Ontogenetic Growth Changes in Mercury and Stable Isotope Ratios of Carbon, Nitrogen, and Oxygen in Male and Female Dalli-Type Dall’s Porpoises (Phocoenoides dalli) Stranded in Hokkaido, Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples of Dalli-Type and Truei-Type Dall’s Porpoises
2.2. Chemical Analyses
2.3. Statistical Analyses
3. Results
3.1. General Information on the Male and Female Dalli-Type Dall’s Porpoises
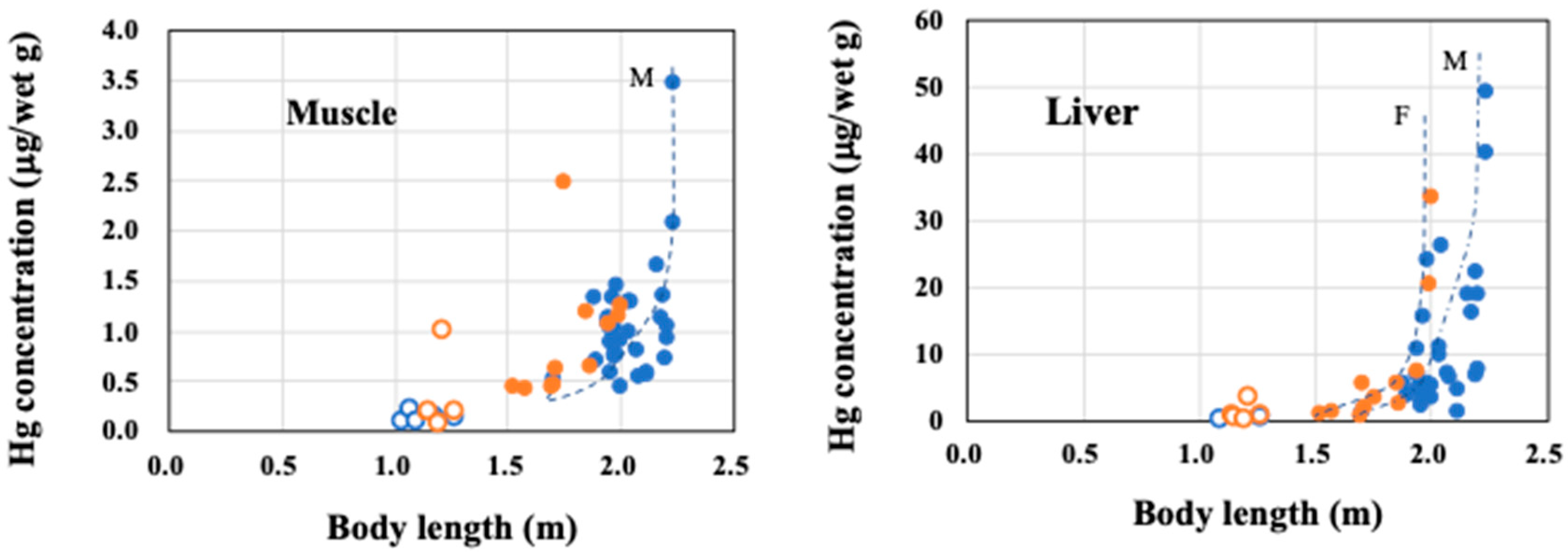
3.2. THg Distribution in the Male and Female Dalli-Type Dall’s Porpoises
3.3. Ontogenetic Growth Changes of δ13C and δ15N Values in the Muscles and Livers of Males and Females
3.4. Relationships Between the δ13C and δ15N Values for Males, Females, and Calves
3.5. Comparison of δ13C and δ15N Values in Liver Samples with Those in Muscle Samples
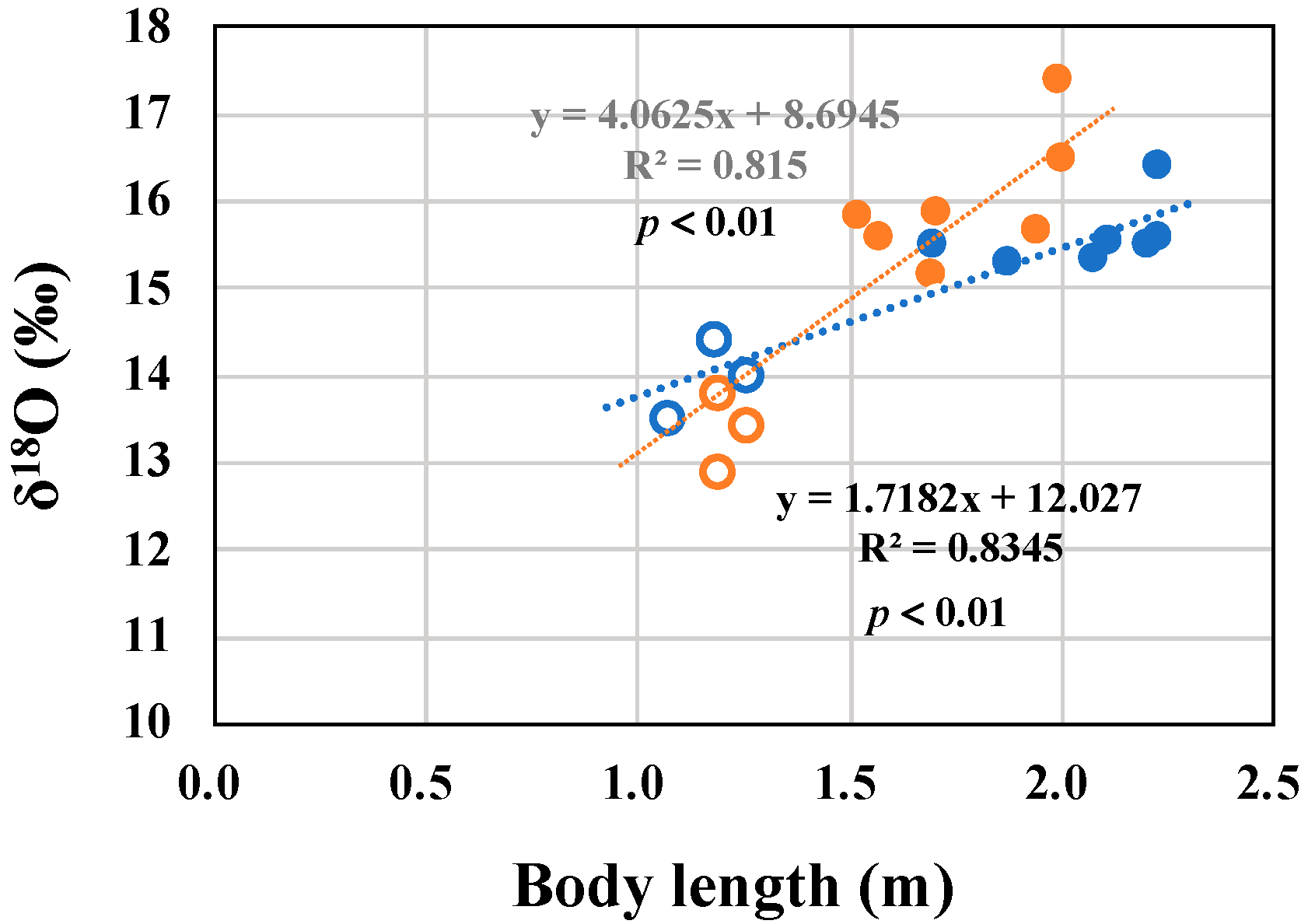
3.6. Ontogenetic Growth Changes of δ18O Values in the Muscles of Males and Females
3.7. Correlations of THg Concentrations in Muscle and Liver with Either δ13C, δ15N, or δ18O Values
3.8. Comparison of THg Concentrations and δ13C and δ15N Values of Dalli-Type with Those of Truei-Type of Dall’s Porpoises
4. Discussion
4.1. THg Concentrations in the Muscle and Liver of Dalli-Type Dall’s Porpoises with Growth
4.2. δ13C, δ15N, and δ18O Values During and After Lactation
4.3. Comparison of δ13C and δ15N Values in Liver Samples with Those in Muscle Samples
4.4. Correlations of THg Concentrations with Wither δ13C, δ15N, or δ18O Values
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Selin, N.E. Global biogeochemical cycling of mercury: A Review. Annu. Rev. Environ. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Atwell, L.; Hobson, K.A.; Welch, H.E. Biomagnification and bioaccumulation of mercury in an Arctic marine food web: Insights from stable nitrogen isotope analysis. Can. J. Fish. Aquat. Sci. 1998, 55, 1114–1121. [Google Scholar] [CrossRef]
- Power, M.; Klein, G.M.; Guiguer, K.R.R.A.; Kwan, M.K.H. Mercury accumulation in the fish community of a subArctic lake in relation to trophic position and carbon sources. J. App. Ecol. 2002, 39, 819–830. [Google Scholar] [CrossRef]
