First Record of a Cannonball Jellyfish Bloom (Stomolophus sp.) in Venezuelan Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Bloom Detection and Stomolophus’ Occurrence in the Western Atlantic
2.2. Environmental Data Acquisition and Visualization
3. Results
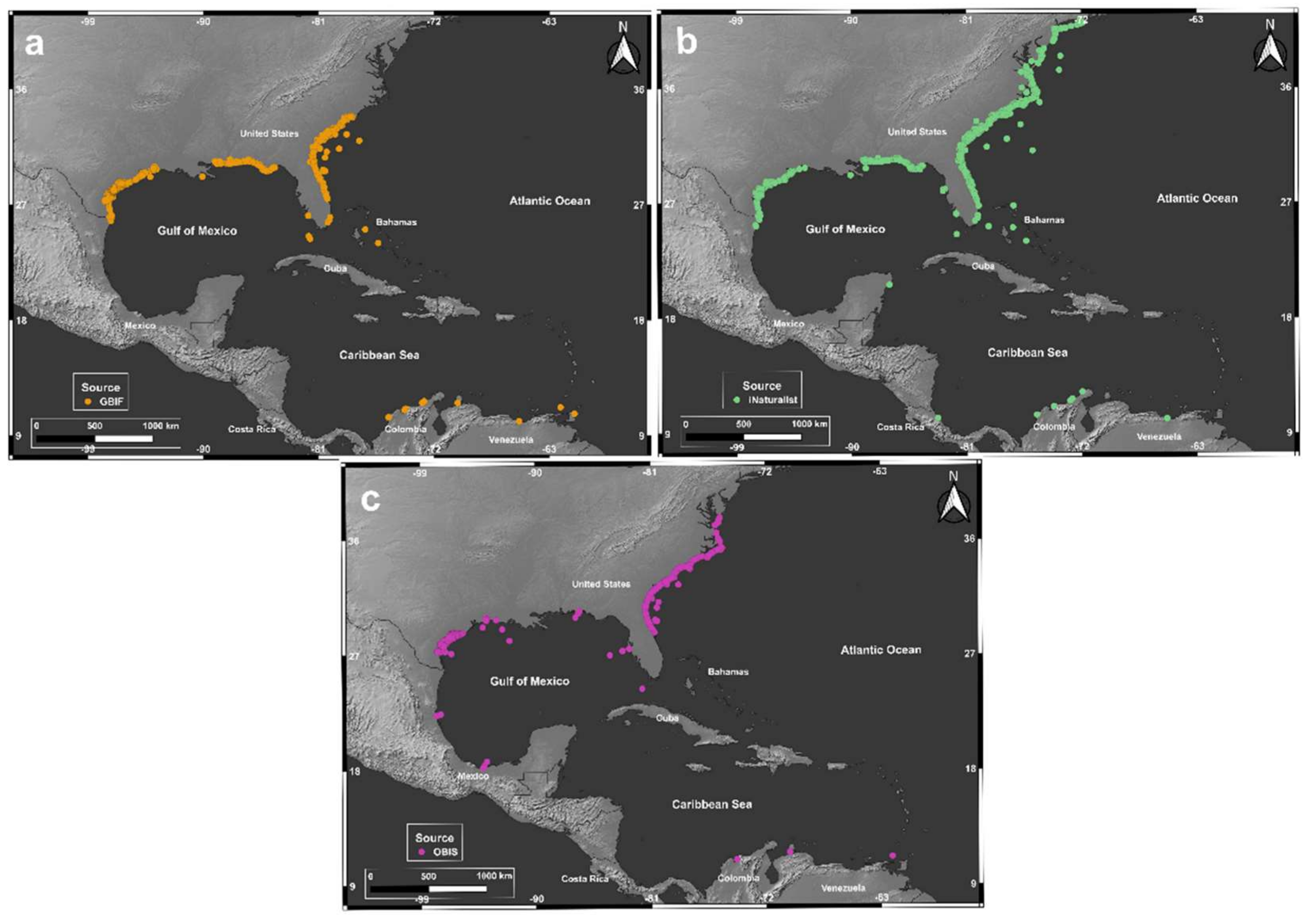
3.1. Bloom Detection and Stomolophus’ Occurrence in the Western Atlantic
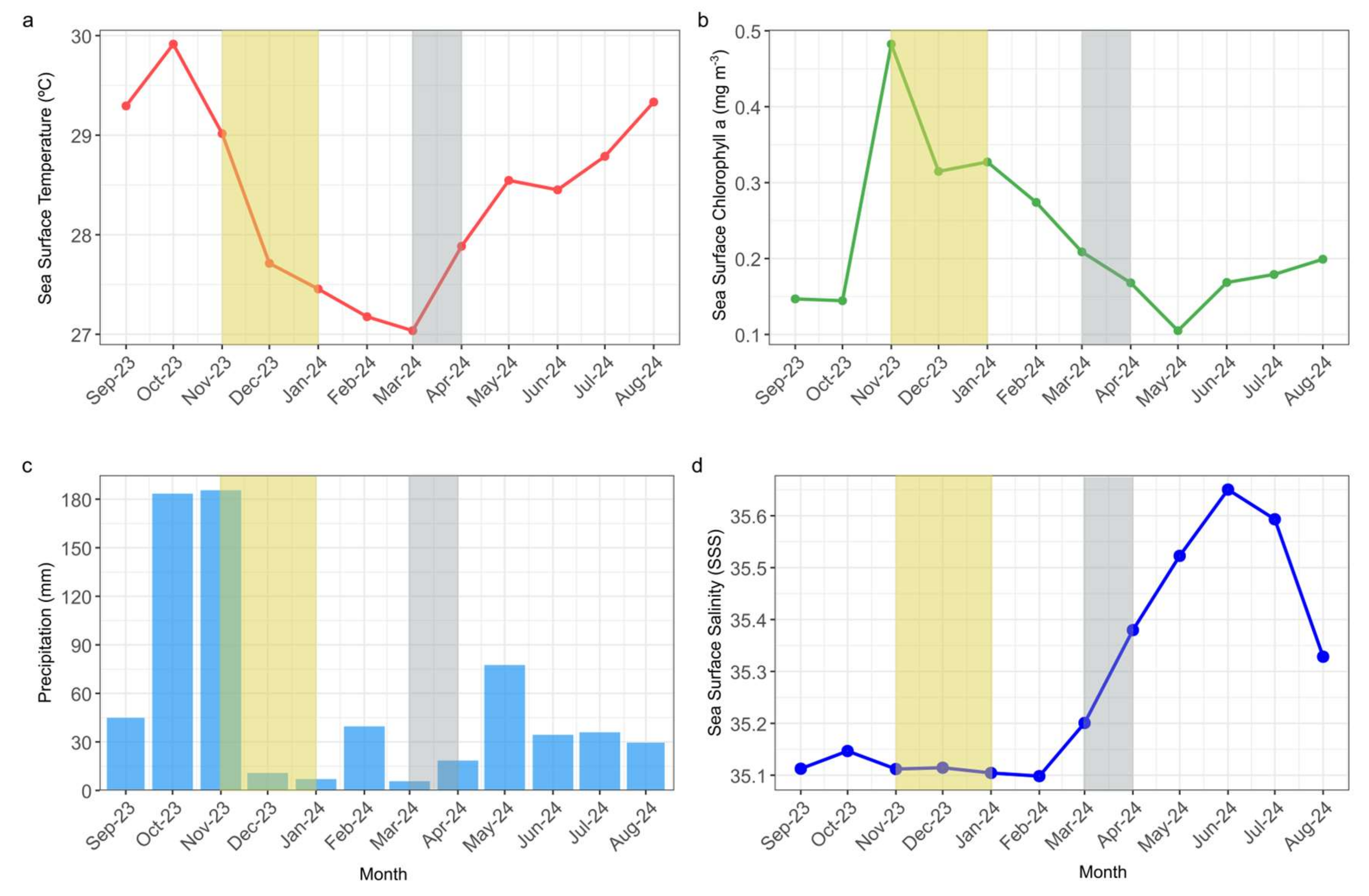
3.2. Environmental Conditions
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purcell, J.E.; Uye, S.-I.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Condon, R.H.; Duarte, C.M.; Pitt, K.A.; Robinson, K.L.; Lucas, C.H.; Sutherland, K.R.; Mianzan, H.W.; Bogeberg, M.; Purcell, J.E.; Decker, M.B.; et al. Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl. Acad. Sci. USA 2013, 110, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Pitt, K.A.; Lucas, C.H.; Condon, R.H.; Duarte, C.M.; Stewart-Koster, B. Claims that anthropogenic stressors facilitate jellyfish blooms have been amplified beyond the available evidence: A systematic review. Front. Mar. Sci. 2018, 5, 451. [Google Scholar] [CrossRef]
- Gamero-Mora, E.; Nevarez-Lopez, C.A.; Llera-Herrera, R.; Muhlia-Almazan, A. Transcriptomic modifications induced by short-term temperature exposure reveal jellyfish adaptive energetic responses. Hydrobiologia 2024, 852, 1789–1803. [Google Scholar] [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Pérez-Ruzafa, A. The unpredictability of scyphozoan jellyfish blooms. Front. Mar. Sci. 2024, 11, 1349956. [Google Scholar] [CrossRef]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Q.; Yang, L.; Jia, C.; Guan, C.; Wang, H.; Guo, H. Aggregation process of two disaster-causing jellyfish species, Nemopilema nomurai and Aurelia coerulea, at the intake area of a nuclear power cooling-water system in Eastern Liaodong Bay, China. Front. Mar. Sci. 2023, 9, 1098232. [Google Scholar] [CrossRef]
- Breitburg, D.L.; Loher, T.; Pacey, C.A.; Gerstein, A. Varying effects of low dissolved oxygen on trophic interactions in an estuarine food web. Ecol. Monogr. 1997, 67, 489–507. [Google Scholar] [CrossRef]
- Purcell, J.E.; Baxter, E.J.; Fuentes, V.L. Jellyfish as products and problems of aquaculture. In Advances in Aquaculture Hatchery Technology, 1st ed.; Allan, G., Burnell, G., Eds.; Woodhead Publishing: Oxford, UK, 2013; pp. 404–430. [Google Scholar] [CrossRef]
