraction and cultivation process of hydrobiological species in coastal marine waters.
3.3. Microbiological Quality and Proximal Composition of C. chamissoi
The evaluation of the microbiological quality and proximal chemical composition was carried out on the fresh algae C. chamissoi, which grew on the natural bank and in the suspended culture of La Ramada beach with the purpose of evaluating whether there are differences between the products that affect their potential use in human nutrition (Table 3 and Table 4).

Table 3.
Evaluation of microbiological quality requirements for fresh hydrobiological products.

Table 4.
Proximal chemical composition of fresh hydrobiological products.
3.4. Growth Rate of C. chamissoi
The growth rate evaluated for each propagule propagation system and in two different periods showed that at the end of 60 days, there was a slight difference in the systems that applied Bayfolan at 1% (Figure 5). Similarly, in the statistical analysis with Student’s t-test, it was shown that there was a slight significant difference between both groups (Table 5).
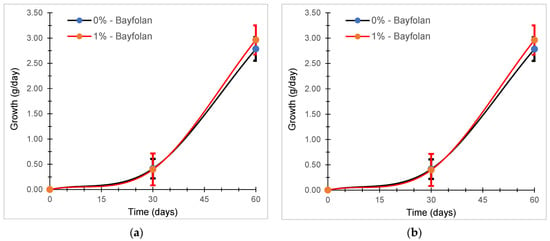
Figure 5.
Growth rate of C. chamissoi evaluated in propagation systems with 0 and 1% Bayfolan. (a) Evaluation carried out between June and August 2022 and (b) evaluation carried out between September and November 2022.

Table 5.
Student’s t-test applied to the data obtained during the propagation of the C. chamissoi with 0 and 1% Bayfolan carried out in two different periods.
4. Discussion
An important consideration for developing any seaweed crop is understanding whether the intended crop can thrive in the natural conditions of future productive areas. In this context, the presence of C. chamissoi has been reported along the northern coast of Peru [22,44]. In the La Libertad region, C. chamissoi has been observed in the intertidal and subtidal zones of Huanchaco and Salaverry beaches, recording maximum volumes between August and December, and in a very similar way to other coastal areas of Peru and Chile [21,45]. The cyclic presence and abundance of this algae have implications for extractive activities, often leading to overexploitation. Despite government regulations, enforcement remains challenging due to inadequate control and compliance. La Ramada beach in the Salaverry district, La Libertad, was chosen based on several criteria, including access, natural bank availability for specimen collection, seawater quality (free from microbiological, chemical, and anthropogenic contaminants), and nutrient availability for optimal C. chamissoi growth. This statement is corroborated by the results shown in Table 2.
Experimental studies initiated in Chile and replicated on the southern coast of Peru have demonstrated C. chamissoi is highly adaptable to variations in light and temperature. Various studies show that this macroalga has an optimal growth range between 20 °C and 25 °C and irradiances of 70 to 120 μmol photons m−2 [29]. In Peru, a tropical and subtropical climate prevails along its coastal areas, and the extraction of red and brown algae is mainly concentrated in the regions of Arequipa, Nazca, Lima, and La Libertad. Pisco stands out as an area of high productivity for C. chamissoi, where the sea surface temperature during the summer season is around 21.0 ± 1.0 °C. In the northern region of Peru, La Libertad is one of the main regions that contribute to the production of C. chamissoi. For instance, in 2019, 260.3 tons of this algae were marketed, and Salaverry contributed 23.2% of this production, recording 18 °C as the average seawater temperature [46]. Subsequent years saw commercialization figures of 320.03 tons in 2020, with Salaverry contributing only 13.3% and an average seawater temperature of 16.0 °C [47]; 93.48 tons in 2021, with Salaverry and Huanchaco contributing 50% and an average seawater temperature of 16.5 °C; and 484.93 tons in 2022, with Salaverry and Huanchaco contributing 40% and seawater temperatures ranging between 14.3 °C and 20.8 °C [48,49]. Data from the last 5 years indicate that C. chamissoi has developed well on the beaches of Salaverry, contributing up to 25% of the productive quota of extraction and commercialization. The data obtained throughout this study show that the temperature on La Ramada beach has ranged between 17.5 °C and 22.5 °C. These data show that the seawater is within the optimal range for the development of this macroalgae. During the coastal Niño times, it has been recorded that the seawater temperature can increase up to 25.5 ± 1.0 °C, negatively affecting the natural meadows where the algae is found and decreasing its biomass. However, the effect of the coastal El Niño has not caused an increase in the average temperature of seawater on the beaches of Salaverry, since IMARPE reports indicate average temperatures between 16.5 °C and 22.8 °C on Salaverry beach, near La Ramada beach, where the natural bank of C. chamissoi is located [47,48,49].