- Pethybridge, H.; Butler, E.C.V.; Cossa, D.; Daley, R. Trophic structure and biomagnification of mercury in an assemblage of deepwater chondrichthyans from southeastern Australia. Mar. Ecol. Prog. Ser. 2012, 451, 163–174. [Google Scholar] [CrossRef]
- Chouvelon, T.; Caurant, F.; Cherel, Y.; Simon-Bouhet, B.; Spitz, J.; Bustamante, P. Species- and size-related patterns in stable isotopes and mercury concentrations in fish help refine marine ecosystem indicators and provide evidence for distinct management units for hake in the Northeast Atlantic. ICES Mar. Sci. 2014, 71, 1073–1087. [Google Scholar] [CrossRef]
- Honda, K.; Tatsukawa, R.; Itano, K.; Miyazaki, N.; Fujiyama, T. Heavy metal concentrations in muscle, liver and kidney tissue of striped dolphin, Stenella coeruleoalba, and their variations with body length, weight, age, and sex. Agric. Biol. Chem. 1983, 47, 1219–1228. [Google Scholar] [CrossRef]
- Endo, T.; Kimura, O.; Hisamichi, Y.; Minoshima, Y.; Haraguchi, K. Age-dependent accumulation of heavy metals in a pod of killer whales (Orcinus orca) stranded in the northern area of Japan. Chemosphere 2007, 67, 51–59. [Google Scholar] [CrossRef]
- Endo, T.; Hisamichi, Y.; Kimura, O.; Haraguchi, K.; Baker, C.S. Contamination levels of mercury and cadmium in melon-headed whales (Peponocephala electra) from a mass stranding on the Japanese coast. Sci. Total Environ. 2008, 401, 73–80. [Google Scholar] [CrossRef]
- Endo, T.; Hisamichi, Y.; Haraguchi, K.; Kato, Y.; Ohta, C.; Koga, N. Hg, Zn and Cu levels in the muscle and liver of tiger sharks (Galeocerdo cuvier) from the coast of Ishigaki Island, Japan: Relationship between metal concentrations and body length. Mar. Pollut. Bull. 2008, 56, 1774–1780. [Google Scholar] [CrossRef]
- Endo, T.; Ohta, C.; Hisamichi, Y.; Kimura, O.; Ogasawara, H.; Koga, N.; Kato, Y.; Haraguchi, Y. Levels of mercury in muscle and liver of star-spotted dogfish (Mustelus manazo) from the northern region of Japan: A comparison with spiny dogfish (Squalus acanthias). Arch. Environ. Contam. Toxicol. 2013, 64, 467–474. [Google Scholar] [CrossRef]