- Duarte, C.M.; Pitt, K.A.; Lucas, C.H.; Purcell, J.E.; Uye, S.I.; Robinson, K.; Brotz, L.; Decker, M.B.; Sutherland, K.R.; Malej, A.; et al. Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ. 2013, 11, 91–97. [Google Scholar] [CrossRef]
- Thiebot, J.B.; McInnes, J.C. Why do marine endotherms eat gelatinous prey? ICES J. Mar. Sci. 2020, 77, 58–71. [Google Scholar] [CrossRef]
- Arai, M.N. The potential importance of podocysts to the formation of scyphozoan blooms: A review. Jellyfish Blooms Causes Conseq. Recent Adv. Dev. Hydrobiol. 2009, 206, 193–199. [Google Scholar] [CrossRef]
- Lucas, C.H.; Graham, W.M.; Widmer, C. Jellyfish life histories: Role of polyps in forming and maintaining scyphomedusa populations. Adv. Mar. Biol. 2012, 63, 133–196. [Google Scholar] [CrossRef]
- Boero, F.; Bouillon, J.; Gravili, C.; Miglietta, M.P.; Parsons, T.; Piraino, S. Gelatinous plankton: Irregularities rule the world (sometimes). Mar. Ecol. Prog. Ser. 2008, 356, 299–310. [Google Scholar] [CrossRef]
- Wang, L.; Sun, T.; Jiang, H.; Zhang, W.; He, J.; Ma, Y.; Zhao, J.; Dong, Z. Coastal aquaculture ponds represent a notable source of the blooming jellyfish Aurelia coerulea. Front. Ecol. Evol. 2025, 13, 1528335. [Google Scholar] [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Pérez-Ruzafa, A. Larger scyphozoan species dwelling in temperate, shallow waters show higher blooming potential. Mar. Pollut. Bull. 2021, 173, 113100. [Google Scholar] [CrossRef]
- Brotz, L.; Cheung, W.W.; Kleisner, K.; Pakhomov, E.A.; Pauly, D. Increasing jellyfish populations: Trends in large marine ecosystems. Jellyfish Blooms IV Dev. Hydrobiologia 2012, 220, 3–22. [Google Scholar] [CrossRef]
- Sigurdsson, G.M.; Lüskow, F.; Gislason, A.; Svavarsson, J. Detached tentacles of lion’s mane jellyfish Cyanea capillata can injure aquaculture fish. Aquacult. Environ. Interact. 2024, 16, 263–266. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.P.; Leong, F.-M.; Rudloe, J. Jellyfish as food. Hydrobiologia 2001, 451, 11–17. [Google Scholar] [CrossRef]
- Brotz, L.; Schiariti, A.; López-Martínez, J.; Álvarez-Tello, J.; Hsieh, Y.H.P.; Jones, R.P.; Quiñones, J.; Dong, Z.; Morandini, A.C.; Preciado, M.; et al. Jellyfish fisheries in the Americas: Origin, state of the art, and perspectives on new fishing grounds. Rev. Fish Biol. Fish. 2017, 27, 1–29. [Google Scholar] [CrossRef]