The quality of the coastal marine environment is an important factor to know and consider before implementing future seaweed productive areas; Table 2 shows the physical, chemical, and microbiological quality of the water from La Ramada beach in the Salaverry district. The results of dissolved oxygen, pH, TDS (total dissolved solids), nitrates, BOD-5, detergents, oils and greases, cadmium, and thermotolerant coliforms fall below the maximum limits allowed for extraction and cultivation activities of hydrobiological species in marine–coastal areas, according to current national regulations established by MINAM [43]. These results coincide with what was reported by IMARPE in 2021 and 2022 for the beaches of Salaverry. The good quality of the seawater on La Ramada beach is attributed to the fact that this area of Salaverry has a low influx of population because it is not very crowded and is mainly oriented towards artisanal fishing activity. Consequently, minimal anthropogenic contamination helps maintain the natural ecosystem that is the source of income for local fishermen. In contrast, the beaches of Malabrigo and Huanchaco have high anthropogenic contamination, and microbiological values usually reach between 103 and 104 thermotolerant coliforms per 100 mL of seawater [46,47,48,49].
The environmental conditions recorded during propagule propagation and in the semi-suspended culture are favorable for the growth of C. chamissoi, despite the fluctuations in surface water temperature between 18 °C and 25 °C, typical of an “El Niño” phenomenon, where the viability of the semi-suspended culture was not affected. The seagrass La Ramada offers the appropriate physical–chemical and microbiological conditions for the extraction and cultivation activities of hydrobiological species in marine–coastal areas, according to the current national regulations established by the MINAM [43]. Furthermore, the fresh C. chamissoi product obtained from both the natural bank and the semi-suspended cultivation in La Ramada maintains its nutritional values and microbiological quality within the maximum permissible limits to be considered suitable for human diet and consumption. Therefore, it has been shown that it is feasible to implement the continuous cultivation of C. chamissoi in the La Ramada de Salaverry meadow, using simple and economical techniques such as vegetative propagation and semi-suspended cultivation under natural conditions. Likewise, this study provides essential information for the cultivation, management, application, and preservation of C. chamissoi in La Ramada, becoming a viable alternative against the seasonal overexploitation of this resource. Moreover, it could generate new economic opportunities for families in the Salaverry area.
The propagation of edible seaweed can be carried out by various methods, with harvesting and controlled cultivation being the most common techniques. In the harvesting process, algae are extracted from their natural environment, which can have environmental implications, such as overexploitation, if not managed sustainably. On the other hand, controlled cultivation involves planting and harvesting algae in specific facilities, such as marine farms. These facilities allow for the precise control of environmental conditions, optimizing growth and product quality. The choice of propagule propagation under laboratory conditions was based on the fact that this is a commonly used alternative method due to its low mortality rate, low operating cost, and high profitability. This process consists of placing the algae on a natural or artificial substrate [27,29,40,50].
Nutrient availability during algae propagation, whether in natural or laboratory conditions, plays a crucial role in successful cultivation. Recent research has explored the use of agricultural fertilizers for cultivating species like Chondracanthus squarrulosus, Pyropia/Porphyra spp., Sarcothalia crispata, and C. chamissoi (Rhodophyta) [20,32]. In Peru, studies have specifically examined the commercial fertilizer Bayfolan® (Bayer), supporting that its content of vitamins and indole acetic acid, which stimulate plant growth, can also induce the same effect on algae. The existing literature and background highlight the importance of using Bayfolan due to its nitrogen and micronutrient content. Colque (2017) [36] and Arbaiza et al. (2018, 2023) [20,37] have demonstrated that Bayfolan® promotes the growth of marine macroalgae. In the context of aquaculture, it is suggested that the use of Bayfolan® in the propagation of C. chamissoi could significantly improve cultivation efficiency, allowing for faster and more uniform production of biomass. This would reduce propagation time, decrease operational costs, and increase the profitability of the cultivation, making the aquaculture of C. chamissoi a viable and attractive option for the industry.