- Le Bourg, B.; Kiszka, J.J.; Bustamante, P.; Heithaus, M.R.; Jaquemet, S.; Humber, F. Effect of body length, trophic position and habitat use on mercury concentrations of sharks from contrasted ecosystems in the southwestern Indian Ocean. Environ. Res. 2019, 169, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Haraguchi, K.; Sakata, M. Mercury and selenium concentrations in the internal organs of toothed whales and dolphins marketed for human consumption in Japan. Sci. Total Environ. 2002, 300, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kimura, O.; Hisamichi, Y.; Minoshima, Y.; Haraguchi, K.; Kakumoto, C.; Kobayashi, M. Distribution of total mercury, methyl mercury and selenium in pod of killer whales (Orcinus orca) stranded in the northern area of Japan: Comparison of mature females with calves. Environ. Pollut. 2006, 144, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kunito, T.; Tanabe, S.; Miyazaki, N. Mercury and its relation with selenium in the liver of Dall’s porpoises (Phocoenoides dalli) off the Sanriku coast of Japan. Environ. Pollut. 2007, 148, 669–673. [Google Scholar] [CrossRef]
- Dietz, R.; Letcher, R.J.; Desforges, J.P.; Eulaers, I.; Sonne, C.; Wilson, S.; Andersen-Ranberg, E.; Basu, N.; Barst, B.D.; Bustnes, J.O.; et al. Current state of knowledge on biological effects from contaminants on arctic wildlife and fish. Sci. Total Environ. 2019, 696, 133792. [Google Scholar] [CrossRef]
- WHO. Methylmercury. Environmental Health Criteria 101; WHO: Geneva, Switzerland, 1990. [Google Scholar]
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of mercury in aquatic food webs: A worldwide meta-analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005, 204, 274–308. [Google Scholar] [CrossRef]
- Amano, M.; Hayano, A. Intermingling of dalli-type Dall’s porpoises into a wintering truei-type population off Japan: Implications from color patterns. Mar. Mamm. Sci. 2007, 23, 1–14. [Google Scholar] [CrossRef]
- Kasuya, T. Small Cetaceans of Japan. Exploitation and Biology; CRC Press: Boca Raton, FL, USA, 2017; pp. 219–244. [Google Scholar]
- FRA (Japan Fisheries Research and Education Agency). Dall’s Porpoise (Phocoenoides dalli). 2022. Available online: https://kokushi.fra.go.jp/R03/R03_49_PDA.pdf (accessed on 22 April 2025).