- Edelist, D.; Angel, D.L.; Canning-Clode, J.; Gueroun, S.K.; Aberle, N.; Javidpour, J.; Andrade, C. Jellyfishing in Europe: Current status, knowledge gaps, and future directions towards a sustainable practice. Sustainability 2021, 13, 12445. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, L.X.; Tan, K.F.; Liu, H.Y.; Purcell, J.E.; Li, P.J.; Ye, C.C. Stock enhancement of the edible jellyfish (Rhopilema esculentum Kishinouye) in Liaodong Bay, China: A review. In Jellyfish Blooms: Causes, Consequences, and Recent Advances. Developments in Hydrobiology; Pitt, K.A., Purcell, J.E., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 206, pp. 113–118. [Google Scholar] [CrossRef]
- Brotz, L.; Cisneros-Montemayor, A.M.; Cisneros-Mata, M.Á. The race for jellyfish: Winners and losers in Mexico’s Gulf of California. Mar. Pol. 2021, 134, 104775. [Google Scholar] [CrossRef]
- D’Ambra, I.; Merquiol, L. Jellyfish from fisheries by-catches as a sustainable source of high-value compounds with biotechnological applications. Marine Drugs 2022, 20, 266. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Tseng, L.C.; Yoon, Y.H.; Ramirez-Romero, E.; Hwang, J.S.; Molinero, J.C. The global spread of jellyfish hazards mirrors the pace of human imprint in the marine environment. Environ. Internat. 2023, 171, 107699. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhao, Y.; Jiang, S. The morphology and structure of Stomolophus meleagris L. Agassiz. Fish. Sci. 1992, 11, 5–8. (In Chinese) [Google Scholar]
- Jarms, G.; Morandini, A.C. World Atlas of Jellyfish; Dölling und Galitz Verlag: Hamburg, Germany, 2019; p. 816. [Google Scholar]
- Morandini, A.C. Morphology of Rhizostomeae jellyfishes: What is known and what we advanced since the 1970s. Adv. Mar. Biol. 2024, 98, 61–97. [Google Scholar] [CrossRef]
- Getino-Mamet, L.N.; Daglio, L.G.; García-De León, F.J. High genetic differentiation in the edible cannonball jellyfish (Cnidaria: Scyphozoa: Stomolophus spp.) from the Gulf of California, Mexico. Fish. Res. 2019, 219, 105328. [Google Scholar] [CrossRef]