Under controlled conditions of pH, dissolved oxygen (%OD), and temperature, two algae propagule propagation systems were established: one without fertilizer and another with 1% Bayfolan (see Table 1). Both systems underwent regular changes of 0.45 μm filtered seawater every 6 days. Statistical analysis revealed that the data obtained followed a normal distribution, and the Student’s t-test for independent variables demonstrated a slightly significant difference in final C. chamissoi weight at 60 days between the groups treated with 0% and 1% Bayfolan® (see Table 5, p = 0.000 in both experiments). The results of 0.038 and 0.036 g/day obtained in growth rate are similar to the values of 0.05 g/day reported by Arbaiza et al. (2018) [37]. Furthermore, this author reported that the use of Bayfolan® led to an increase in algal biomass of up to 50% during periods with longer daylight hours. However, this was not possible to verify in our study because the experimental conditions were not similar [37]. The same author indicated in 2023 that seawater fertilized with Bayfolan® (0.2 mL L−1) and subjected to weekly substrate exchanges provided optimal conditions for maintaining C. chamissoi leaves before vegetative propagation, regardless of inoculum density [20]. However, evaluating growth rates was challenging due to biomass loss during medium exchanges, particularly within the first 30 days when algae healing occurs.
There are very few works that report the growth rate of C. chamissoi during the propagule propagation or vegetative growth stage. This is because this Rhodophyta is primarily found along the coasts of Chile and Peru, where it holds significant economic importance. However, studies in other parts of the world also evaluate the influence of fertilizers or algae extracts on the growth rate of other Rhodophyta species with economic relevance in their respective regions. The experimental results indicate that the growth rate of C. chamissoi during propagule propagation or vegetative growth stage exhibits an increasing trend (see Figure 5). Specifically, after 30 and 60 days, the maximum growth rates were 0.0369 and 0.0388 cm per day-1 for the group treated with 0% Bayfolan, and 0.0397 and 0.0399 cm per day−1 for the group treated with 1% Bayfolan (see Table 5). These findings diverge from those reported by Colque (2017) in a study conducted in Tacna, Peru. Colque used 0.1 and 0.2 mL of Bayfolan per liter of filtered seawater in a controlled system with specific parameters (17.0 ± 0.5 °C, 5.70 ± 0.15, % OD, and 8.14 ± 0.5 pH), resulting in a growth rate of 0.1% d−1 with 0.1 mL per liter of fertilizer. However, for the concentration of 0.2 mL per liter and the control, no growth rate was reported due to biomass loss resulting from excessive manipulation during medium exchanges throughout the experiment. Additionally, microscopic examination revealed a shortened healing process by seven days in the system containing Bayfolan fertilizer [36].
In other latitudes, we can mention studies conducted on other rhodophytes. Dawange and Jaiswar evaluated the effect of powdered Ascophyllum extract on the growth of Gracilaria corticata (Rhodophyta). After three weeks, they reported growth rates ranging from 3.7% to 7.6% in treatments where propagules were immersed for 15, 30, and 60 min in Ascophyllum extract at concentrations of 0.01, 0.1, and 1.0 g L⁻1 [51]. Another study, conducted by Hlaing and Jarukamjorn, evaluated the growth of Kappaphycus alvarezii (Rhodophyta) propagules using 6-benzylaminopurine and indole 3-acetic acid. Their results showed an increase in propagule length, ranging from 10 to 15 mm, achieving propagules measuring between 3.0 and 3.5 cm, which were later transplanted into the sea. The evaluation continued for 45 days, during which individual plantlets, initially weighing 0.67 ± 0.15 g (wet weight), reached a net weight of 42.35 ± 10.28 g, with a daily growth rate of 9.70 ± 0.25% [52]. In contrast, the study by Balar et al., which aimed to produce Gracilaria dura (Rhodophyta) propagules on a large scale, employed a combination of 6-benzylaminopurine (0.25 mg L⁻1) and Ascophyllum extract (1.0 g L⁻1). Propagules were immersed for 60 min, and the results showed successful propagation with an increase in weight from 126.33 ± 14.65 g to 147.5 ± 13.11 g over 112 days [53]. Using the data from this study, the growth rate formula was applied, yielding a value of 0.138. Although not reported in this study, this result proved to be 3.5 times higher than the growth rate observed in our study on C. chamissoi. Based on the information from studies on other Rhodophyta species, we can broaden our perspective to continue investigating the effects of fertilizers and algae extracts on the growth of C. chamissoi.