- Endo, T.; Hotta, Y.; Haraguchi, K.; Sakata, M. Mercury contamination in the red meat of whales and dolphins marketed for human consumption in Japan. Environ. Sci. Technol. 2003, 37, 2681–26815. [Google Scholar] [CrossRef]
- Endo, T.; Haraguchi, K.; Hotta, Y.; Hisamichi, Y.; Lavery, S.; Dalebout, M.L.; Baker, C.S. Total mercury, methyl mercury, and selenium levels in the red meat of small cetaceans sold for human consumption in Japan. Environ. Sci. Technol. 2005, 39, 5703–5708. [Google Scholar] [CrossRef]
- Fisheries Agency of Japanese Government. Small Cetacean Hunting Quotas for FY 2023. 2023. Available online: https://www.jfa.maff.go.jp/j/whale/attach/pdf/index-15.pdf (accessed on 22 April 2025).
- MHLW (Ministry of Health, Labour and Welfare). Advice for Pregnant Women on Fish Consumption and Mercury. 2005. Available online: https://www.mhlw.go.jp/english/wp/other/councils/mercury/index.html (accessed on 22 April 2025).
- Fujise, Y.; Honda, K.; Tatsukawa, R.; Mishima, S. Tissue distribution of heavy metals in Dall’s porpoise in the northwestern Pacific. Mar. Pollut. Bull. 1988, 19, 226–230. [Google Scholar] [CrossRef]
- Yang, J.; Kunito, T.; Tanabe, S.; Miyazaki, N. Mercury in tissues of Dall’s porpoise (Phocoenoides dalli) collected off Sanriku coast of Japan. Fish. Sci. 2002, 68, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 2000, 78, 1–27. [Google Scholar] [CrossRef]
- O’Brien, D.M. Stable isotope ratios as biomarkers of diet for health research. Ann. Rev. Nutr. 2015, 35, 565–595. [Google Scholar] [CrossRef]
- Cherel, Y.; Hobson, K.A. Geographical variation in carbon stable isotope signatures of marine predators: A tool to investigate their foraging areas in the Southern Ocean. Mar. Ecol. Prog. Ser. 2007, 329, 281–287. [Google Scholar] [CrossRef]
- Baylis, A.M.M.; Orben, R.A.; Arnould, J.P.Y.; Peters, K.; Knox, T.; Costa, D.P.; Staniland, I.J. Diving deeper into individual foraging specializations of a large marine predator, the southern sea lion. Oecologia 2015, 179, 1053–1065. [Google Scholar] [CrossRef]
- Chouvelon, T.; Spitz, J.; Caurant, F.; Mèndez-Fernandez, P.; Autier, J.; Lassus-Débat, A.; Chappuis, A.; Bustamante, P. Enhanced bioaccumulation of mercury in deep-sea fauna from the Bay of Biscay (north-east Atlantic) in relation to trophic positions identified by analysis of carbon nitrogen stable isotopes. Deep-Sea Res. Part I 2012, 65, 113–124. [Google Scholar] [CrossRef]
- Kalish, J.M. 13C and 18O isotopic disequilibria in fish otoliths: Metabolic and kinetic effects. Mar. Ecol. Prog. Ser. 1991, 75, 191–203. [Google Scholar] [CrossRef]
- Lukeneder, A.; Harzhauser, M.; Mullegger, S.; Piller, W.E. Stable isotopes (δ18O and δ13C) in Spirula spirula shells from three major oceans indicate developmental changes paralleling depth distributions. Mar. Boil. 2008, 154, 175–182. [Google Scholar] [CrossRef]
- Lin, H.Y.; Shiao, C.; Chen, Y.G.; Iizuka, Y. Ontogenetic vertical migration of grenadiers revealed by otolith microstructures stable isotopic composition. Deep-Sea Res. Part I 2012, 61, 123–130. [Google Scholar] [CrossRef]
- Méndez-Fernandez, P.; Pierce, G.J.; Bustamante, P.; Chouvelon, T.; Ferreira, M.; González, A.F.; López, A.; Read, F.L.; Santos, M.B.; Spitz, J.; et al. Ecological niche segregation among five toothed whale species off the NW Iberian Peninsula using ecological tracers as multiapproach. Mar. Biol. 2013, 160, 2825–2840. [Google Scholar] [CrossRef]