- Nevárez-López, C.; Hernández-Saavedra, N.; Sánchez-Paz, A.; Rojas-Posadas, D.; Muhlia-Almazán, A.; López-Martínez, J. Colour polymorphism and genetic structure in the cannonball jellyfish (Stomolophus meleagris, L. Agassiz, 1860) in the Gulf of California. Mar. Biol. Res. 2021, 16, 714–728. [Google Scholar] [CrossRef]
- López-Martínez, J.; Arzola-Sotelo, E.A.; Nevárez-Martínez, M.O.; Álvarez-Tello, F.J.; Morales-Bojórquez, E. Modeling growth on the cannonball jellyfish Stomolophus meleagris based on a multi-model inference approach. Hydrobiologia 2020, 847, 1399–1422. [Google Scholar] [CrossRef]
- Calder, D.R. Life history of the cannonball jellyfish Stomolophus meleagris L. Agassiz, 1860 (Scyphozoa, Rhizostomida). Biol. Bull. 1982, 162, 149–162. [Google Scholar] [CrossRef]
- Fuentes, V.; Straehler-Pohl, I.; Atienza, D.; Franco, I.; Tilves, U.; Gentile, M.; Acevedo, M.; Olariaga, A.; Gili, J.M. Life cycle of the jellyfish Rhizostoma pulmo (Scyphozoa: Rhizostomeae) and its distribution, seasonality and inter-annual variability along the Catalan coast and the Mar Menor (Spain, NW Mediterranean). Mar. Biol. 2011, 158, 2247–2266. [Google Scholar] [CrossRef]
- Schiariti, A.; Morandini, A.C.; Jarms, G.; von Glehn-Paes, R.; Franke, S.; Mianzan, H. Asexual reproduction strategies and blooming potential in Scyphozoa. Mar. Ecol. Prog. Ser. 2014, 510, 241–253. [Google Scholar] [CrossRef]
- Treible, L.M.; Condon, R.H. Temperature-driven asexual reproduction and strobilation in three scyphozoan jellyfish polyps. J. Exp. Mar. Biol. Ecol. 2019, 520, 151204. [Google Scholar] [CrossRef]
- Girón-Nava, A.; López-Sagástegui, C.; Aburto-Oropeza, O. On the conditions of the 2012 cannonball jellyfish (Stomolophus meleagris) bloom in Golfo de Santa Clara: A fishery opportunity? Fish. Manag. Ecol. 2015, 22, 261–264. [Google Scholar] [CrossRef]
- Larson, R.J. Diet, prey selection and daily ration of Stomolophus meleagris, a filter-feeding scyphomedusa from the NE Gulf of Mexico. Estuar. Coast. Shelf. 1991, 32, 511–525. [Google Scholar] [CrossRef]
- Álvarez-Tello, F.J.; López-Martínez, J.; Lluch-Cota, D.B. Trophic spectrum and feeding pattern of cannonball jellyfish Stomolophus meleagris (Agassiz, 1862) from central Gulf of California. J. Mar. Biol. Assoc. 2016, 96, 1217–1227. [Google Scholar] [CrossRef]
- Nagata, R.M.; D’Ambra, I.; Lauritano, C.; von Montfort, G.M.; Djeghri, N.; Jordano, M.A.; Colin, S.P.; Costello, J.H.; Leoni, V. Physiology and functional biology of Rhizostomeae jellyfish. Adv. Mar. Biol. 2024, 98, 255–360. [Google Scholar] [CrossRef]