One of the main reasons behind the spread of edible seaweed lies in the nutritional value they contribute to the human diet. Numerous studies highlight that marine macroalgae are an excellent source of high-quality proteins, vitamins, minerals, antioxidants, and omega-3 fatty acids, beneficial for cardiovascular health and brain function. From a nutritional point of view, knowing the proximal value and microbiological quality of fresh algae is crucial. The proximal value refers to the content of carbohydrates, proteins, lipids, and micronutrients. Notably, C. chamissoi and Porphyra spp. are two species known for their nutritional content and potential use in the human diet. The results from suspended cultivation at La Ramada beach slightly exceed those from the natural bank, showing up to a 3% increase in protein content. The results of the nutritional content of C. chamissoi are similar to those reported by other researchers, where protein content ranged between 20.2% and 24.1% [33,54,55,56]. A high protein content (>20%) is a characteristic that positions edible algae as an important food source in human nutrition. Regarding microbiological quality, the product obtained from La Ramada beach is microbiologically suitable, because the values obtained do not exceed the maximum limit established in the Sanitary Standard that defines the microbiological criteria for the sanitary quality and safety of foods and beverages for human consumption according to Ministerial Resolution No. 591-2008-MINSA-Peru.
In Peru, for more than a decade, public policies have been promoted to strengthen small aquaculture producers with the aim of generating better opportunities to obtain new products for the national and international markets. In 2019, the National Superintendency of Customs and Tax Administration (SUNAT-Peru) reported that 34 thousand tons of dried macroalgae were exported, generating more than 21 million US dollars. With this background, it is important to continue with research and projects aimed at the cultivation and repopulation of macroalgae using cultivation techniques such as vegetative propagation, spore fragmentation, and spore cultivation. The purpose is to expand the cultivation of macroalgae on the coasts of Peru, reducing the illegal overexploitation in natural seagrass and generating new economic livelihoods for people and small associations of fishermen dedicated to this sector. The participation of private companies is important to achieve this objective, and the joint work between the private sector and artisanal fishermen’s associations, which in recent years has generated repopulation projects in the southern part of our country, must be replicated.
5. Conclusions
It has been evidenced that it is feasible to implement the continuous cultivation of Chondracanthus chamissoi in the La Ramada area, Salaverry, using simple and economical techniques such as vegetative propagation and semi-suspended cultivation in the sea. This study has provided essential information for the cultivation, management, application, and preservation of C. chamissoi in La Ramada, becoming a viable alternative against the seasonal overexploitation of this resource and potentially generating new economic income for many families in the Salaverry area.
The environmental conditions recorded during propagule propagation and semi-suspended cultivation are favorable for the growth of C. chamissoi. Despite fluctuations in surface water temperature between 18 and 25 °C, typical of an El Niño phenomenon, the viability of semi-suspended cultivation was not affected.
The La Ramada area offers suitable physicochemical and microbiological conditions for the extraction and cultivation activities of hydrobiological species in marine–coastal areas, according to the national regulations established in 2019 by the MINAM. Additionally, the fresh C. chamissoi product obtained both from the natural bank and from the semi-suspended cultivation in this area maintains its nutritional and microbiological properties within the maximum permissible limits (LMP) to be considered suitable for diet and human consumption.
In the context of aquaculture, it is essential to continue investigating the impact of agricultural fertilizers like Bayfolan and others to evaluate their effects on various stages of growth and nutrient absorption. These studies will generate valuable knowledge to optimize their application, especially in northern Peru, where the sea temperature is slightly higher. These studies will significantly reinforce the results obtained in the southern part of the country, which indicates that these products reduce the propagation time of the crops. As a consequence, operational costs decrease, and the profitability of the cultivation increases, making the cultivation of C. chamissoi a viable and attractive option for the aquaculture industry.
The present study, initiated by professionals from the César Vallejo University with the objective of acquiring new knowledge about the viable cultivation techniques of C. chamissoi in the La Libertad region, must continue and include strategic partners such as the Alguera communities and artisanal fishing associations in order to request the necessary government support from the authorities of the Regional Government of La Libertad and the Ministry of Production to implement in situ a project that promotes the propagation, cultivation, and repopulation of C. chamissoi in La Ramada. This project to be implemented will seek, in the medium term, to continuously cover the demand for this product in the national market and, in the long term, to become one of the main production centers of C. chamissoi and its derivatives for the international market.