- Clementz, M.T.; Koch, P.L. Differentiating aquatic mammal habitat and foraging ecology with stable isotopes in tooth enamel. Oecologia 2001, 129, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.J.D.; Longstaffe, F.J.; Ferguson, S.H. Dentine oxygen isotopes (δ18O) as a proxy for odontocete distributions and movements. Ecol. Evol. 2016, 6, 4643–4653. [Google Scholar] [CrossRef] [PubMed]
- Ciner, B.; Wang, Y.; Parker, W. Oxygen isotopic variations in modern cetacean teeth and bones: Implications for ecological, paleoecological, and paleoclimatic studies. Sci. Bull. 2016, 61, 92–104. [Google Scholar] [CrossRef]
- Le Croizier, S.G.; Schaal, G.; Point, D.; Le Loc’h, F.; Machu, E.; Fall, M.; Munaron, J.M.; Boyé, A.; Walter, P.; Laë, R.; et al. Stable isotope analyses revealed the influence of foraging habitat on mercury accumulation in tropical coastal marine fish. Sci. Total Environ. 2019, 650, 2129–2140. [Google Scholar] [CrossRef]
- Das, K.; Holsbeek, L.; Browning, J.; Siebert, U.; Birkun, A., Jr.; Bouquegneau, J.M. Trace metal and stable isotope measurements (δ13C and δ15N) in the harbour porpoise Phocoena phocoena relicta from the Black Sea. Environ. Pollut. 2004, 13, 197–204. [Google Scholar] [CrossRef]
- Endo, T.; Kimura, O.; Terasaki, M.; Kato, Y.; Fujii, Y.; Haraguchi, K. Comparison of carbon, nitrogen, and oxygen stable isotope ratios and mercury concentrations in muscle tissues of five beaked whale species and sperm whales stranded in Hokkaido, Japan. Isot. Environ. Health Stud. 2024, 60, 251–271. [Google Scholar] [CrossRef]
- Newsome, S.D.; Clementz, M.T.; Koch, P.L. Using stable isotope biogeochemistry to study marine mammal ecology. Mar. Mamm. Sci. 2010, 26, 509–572. [Google Scholar] [CrossRef]
- Valenzuela, L.O.; Sironi, M.; Rowntree, V.J. Interannual variation in the stable isotope differences between mothers and their calves in southern right whales (Eubalaena australis). Aquat. Mamm. 2010, 36, 138–147. [Google Scholar] [CrossRef]
- Drago, M.; Franco-Trecu, V.; Cardona, L.; Inchausti, P. Diet-to-female and female-to-pup isotopic discrimination in South American sea lions. Rapid Commun. Mass. Spectrom. 2015, 29, 1513–1520. [Google Scholar] [CrossRef]
- Borrell, A.; Gómez-Campos, E.; Aguilar, A. Influence of reproduction on stable-isotope ratios: Nitrogen and carbon isotope discrimination between mothers, fetuses, and milk in the fin whale, a capital breeder. Physiol. Biochem. Zool. 2016, 89, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kimura, O.; Terasaki, M.; Kobayashi, M. Body length, stable carbon and nitrogen isotope ratios and mercury levels in common minke whales (Balaenoptera acutorostrata) stranded along the coast of Hokkaido, Japan. Aquat. Mamm. 2021, 47, 86–95. [Google Scholar] [CrossRef]
- Endo, T.; Terasaki, M.; Kimura, O. Stable isotope ratios of carbon, nitrogen, oxygen, and mercury concentrations in North Pacific baleen whales and the comparison of their calves with toothed whale calves. J. Veter Sci. Med. 2022, 10, 10. [Google Scholar] [CrossRef]