- Pico-Vargas, A.; Quirós-Rodríguez, J.; Cedeño-Posso, C. Primer registro de medusas Stomolophus meleagris (Cnidaria: Scyphozoa) en la bahía de Cispatá, Córdoba, Colombia. Rev. Biol. Mar. Oceanogr. 2016, 51, 709–712. [Google Scholar] [CrossRef]
- Banha, T.N.S.; Morandini, A.C.; Rosário, R.P.; Martinelli Filho, J.E. Scyphozoan jellyfish (Cnidaria, Medusozoa) from Amazon coast: Distribution, temporal variation and length-weight relationship. J. Plank. Res. 2020, 42, 767–778. [Google Scholar] [CrossRef]
- Faulk, L.G.; Smart, T.; Stone, J.P. Temporal and spatial distribution of the cannonball jellyfish Stomolophus meleagris in the South Atlantic Bight, USA. Mar. Ecol. Prog. Ser. 2023, 717, 51–65. [Google Scholar] [CrossRef]
- Gómez-Daglio, L.; Dawson, M.N. Species richness of jellyfishes (Scyphozoa: Discomedusae) in the Tropical Eastern Pacific: Missed taxa, molecules, and morphology match in a biodiversity hotspot. Invertebr. Syst. 2017, 31, 635–663. [Google Scholar] [CrossRef]
- Sastré-Velásquez, C.D.; Rodríguez-Armenta, C.; Minjarez-Osorio, C.; La Re-Vega, D. Current status of the knowledge of the cannonball Jellyfish (Stomolophus meleagris). Epistemus 2022, 16, 75–83. [Google Scholar] [CrossRef]
- Morejón-Arrojo, R.D.; Lüskow, F.; Miglietta, M.P.; Pakhomov, E.A.; Rodríguez-Viera, L. Are marine heatwaves driving increases in scyphozoan jellyfish abundance across the Caribbean Sea and Gulf of Mexico? Discover Ocean 2025. submitted. [Google Scholar]
- Gómez-Salinas, L.C.; López-Martínez, J.; Morandini, A.C. The young stages of the cannonball jellyfish (Stomolophus sp. 2) from the central Gulf of California (Mexico). Diversity 2021, 13, 229. [Google Scholar] [CrossRef]
- Trinci, G. Sopra una discomedusa des Golfo di Paria (America del Sud). Ann. Mus. Zool. Napoli. 1906, 9, 1–4. [Google Scholar]
- Stiasny, G. Die Rhizostomeen-Sammlung des British Museum (Natural History) in London. Zool. Meded. 1931, 14, 137–178. [Google Scholar]
- Chamberlain, S. Package Rnoaa; National Oceanic and Atmospheric Administration: Silver Spring, MA, USA, 2019; Available online: https://docs.ropensci.org/rnoaa/ (accessed on 21 January 2025).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 21 January 2025).
