Development of Anti-Peptide Antibody Specific for IgM Heavy Chain of Oryzias latipes and Its Application to Assay of Immune Response Triggered by BSA-Coated Microplastics
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
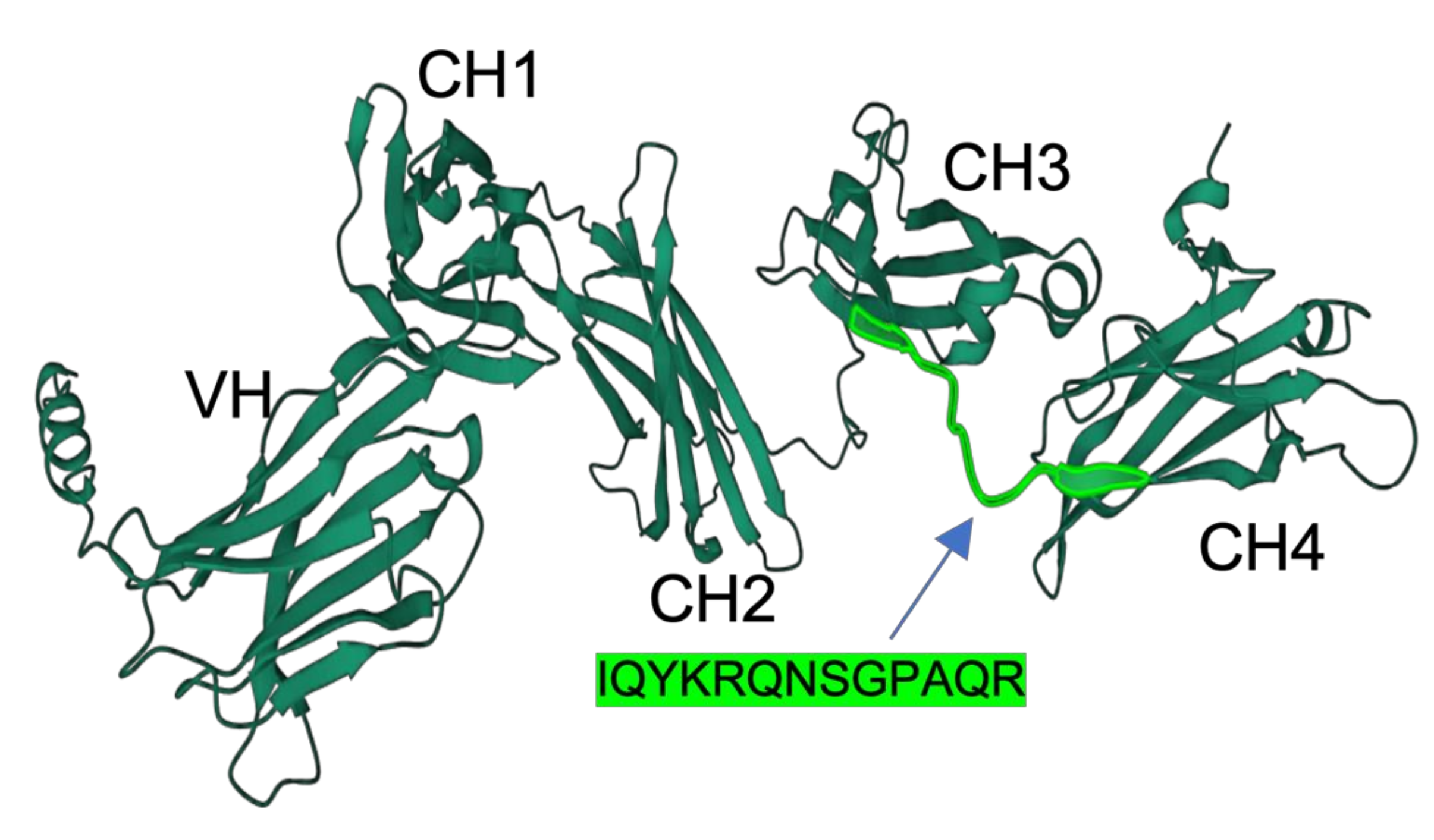