- Endo, T.; Kimura, O.; Terasaki, M.; Kato, Y.; Fujii, Y.; Haraguchi, K. Changes in carbon, nitrogen, and oxygen stable isotope ratios and mercury concentrations in killer whales (Orcinus orca) during and after lactation. J. Mar. Sci. Eng. 2024, 12, 623. [Google Scholar] [CrossRef]
- Oftedal, O.T. Lactation in whales and dolphins: Evidence of divergence between baleen- and toothed-species. J. Mammary Gland Biol. Neoplasia 1997, 2, 205–230. [Google Scholar] [CrossRef]
- Kurle, C.M.; Worthy, G.A.J. Stable nitrogen and carbon isotope ratios in multiple tissues of the northern fur seal Callorhinus ursinus: Implications for dietary and migratory reconstructions. Mar. Ecol. Prog. Ser. 2002, 236, 289–300. [Google Scholar] [CrossRef]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Borrell, A.; Abad-Oliva, N.; Gómez-Campos, E.; Giménez, J.; Aguilar, A. Discrimination of stable isotopes in fin whale tissues and application to diet assessment in cetaceans. Rapid Commun. Mass. Spectrom. 2012, 26, 1596–1602. [Google Scholar] [CrossRef]
- Vanderklift, M.A.; Ponsard, S. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia 2003, 136, 169–182. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Perini, M.; Piasentier, E. Stable isotope ratio analysis for assessing the authenticity of food animal origin. Compr. Rev. Food Sci. Food Saf. 2016, 15, 868–877. [Google Scholar] [CrossRef]
- Endo, T.; Kobayashi, M. Typical changes in carbon and nitrogen stable isotope ratios and mercury concentration during the lactation of marine mammals. In Marine Mammals; Kaoud, H.A.E., Ed.; IntechOpen: London, UK, 2022; pp. 55–78. Available online: https://www.intechopen.com/predownload/81844 (accessed on 22 April 2025).
- Logan, J.M.; Lutcavage, M.E. A comparison of carbon and nitrogen stable isotope ratios of fish tissues following lipid extractions with non-polar and traditional chloroform/methanol solvent systems. Rapid Commun. Mass. Spectrom. 2008, 22, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Hotta, Y.; Hisamichi, Y.; Kimura, O.; Sato, R.; Haraguchi, K.; Funahasi, N.; Baker, C.S. Stable isotope ratios and mercury levels in red meat products from six baleen whales sold in Japanese markets. Ecotoxicol. Environ. Saf. 2012, 79, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Hisamichi, Y.; Kimura, O.; Haraguchi, K.; Lavery, S.; Dalebout, M.L.; Funahashi, N.; Baler, C.S. Stable isotope ratios of carbon and nitrogen and mercury concentrations in 13 toothed whale species taken from the western Pacific Ocean off Japan. Environ. Sci. Technol. 2010, 44, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Itano, K.; Kawai, S.; Miyazaki, N.; Tatsukawa, R.; Fujiyama, T. Mercury and selenium levels at the fetal and suckling stages of striped dolphin, Stenella coeruleoalba. Agric. Biol. Chem. 1984, 48, 1691–1698. [Google Scholar] [CrossRef][Green Version]
- Caon, G.; Secchi, E.R.; Capp, E.; Kucharski, L.C. Milk composition of franciscana dolphin (Pontoporia blainvillei) from Rio Grande do Sul, southern Brazil. J. Mar. Biol. Assoc. U. K. 2008, 88, 1099–1101. [Google Scholar] [CrossRef]
- Yang, J.; Kunito, T.; Anan, Y.; Tanabe, S.; Miyazaki, N. Total and subcellular distribution of trace elements in the lever of a mother–fetus pair of Dall’s porpoises (Phocoenoides dalli). Mar. Pollut. Bull. 2004, 48, 1122–1129. [Google Scholar] [CrossRef]