- QGIS Development Team. QGIS Geographic Information System; Open-Source Geospatial Foundation Project: Beaverton, OR, USA, 2022; Available online: http://qgis.osgeo.org/ (accessed on 21 January 2025).
- Helm, R.R. Evolution and development of scyphozoan jellyfish. Biol. Rev. 2018, 93, 1228–1250. [Google Scholar] [CrossRef]
- Mayer, A.G. Medusae of the World. Publ. Carneg. Inst. Wash. 1910, 109, 1–735. [Google Scholar]
- Gutsell, J.S. The spider crab, Libinia dubia, and the jellyfish, Stomolophus meleagris, found associated at Beauford, North Carolina. Ecology 1928, 9, 358–359. [Google Scholar]
- Calder, D.R.; Hester, B.S. Phylum Cnidaria. In An Annotated Checklist of the Biota of the Coastal Zone of South Carolina; Zingmark, R.G., Ed.; University of South Carolina Columbia: Columbia, SC, USA, 1978; pp. 87–93. [Google Scholar]
- Boone, L. Scientific results of the cruises of the yachts Eagle and Ara, 1921–1928; Coelenterata, Echinodermata, and Mollusca. Bull. Vanderbilt Marine Mus. 1933, 217. [Google Scholar]
- Burke, W.D. Pelagic cnidaria of Mississippi Sound and adjacent waters. Gulf Caribb. Res. 1975, 5, 23–38. [Google Scholar] [CrossRef]
- Burke, W.D. Biology and distribution of the macrocoelenterates of Mississippi Sound and adjacent waters. Gulf Caribb. Res. 1976, 5, 17–28. [Google Scholar] [CrossRef]
- Colby, M.J. Poisonous marine animals in the Gulf of Mexico. Proc. Texas Acad. Sci. 1943, 26, 62–70. [Google Scholar]
- Corrington, J.D. Commensal association of a spider crab and a medusa. Biol. Bull. 1927, 53, 346–350. [Google Scholar] [CrossRef]
- Cortés, J. Biodiversidad marina de Costa Rica: Filo Cnidaria. Rev. Biol. Trop. 1996, 44, 323–334. [Google Scholar]
- Durán-Fuentes, J.; Gracia, A.C.; Osario, C.M.; Cedeño-Posso, C. Aporte al conocimiento de las medusas (Cnidaria: Medusozoa) en el departamento del Atlántico, Colombia. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2018, 42, 49–57. [Google Scholar] [CrossRef]
- Félix-Torres, F.J.; Garrido-Mora, A.; Sánchez-Alcudia, Y.; Sánchez-Martínez, A.J.; Granados-Berber, A.A.; Ramos-Palma, J.L. Distribución y abundancia espacial y temporal de Stomolophus meleagris (Rhizostomae: Stomolophidae) en un sistema lagunar del sur del Golfo de México. Rev. Biol. Trop. 2017, 65, 167–179. [Google Scholar] [CrossRef][Green Version]
- Gómez-Aguirre, S. Variación estacional de grandes medusas (Scyphozoa) en un sistema de lagunas costeras del sur del golfo de México (1977/1978). Bol. Inst. Oceanogr. 1980, 29, 183–185. [Google Scholar]
- Grana-Raffucci, F.A. Nomenclatura de los Organismos Acuáticos y Marinos de Puerto Rico e Islas Vírgenes, Vol. 1: Ctenóforos y Cnidarios de Puerto Rico e Islas Vírgenes; Puerto Rico Department of Natural & Environmental Resources: San Juan, Puerto Rico, 2007; p. 99. Available online: https://www.drna.pr.gov/historico-2006-2015/biblioteca/publicaciones/tecnicas/cnid01.pdf/view (accessed on 2 February 2025).