2.2. Peptide Sequence and Polyclonal Antiserum Production
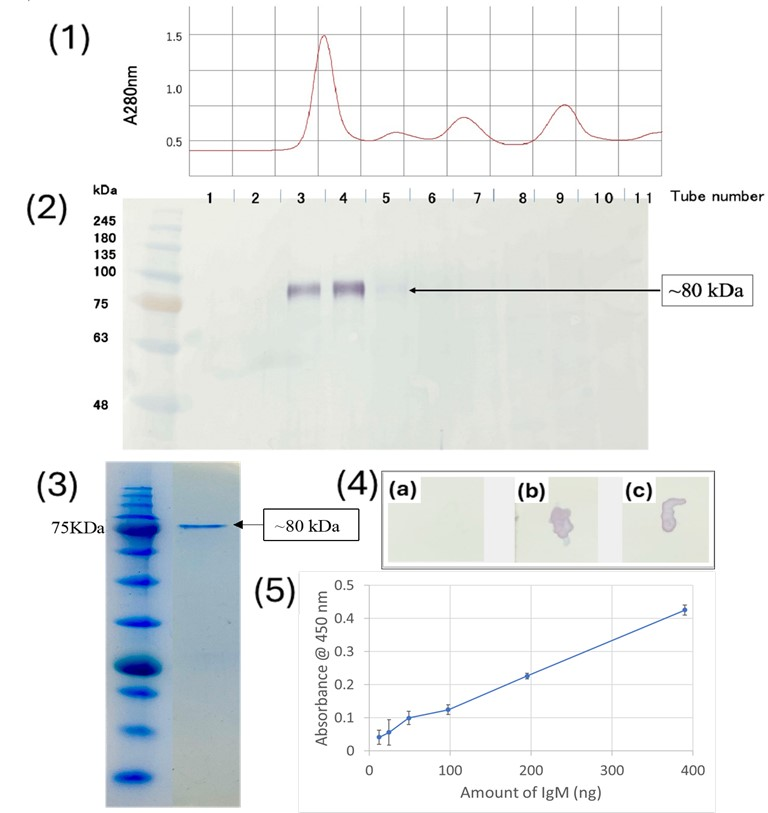
2.3. Purification of Antiserum
2.4. Size Exclusion Chromatography of Medaka Serum
2.5. SDS-PAGE and Western Blot
2.6. Dot Blot Analysis
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Adsorption of BSA to Microplastics