- Vales, D.G.; Cardona, L.; Garcia, N.A.; Zenteno, L.; Crespo, E.A. Ontogenetic dietary changes in male South American fur seals Arctocephalus australis in Patagonia. Mar. Ecol. Prog. Ser. 2015, 525, 245–260. [Google Scholar] [CrossRef]
- Hisamichi, Y.; Haraguchi, K.; Endo, T. Levels of mercury and organochlorine compounds and stable isotope ratios in three tuna species taken from different regions of Japan. Environ. Sci. Technol. 2010, 44, 5971–5978. [Google Scholar] [CrossRef]
- Ohizumi, H.; Myasaki, N. Differences in stable isotope ratios of Dall’s porpoises (Phocoenoides dalli) between coastal and oceanic areas of the North Pacific. Fish. Oceanogr. 2010, 19, 257–261. [Google Scholar] [CrossRef]
- Ryan, R.C.; McHugh, B.; Trueman, C.N.; Harrod, C.; Berrow, S.D.; O’Connor, I. Accounting for the effects of lipids in stable isotope (δ13C and δ15N values) analysis of skin and blubber of balaenopterid whales. Rapid Commun. Mass. Spectrom. 2012, 26, 2745–2754. [Google Scholar] [CrossRef]
- Choy, C.A.; Popp, B.N.; Kaneko, J.J.; Drazen, J.C. The influence of depth on mercury levels in pelagic fishes and their prey. Proc. Natl. Acad. Sci. USA 2009, 106, 13865–13869. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.M.; Lutcavage, M.E. Assessment of trophic dynamics of cephalopods large pelagic fishes in the central North Atlantic Ocean using stable isotope analysis. Deep-Sea Res. Part II 2013, 95, 63–73. [Google Scholar] [CrossRef]
- Tsui, M.T.-K.; Blum, J.D.; Kwon, S.Y. Review of stable mercury isotopes in ecology and biogeochemistry. Sci. Total Environ. 2020, 716, 135386. [Google Scholar] [CrossRef] [PubMed]
- Médieu, A.; Spitz, J.; Point, D.; Sonke, J.E.; Loutrage, L.; Laffont, L.; Chouvelon, T. Mercury stable isotopes reveal the vertical distribution and trophic ecology of deep-pelagic organisms over the North-East Atlantic Ocean continental slope. Environ. Sci. Technol. 2024, 58, 18733–18743. [Google Scholar] [CrossRef]




| Male | Female | ||
|---|---|---|---|
| Body length (m) | 1.91 ± 0.34 (n = 37) | 1.57 ± 0.32 (n = 17) | |
| THg concentrations | Muscle | 0.95 ± 0.62 (n = 37) | 0.71 ± 0.60 (n = 17) |
| (μg/wet g) | Liver | 10.3 ± 11.1 (n = 35) | 5.24 ± 8.78 (n = 17) |
| Kidney | 2.08 ± 2.26 (n = 33) | 1.05 ± 0.82 (n = 15) | |
| Lung | 0.60 ± 0.56 (n = 23) | 0.37 ± 0.34 (n = 14) | |
| Spleen | 0.82 ± 1.08 (n = 33) | 0.42 ± 0.49 (n = 12) | |
| Heart | 0.58 ± 0.29 (n = 25) | 0.41 ± 0.29 (n = 14) | |
| Blood | 0.24 ± 0.11 (n = 29) | 0.20 ± 0.11 (n = 14) |
| δ13C (‰) | δ15N (‰) | δ18O (‰) | |||
|---|---|---|---|---|---|
| Muscle | All animals | Male | −19.4 ± 0.5 (n = 37) | 13.0 ± 0.8 (n = 37) | 15.1 ± 0.9 (n = 10) |
| Female | −19.3 ± 0.3 (n = 17) | 13.4 ± 1.2 (n = 17) | 15.2 ± 1.4 (n = 10) | ||
| Total | −19.4 ± 0.4 (n = 54) | 13.1 ± 0.9 (n = 54) | 15.2 ± 1.2 (n = 20) | ||
| Calves | Male | −19.5 ± 0.8 (n = 5) | 14.4 ± 0.7 (n = 5) | 14.0 ± 0.4 ** (n = 3) | |
| Female | −19.2 ± 0.2 (n = 6) | 14.8 ± 0.5 (n = 6) | 13.3 ± 0.5 ** (n = 3) | ||
| Total | −19.4 ± 0.6 (n = 11) | 14.6 ± 0.6 * (n = 11) | 13.7 ± 0.5 ** (n = 6) | ||
| Weaned | Male | −19.4 ± 0.4 (n = 32) | 12.8 ± 0.6 (n = 32) | 15.6 ± 0.9 (n = 7) | |
| Animals | Female | −19.4 ± 0.3 (n = 11) | 12.6 ± 0.5 (n = 11) | 15.6 ± 1.1 (n = 8) | |