- Haeckel, E. System der Acraspeden: Zweite Hälfte des Systems der Medusen. Denkschr. Med. Naturw. Ges. Jena 1880, 2, 361–672. [Google Scholar]
- Hedgpeth, J.W. Scyphozoa. Fish. Bull. U. S. Fish Wildl. Serv. 1954, 55, 277–278. [Google Scholar]
- Hoese, H.D.; Copeland, B.J.; Miller, J.M. Seasonal occurrence of Cyanea medusae in the Gulf of Mexico at Port Aransas, Texas. Tex. J. Sci. 1964, 16, 391–393. [Google Scholar]
- Kraeuter, J.N.; Setzler, E.M. The seasonal cycle of Scyphozoa and Cubozoa in Georgia estuaries. Bull. Mar. Sci. 1975, 25, 66–74. [Google Scholar]
- Kramp, P.L. The Medusae of the tropical west coast of Africa. Atlantide Rep. 1955, 3, 239–324. [Google Scholar]
- Larson, R.J. Marine Flora and Fauna of the Northeastern United States. Cnidaria: Scyphozoa; United States Government Printing Office: Washington, DC, USA, 1976; Volume 397, pp. 1–18. [Google Scholar]
- Page, J.W. Characterization of bycatch in the cannonball jellyfish fishery in the coastal waters off Georgia. Mar. Coast. Fish. 2015, 7, 190–199. [Google Scholar] [CrossRef]
- Phillips, P.J. The Pelagic Cnidaria of the Gulf of Mexico: Zoogeography, Ecology and Systematics. Ph.D. Thesis, Texas A.&M. University, College Station, TX, USA, 1972; 212p. [Google Scholar]
- Phillips, P.J.; Burke, W.D.; Keener, E.J. Observations on the trophic significance of jellyfishes in Mississippi Sound with quantitative data on the associative behavior of small fishes with medusae. Trans. Am. Fish. Soc. 1969, 98, 703–712. [Google Scholar]
- Pratt, H.S. A Manual of the Common Invertebrate Animals: Exclusive of Insects, 2nd ed.; AC McClurg & Company, University of Minnesota: Minneapolis, MN, USA, 1916; p. 737. [Google Scholar]
- Ranson, G. Les Scyphoméduses de la collection du Muséum National d’Histoire Naturelle de Paris.—II. Catalogue raisonné; origine des récoltes. Bull. Mus. Natl. Hist. Nat. 1945, 17, 312–320. [Google Scholar]
- Tunberg, B.G.; Reed, S.A. Mass occurrence of the jellyfish Stomolophus meleagris and an associated spider crab Libinia dubia, Eastern Florida. Available online: https://www.jstor.org/stable/24321204 (accessed on 2 February 2025).
- Bigelow, H.B. Note on the medusan genus Stomolophus from San Diego. Univ. Calif. Publ. Zool. 1914, 13, 239–241. [Google Scholar]
- Stiasny, G. Studien über Rhizostomeen mit besonderer Berücksichtigung der Fauna des Malaiischen Archipels nebst einer Revision des Systems. Capita Zool. 1921, 1, 1–179. [Google Scholar]
- Stiasny, G. Ergebnisse der Nachuntersuchung einiger Rhizostomeen-Typen Haeckel’s und Chun’s aus dem Zoologischen Museum in Hamburg. Zool. Meded. 1922, 7, 41–60. [Google Scholar]
- Fernández-Alías, A.; Molinero, J.C.; Quispe-Becerra, J.I.; Bonnet, D.; Marcos, C.; Pérez-Ruzafa, A. Phenology of scyphozoan jellyfish species in a eutrophication and climate change context. Mar. Poll. Bull. 2023, 194, 115286. [Google Scholar] [CrossRef]
- Chollett, I.; Mumby, P.J.; Müller-Karger, F.E.; Hu, C. Physical environments of the Caribbean Sea. Limnol. Oceanogr. 2012, 57, 1233–1244. [Google Scholar] [CrossRef]
- Leoni, V.; González, S.; Ortega, L.; Scarabino, F.; Failla-Siquier, G.; Dutra, A.; Rubio, L.; Abreu, M.; Serra, W.; Alonzo-Campo, A.G.; et al. Tamoya haplonema (Cnidaria: Cubozoa) from Uruguayan and adjacent waters: Oceanographic context of new and historical findings. Mar. Biodivers. Rec. 2016, 9, 1–9. [Google Scholar] [CrossRef][Green Version]
- Robinson, L.M.; Hobday, A.J.; Possingham, H.P.; Richardson, A.J. Trailing edges projected to move faster than leading edges for large pelagic fish habitats under climate change. Deep-Sea Res. II Top. Stud. Oceanogr. 2015, 113, 225–234. [Google Scholar] [CrossRef]
- La República. Available online: https://larepublica.pe/ (accessed on 2 February 2025).