Author Contributions
Conceptualization, N.S.-D. and L.C.-C.; methodology, N.S.-D. and L.C.-C.; validation, N.S.-D., L.C.-C. and N.T.-R.; formal analysis, N.S.-D.; investigation, L.C.-C. and N.T.-R.; resources, N.S.-D. and L.C.-C.; data curation, L.C.-C.; writing—original draft preparation, N.S.-D. and L.C.-C.; writing—review and editing, L.C.-C.; visualization, N.S.-D.; supervision, N.S.-D.; project administration, N.S.-D.; funding acquisition, N.S.-D. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded through Teaching Research Project No. P-2022-56, presented at the University Cesar-Vallejo SAC.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Thanks to the association of artisanal fishermen of La Ramada for helping us with the information and processes of collecting C. chamissoi “yuyo”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Iqbal, M.S. Seaweed farming-global scenario: Socio-economic aspects. In Sustainable Global Resources of Seaweeds; Springer International Publishing: Cham, Switzerland, 2022; Volume 1, pp. 313–327. ISBN 9783030919542. [Google Scholar]
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.-S.; Jena, M. Beneficial Effects of Seaweeds and Seaweed-Derived Bioactive Compounds: Current Evidence and Future Prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242. [Google Scholar] [CrossRef]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as Valuable Sources of Essential Fatty Acids for Human Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, A.S.; Meena, S.N. Seaweed farming: A perspective of sustainable agriculture and socio-economic development. In Natural Resources Conservation and Advances for Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 493–501. [Google Scholar]
- Torres, M.D.; Kraan, S.; Dominguez, H. Sustainable Seaweed Technologies: Cultivation, Biorefinery, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128179444. [Google Scholar]
- Pati, M.P.; Sharma, S.D.; Nayak, L.; Panda, C.R. Uses of seaweed and its application to human welfare: A review. Int. J. Pharm. Pharm. Sci. 2016, 8, 12. [Google Scholar] [CrossRef]
- Prasad Behera, D.; Vadodariya, V.; Veeragurunathan, V.; Sigamani, S.; Moovendhan, M.; Srinivasan, R.; Kolandhasamy, P.; Ingle, K.N. Seaweeds Cultivation Methods and Their Role in Climate Mitigation and Environmental Cleanup. Total Environ. Res. Themes 2022, 3–4, 100016. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Afflerbach, J.C.; Frazier, M.; Halpern, B.S. Blue Growth Potential to Mitigate Climate Change through Seaweed Offsetting. Curr. Biol. 2019, 29, 3087–3093.e3. [Google Scholar] [CrossRef]
- Cai, J. Global status of seaweed production, trade and utilization. In Proceedings of the Seaweed Innovation Forum Belize, Belize City, Belize, 28 May 2021. [Google Scholar]
- Sultana, F.; Wahab, M.A.; Nahiduzzaman, M.; Mohiuddin, M.; Iqbal, M.Z.; Shakil, A.; Mamun, A.-A.; Khan, M.S.R.; Wong, L.; Asaduzzaman, M. Seaweed Farming for Food and Nutritional Security, Climate Change Mitigation and Adaptation, and Women Empowerment: A Review. Aquac. Fish. 2023, 8, 463–480. [Google Scholar] [CrossRef]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.R.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and Human Health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Illera-Vives, M.; Seoane Labandeira, S.; Fernández-Labrada, M.; López-Mosquera, M.E. Agricultural uses of seaweed. In Sustainable Seaweed Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 591–612. [Google Scholar]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Cassani, L.; Lourenço-Lopes, C.; Barral-Martinez, M.; Chamorro, F.