| Total | −19.4 ± 0.4 (n = 43) | 12.8 ± 0.5 (n = 43) | 15.6 ± 0.8 (n = 15) | ||
| Liver | All animals | Male | −19.5 ± 0.5 (n = 25) | 14.0 ± 0.8 (n = 25) | ND |
| Female | −19.4 ± 0.4 (n = 8) | 13.8 ± 0.7 (n = 8) | ND | ||
| Total | −19.5 ± 0.4 (n = 33) | 13.9 ± 0.8 (n = 33) | ND | ||
| Calves | Male | −19.6 and −19.3 (n = 2) | 13.9 and 14.4 (n = 2) | ND | |
| Female | −18.6 and −18.5 (n = 2) | 14.7 and 5.3 (n = 2) | ND | ||
| Total | −19.0 ± 0.5 (n = 4) | 14.6 ± 0.6 (n = 4) | ND | ||
| Weaned | Male | −19.6 ± 0.4 (n = 23) | 13.9 ± 0.7 (n = 23) | ND | |
| Animals | Female | −19.6 ± 0.5 (n = 6) | 13.7 ± 0.7 (n = 6) | ND | |
| Total | −19.6 ± 0.4 (n = 29) | 13.9 ± 0.7 (n = 29) | ND |
| Muscle | Liver | ||||
|---|---|---|---|---|---|
| R2 | p Value | R2 | p Value | ||
| δ13C | Male (n = 32) | 0.308 | 0.087 | 0.514 | 0.003 |
| Female (n = 11) | 0.183 | 0.590 | 0.610 | 0.046 | |
| δ15N | Male (n = 32) | 0.104 | 0.569 | 0.071 | 0.697 |
| Female (n = 11) | 0.356 | 0.283 | 0.060 | 0.860 | |
| δ18O | Male (n = 7) | 0.484 | 0.083 | 0.283 | 0.219 |
| Female (n = 7) | 0.484 | 0.082 | 0.640 | 0.031 | |
| Dalli-Type (n = 43) | Truei-Type (n = 5) | Type Unknown (n = 8) * | ||
|---|---|---|---|---|
| Stranded in Hokkaido (Weaned Animals) | Hunted in the North Pacific Ocean off Miyagi Prefecture (Mature Animals) | Hunted in the North Pacific Ocean Area and Maturity Not Known | ||
| Muscle | THg (μg/wet g) | 1.04 ± 0.58 | 1.10 ± 0.35 | 1.27 ± 0.33 |
| δ13C (‰) | −19.4 ± 0.4 | −18.4 ± 0.5 | −18.8 ± 0.2 | |
| δ15N (‰) | 12.8 ± 0.5 | 13.1 ± 0.2 | 13.2 ± 0.3 | |
| Liver | THg (μg/wet g) | 10.3 ± 11.0 | 8.74 ± 2.54 | ND |
| δ13C (‰) | −19.6 ± 0.4 | −17.8 ± 0.5 ** | ND | |
| δ15N (‰) | 13.9 ± 0.7 | 13.9 ± 0.7 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, T.; Kimura, O.; Terasaki, M.; Kato, Y.; Fujii, Y.; Haraguchi, K. Ontogenetic Growth Changes in Mercury and Stable Isotope Ratios of Carbon, Nitrogen, and Oxygen in Male and Female Dalli-Type Dall’s Porpoises (Phocoenoides dalli) Stranded in Hokkaido, Japan. J. Mar. Sci. Eng. 2025, 13, 892. https://doi.org/10.3390/jmse13050892
Endo T, Kimura O, Terasaki M, Kato Y, Fujii Y, Haraguchi K. Ontogenetic Growth Changes in Mercury and Stable Isotope Ratios of Carbon, Nitrogen, and Oxygen in Male and Female Dalli-Type Dall’s Porpoises (Phocoenoides dalli) Stranded in Hokkaido, Japan. Journal of Marine Science and Engineering. 2025; 13(5):892. https://doi.org/10.3390/jmse13050892
Chicago/Turabian StyleEndo, Tetsuya, Osamu Kimura, Masaru Terasaki, Yoshihisa Kato, Yukiko Fujii, and Koichi Haraguchi. 2025. "Ontogenetic Growth Changes in Mercury and Stable Isotope Ratios of Carbon, Nitrogen, and Oxygen in Male and Female Dalli-Type Dall’s Porpoises (Phocoenoides dalli) Stranded in Hokkaido, Japan" Journal of Marine Science and Engineering 13, no. 5: 892. https://doi.org/10.3390/jmse13050892
APA StyleEndo, T., Kimura, O., Terasaki, M., Kato, Y., Fujii, Y., & Haraguchi, K. (2025). Ontogenetic Growth Changes in Mercury and Stable Isotope Ratios of Carbon, Nitrogen, and Oxygen in Male and Female Dalli-Type Dall’s Porpoises (Phocoenoides dalli) Stranded in Hokkaido, Japan. Journal of Marine Science and Engineering, 13(5), 892. https://doi.org/10.3390/jmse13050892