- El Meridiano. Available online: https://meridiano.net/ (accessed on 2 February 2025).
- El Impulso. Available online: https://www.elimpulso.com/ (accessed on 2 February 2025).
- López-Martínez, J.; Álvarez-Tello, J. The jellyfish fishery in Mexico. Agricult. Sci. 2013, 4, 57–61. [Google Scholar] [CrossRef]
- Doyle, T.K.; Hays, G.C.; Harrod, C.; Houghton, J.D.R. Ecological and Societal Benefits of Jellyfish. In Jellyfish Blooms; Pitt, K., Lucas, C., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 105–127. [Google Scholar] [CrossRef]
- Raposo, A.; Alasqah, I.; Alfheeaid, H.A.; Alsharari, Z.D.; Alturki, H.A.; Raheem, D. Jellyfish as food: A narrative review. Foods 2022, 11, 2773. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Milisenda, G.; Piraino, S. Mediterranean jellyfish as novel food: Effects of thermal processing on antioxidant, phenolic, and protein contents. Eur. Food Res. Technol. 2019, 245, 1611–1627. [Google Scholar] [CrossRef]
- Elliott, A.; Hobson, V.; Tang, K.W. Balancing fishery and conservation: A case study of the barrel jellyfish Rhizostoma octopus in South Wales. ICES J. Mar. Sci. 2017, 74, 234–241. [Google Scholar] [CrossRef]
- Uye, S.I. Blooms of the giant jellyfish Nemopilema nomurai: A threat to the fisheries sustainability of the East Asian Marginal Seas. Plankton Benthos Res. 2008, 3, 125–131. [Google Scholar] [CrossRef]
- Gibbons, M.J.; Boero, F.; Brotz, L. We should not assume that fishing jellyfish will solve our jellyfish problem. ICES J. Mar. Sci. 2016, 73, 1012–1018. [Google Scholar] [CrossRef]
- Pitt, K.A.; Welsh, D.T.; Condon, R.H. Influence of jellyfish blooms on carbon, nitrogen and trophic dynamics in a coastal lagoon. Hydrobiologia 2009, 616, 51–64. [Google Scholar] [CrossRef]
- Jo, T.S. Utilizing the state of environmental DNA (eDNA) to incorporate time-scale information into eDNA analysis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2023, 290, 20230979. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morejón-Arrojo, R.D.; Lüskow, F.; Fernández-Alías, A.; Ramírez, H.; Cróquer, A. First Record of a Cannonball Jellyfish Bloom (Stomolophus sp.) in Venezuelan Waters. J. Mar. Sci. Eng. 2025, 13, 689. https://doi.org/10.3390/jmse13040689
Morejón-Arrojo RD, Lüskow F, Fernández-Alías A, Ramírez H, Cróquer A. First Record of a Cannonball Jellyfish Bloom (Stomolophus sp.) in Venezuelan Waters. Journal of Marine Science and Engineering. 2025; 13(4):689. https://doi.org/10.3390/jmse13040689
Chicago/Turabian StyleMorejón-Arrojo, Ramón D., Florian Lüskow, Alfredo Fernández-Alías, Humberto Ramírez, and Aldo Cróquer. 2025. "First Record of a Cannonball Jellyfish Bloom (Stomolophus sp.) in Venezuelan Waters" Journal of Marine Science and Engineering 13, no. 4: 689. https://doi.org/10.3390/jmse13040689
APA StyleMorejón-Arrojo, R. D., Lüskow, F., Fernández-Alías, A., Ramírez, H., & Cróquer, A. (2025). First Record of a Cannonball Jellyfish Bloom (Stomolophus sp.) in Venezuelan Waters. Journal of Marine Science and Engineering, 13(4), 689. https://doi.org/10.3390/jmse13040689