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Thermochemical Characterization of Eight Seaweed Species and Evaluation of Their Potential Use as an Alternative for Biofuel Production and Source of Bioactive Compounds. Int. J. Mol. Sci. 2022, 23, 2355. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, L.; Cantarino, S.J.; Critchley, A.T. Challenges to the future domestication of seaweeds as cultivated species: Understanding their physiological processes for large-scale production. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–83. ISBN 9780081027103. [Google Scholar]
- Obando, J.M.C.; dos Santos, T.C.; Martins, R.C.C.; Teixeira, V.L.; Barbarino, E.; Cavalcanti, D.N. Current and Promising Applications of Seaweed Culture in Laboratory Conditions. Aquaculture 2022, 560, 738596. [Google Scholar] [CrossRef]
- Arakaki, N.; Suárez-Alarcón, S.; Márquez-Corigliano, D.; Gil-Kodaka, P.; Tellier, F. The Widely Distributed, Edible Seaweeds in Peru, Chondracanthus chamissoi and Chondracanthus chamissoi f. Glomeratus (Gigartinaceae, Rhodophyta), Are Morphologically Diverse but Not Phylogenetically Distinct. J. World Aquac. Soc. 2021, 52, 1290–1311. [Google Scholar] [CrossRef]
- Arbaiza, S.; Avila-Peltroche, J.; Castañeda-Franco, M.; Mires-Reyes, A.; Advíncula, O.; Baltazar, P. Vegetative Propagation of the Commercial Red Seaweed Chondracanthus chamissoi in Peru by Secondary Attachment Disc during Indoor Cultivation. Plants 2023, 12, 1940. [Google Scholar] [CrossRef]
- Uribe Alzamora, R.; Atoche Suclupe, D.; Paredes Paredes, J. Aspectos ecológicos de la macroalga roja Chondracanthus chamissoi (C. Agardh) Kützing en la región La Libertad, Perú A1.3. ALICIA 2023, 1, 11–13. [Google Scholar] [CrossRef]
- Avila-Peltroche, J.; Padilla-Vallejos, J. The Seaweed Resources of Peru. Bot. Mar. 2020, 63, 381–394. [Google Scholar] [CrossRef]
- Berger, C. La acuicultura y sus oportunidades para lograr el desarrollo sostenible en el Perú. S. Sust. 2020, 1, e003. [Google Scholar] [CrossRef]
- Vivanco, C.; Álvarez, J.; Vodden, K. Extracción de algas en Pisco: Desafíos, oportunidades, adaptación y perspectivas futuras. Ind. Data 2014, 14, 19–27. [Google Scholar] [CrossRef]
- Zuniga-Jara, S.; Soria-Barreto, K. Prospects for the Commercial Cultivation of Macroalgae in Northern Chile: The Case of Chondracanthus chamissoi and Lessonia trabeculata. J. Appl. Phycol. 2018, 30, 1135–1147. [Google Scholar] [CrossRef]
- Avila-Peltroche, J.; Villena-Sarmiento, G. Analysis of Peruvian Seaweed Exports during the Period 1995–2020 Using Trade Data. Bot. Mar. 2022, 65, 209–220. [Google Scholar] [CrossRef]
- Bulboa, C.R.; Macchiavello, J.E.; Oliveira, E.C.; Fonck, E. First Attempt to Cultivate the Carrageenan-Producing Seaweed Chondracanthus chamissoi (C. Agardh) Kutzing (Rhodophyta; Gigartinales) in Northern Chile. Aquac. Res. 2005, 36, 1069–1074. [Google Scholar] [CrossRef]
- Bulboa, C.; Macchiavello, J.; Véliz, K.; Oliveira, E.C. Germination Rate and Sporeling Development of Chondracanthus chamissoi (Rhodophyta, Gigartinales) Varies along a Latitudinal Gradient on the Coast of Chile. Aquat. Bot. 2010, 92, 137–141. [Google Scholar] [CrossRef]
- Macchiavello, J.; Sepúlveda, C.; Basaure, H.; Sáez, F.; Yañez, D.; Marín, C.; Vega, L. Suspended Culture of Chondracanthus chamissoi (Rhodophyta: Gigartinales) in Caleta Hornos (Northern Chile) via Vegetative Propagation with Secondary Attachment Discs. J. Appl. Phycol. 2018, 30, 1149–1155. [Google Scholar] [CrossRef]
- Espinoza, I. Revista Ciencia y Technologia-Para el Desarrollo-UJCM; 2022; pp. 101–107. Available online: https://revistas.ujcm.edu.pe/index.php/rctd/article/view/189 (accessed on 10 May 2024).
- Zapata-Rojas, J.C.; Gonzales Vargas, A.M.; Zevallos-Feria, S.A. Estudio comparativo para propagación vegetativa de Chondracanthus chamissoi “Yuyo” sobre tres tipos de sustrato en ambiente controlado y su viabilidad en la región Moquegua. Enfoque UTE 2020, 11, 37–47. [Google Scholar] [CrossRef]
- Arbaiza, S.; Gil-Kodaka, P.; Arakaki, N.; Alveal, K. Primeros estadios de cultivo a partir de carpósporas de Chondracanthus chamissoi de tres localidades de la costa peruana. Rev. Biol. Mar. Oceanogr. 2019, 54, 204–213. [Google Scholar] [CrossRef]
- Diaz Ruíz, J.T.; Fretell Timoteo, W.J.; Baltazar Guerrero, P.M.; Castañeda Franco, M.; Meza Balvin, S.J.; Ordoñez Suñiga, C.A. Arnaldoa. 2021. Available online: http://www.scielo.org.pe/pdf/arnal/v28n1/2413-3299-arnal-28-01-163.pdf (accessed on 10 May 2024).
- Atoche-Suclupe, D.; Campos, L.; Uribe, R.A.; Buitrón, B.; Veneros, B.; Berríos, F. Describing Factors That Influence Chondracanthus chamissoi (Rhodophyta) Fishery in Northern Peru under the DPSIR Conceptual Framework: Implications for the Design of Integral Management Strategies. Ocean Coast. Manag. 2023, 244, 106814. [Google Scholar] [CrossRef]
- De Oliveira Bastos, E.; Horta, P.A.; Hayashi, L. Strain Selection in Chondracanthus Teedei (Gigartinaceae, Rhodophyta) Using Tetraspore and Carpospore Progeny: Growth Rates, Tolerance to Temperature and Carrageenan Yield. J. Appl. Phycol. 2021, 33, 2379–2390. [Google Scholar] [CrossRef]
- Colque Arce, L.M. Evaluación del Crecimiento de Cultivo Vegetativo de (Chondracanthus chamissoi), Utilizando Fertilizante Comercial Bayfolan y Medio Guillard F/2, en Condiciones de Laboratorio en el Centro de Acuicultura Morro Sama del FONDEPES; Universidad Nacional Jorge Basadre Grohmann: Tacna, Peru, 2017. [Google Scholar]
- Arbaiza, S.; Castañeda, M.; Gerónimo, G.; Munayco, P.; Reynaga, R.; Advíncula, O. Efecto del fotoperiodo y nutriente foliar comercial en el crecimiento (biomasa) de cochayuyo Porphyra spp. bajo condiciones semicontroladas de cultivo. In Proceedings of the Annual Meeting of the Latin American & Caribbean Aquaculture Societies, Bogotá, Colombia, 23–26 October 2018; p. 25. [Google Scholar]
- Ruiz-Ipanaque, W.; Baltazar Guerrero, P.; Casteñeda-Franco, M.S.; Mires-Reyes, A.J. Capacidad regenerativa post-cosecha de la macroalga roja Chondracanthus chamissoi en dos sistemas de cultivo en la costa centro-sur del Perú. Boletín Investig. Mar. Costeras 2024, 53, 67–82. [Google Scholar] [CrossRef]
- Huaman Fernandez, D.; Baltazar Guerrero, P.; Arbaiza-Quispe, S.; Advíncula Zeballos, O. Evaluación en la formación de discos de fijación secundaria de Chondracanthus chamissoi (yuyo) en condiciones de cultivo semi-controladas. Boletín Investig. Mar. Costeras 2024, 53, 31–44. [Google Scholar] [CrossRef]
- Oyarzo, S.; Ávila, M.; Alvear, P.; Remonsellez, J.P.; Contreras-Porcia, L.; Bulboa, C. Secondary Attachment Disc of Edible Seaweed Chondracanthus chamissoi (Rhodophyta, Gigartinales): Establishment of Permanent Thalli Stock. Aquaculture 2021, 530, 735954. [Google Scholar] [CrossRef]
- Arbaiza Quispe, S.J. Manual de Cultivo de Yuyo Para la Bahía de Sechura (Chondracanthus chamissoi); 2022. Available online: https://rnia.produce.gob.pe/wp-content/uploads/2022/05/Manual-de-Cultivo-de-yuyo-FINAL.pdf (accessed on 10 May 2024).
- Yong, Y.S.; Yong, W.T.L.; Anton, A. Analysis of Formulae for Determination of Seaweed Growth Rate. J. Appl. Phycol. 2013, 25, 1831–1834. [Google Scholar] [CrossRef]
- Ministerio del Ambiente Peru (MINAM). Aprueban Estándares de Calidad Ambiental (ECA) Para Agua y Establecen Disposiciones Complementarias; El Peruano, Alfonso Ugarte: Lima, Peru, 2017; Available online: https://sinia.minam.gob.pe/sites/default/files/sinia/archivos/public/docs/ds-004-2017-minam.pdf (accessed on 10 May 2024).
- Arakaki, N.; Carbajal Enzian, P.; Marquez-Corigliano, D.; Suárez Alarcón, S.; Gil-Kodaka, P.; Perez-Araneda, K.; Tellier, F. Genética de Macroalgas en el Perú: Diagnóstico, guía Metodológica y Casos de Estudio. 2021. Available online: https://hdl.handle.net/20.500.12958/3648 (accessed on 10 May 2024).
- Rodríguez, E.; Fernández, M.; Alvítez, E.; Pollack, L.; Luján, L.; Geldres, C.; Paredes, Y. Seaweeds of the coast of La Libertad region, Peru. Sci. Agropecu. 2018, 9, 71–81. [Google Scholar] [CrossRef]
- Instituto del Mar del Perú (IMARPE) (Ed.) Anuario Científico Tecnológico IMARPE 2019; Instituto del Mar del Perú: Callao, Peru, 2020; Volume 19. [Google Scholar]
- Instituto del Mar del Perú (IMARPE) (Ed.) Anuario Científico Tecnológico IMARPE 2020; Instituto del Mar del Perú: Callao, Peru, 2021; Volume 20. [Google Scholar]
- Instituto del Mar del Perú (IMARPE) (Ed.) Anuario Científico Tecnológico IMARPE 2021; Instituto del Mar del Perú: Callao, Peru, 2022; Volume 21. [Google Scholar]
- Instituto del Mar del Perú (IMARPE) (Ed.) Anuario Científico Tecnológico IMARPE 2022; Instituto del Mar del Perú: Callao, Peru, 2023; Volume 22. [Google Scholar]
- Bulboa, C.; Macchiavello, J.; Oliveira, E.; Véliz, K. Growth Rate Differences between Four Chilean Populations of Edible Seaweed Chondracanthus chamissoi (Rhodophyta, Gigartinales). Aquac. Res. 2008, 39, 1550–1555. [Google Scholar] [CrossRef]
- Dawange, P.; Jaiswar, S. Effects of Ascophyllum Marine Plant Extract Powder (AMPEP) on Tissue Growth, Proximate, Phenolic Contents, and Free Radical Scavenging Activities in Endemic Red Seaweed Gracilaria Corticata Var. Cylindrica from India. J. Appl. Phycol. 2020, 32, 4127–4135. [Google Scholar] [CrossRef]
- Hlaing, W.M.M.; Jarukamjorn, K. Plantlet Regeneration from Callus Cultures of Kappaphycus Alvarezii for Cultivation in Coastal Waters at Myeik Archipelago, Myanmar. Pak. J. Biol. Sci. 2024, 27, 479–486. [Google Scholar] [CrossRef]
- Balar, N.; Depani, P.; Baraiya, M.; Jaiswar, S.; Rathore, M.S.; Singh, V.; Mantri, V.A. Scaled-up Clonal Propagule Production in Gracilaria Dura (Rhodophyta) for Sustainable Feedstock Production and Implications for Circular Economy. Aquac. Int. 2025, 33, 20. [Google Scholar] [CrossRef]
- Arakaki, N.; Flores Ramos, L.; Oscanoa Huaynate, A.I.; Ruíz Soto, A.; Ramírez, M.E. Biochemical and Nutritional Characterization of Edible Seaweeds from the Peruvian Coast. Plants 2023, 12, 1795. [Google Scholar] [CrossRef]
- Centro Tecnológico del Mar—Fundación CETMAR Las Algas Como Recurso Industriales y Tendencias, Valorización Aplicaciones. Available online: https://cetmar.org/wp-content/uploads/2022/11/Las-algas-como-recurso.pdf (accessed on 10 May 2024).
- Gamero-Vega, G.; Vásquez-Corales, E.; Ormeño-Llanos, M.; Cordova-Ruiz, M.; Quitral, V. Characterization of Red Seaweed Chondracanthus chamissoi from the Coasts of Perú: Chemical Composition, Antioxidant Capacity and Functional Properties. Plant Foods Hum. Nutr. 2024, 79, 137–142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).