2.9. Exposure Experiments
2.10. Induction of Body Transparency and Fluorescent Microscopy
2.11. Statistical Analysis
3. Results
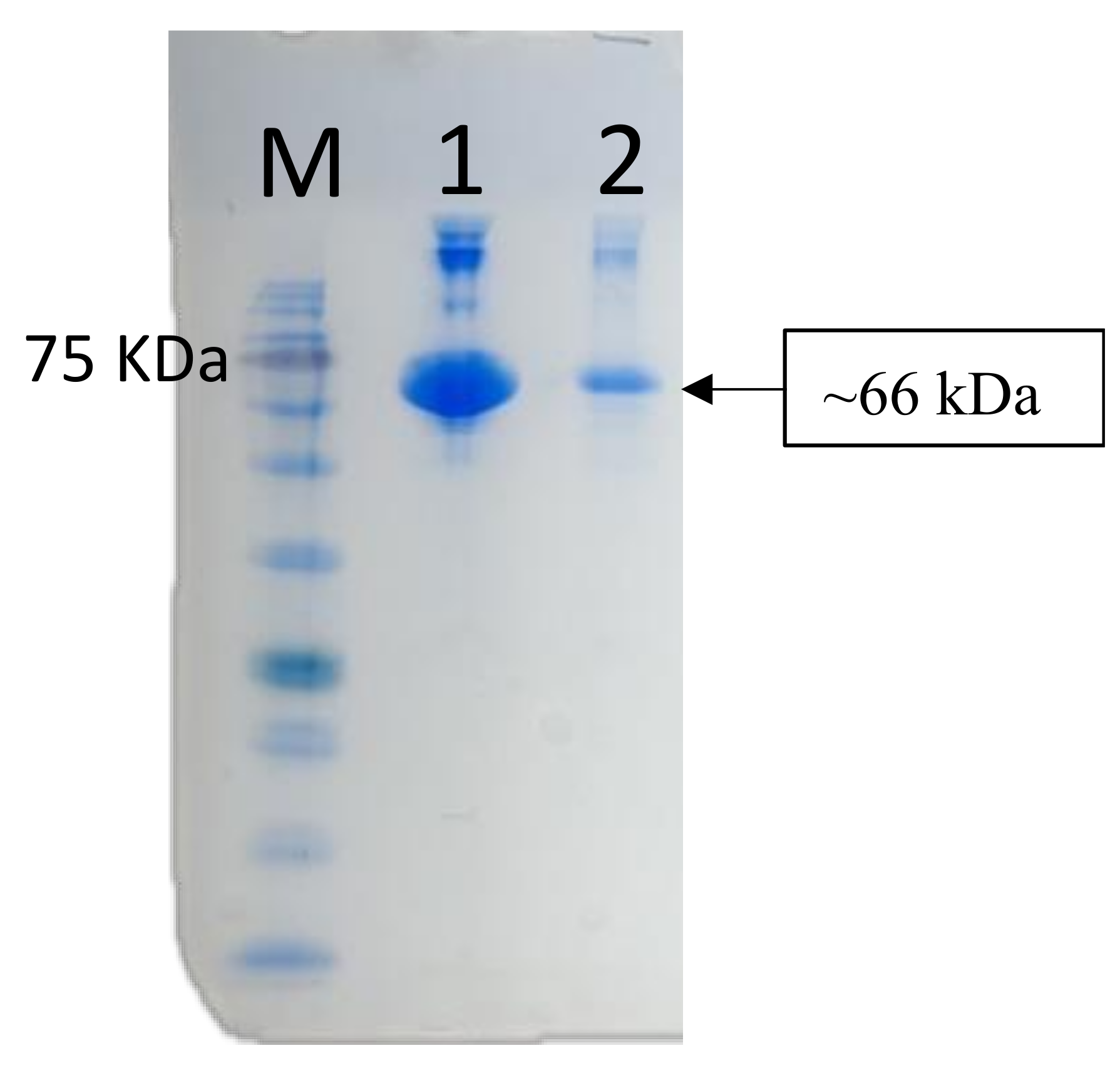
3.1. Specificity and Reactivity of Anti-Medaka IgM Anti-Peptide Antibody
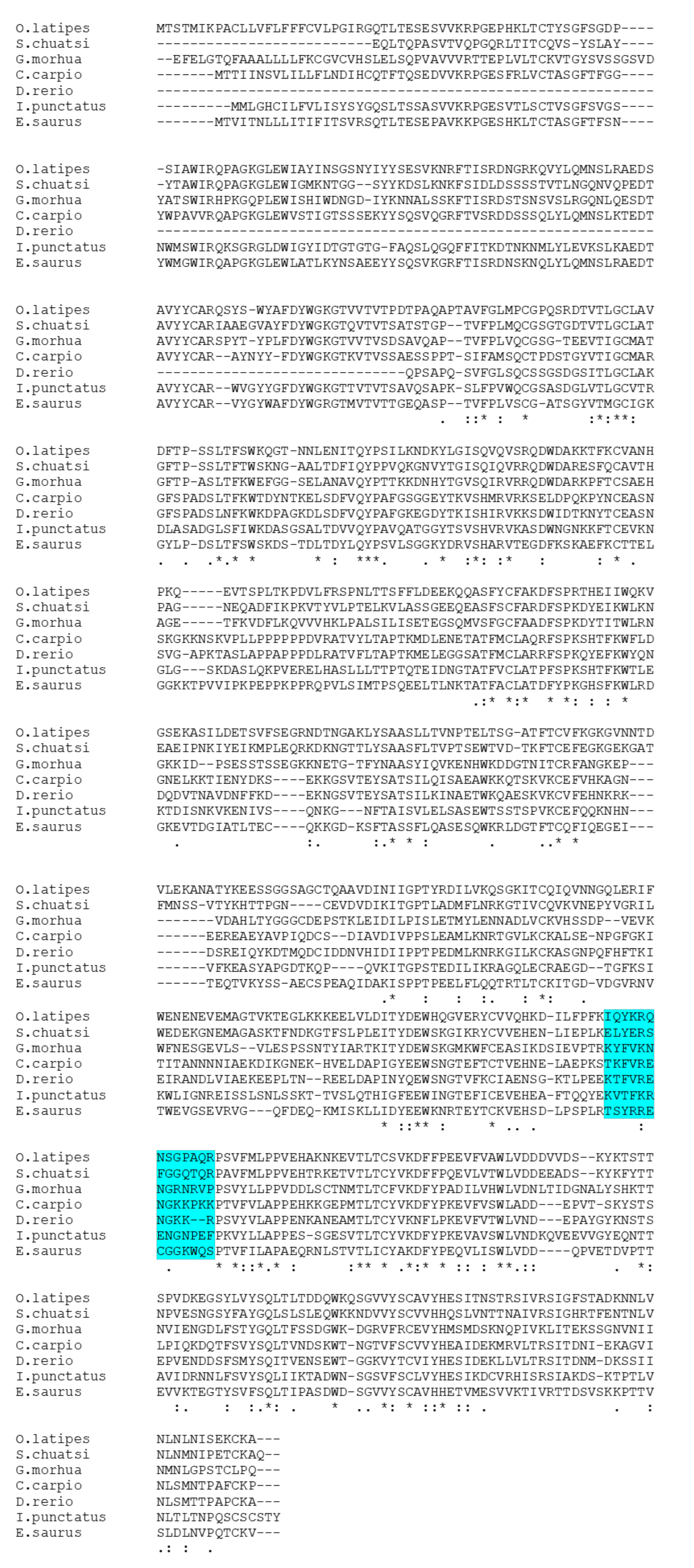
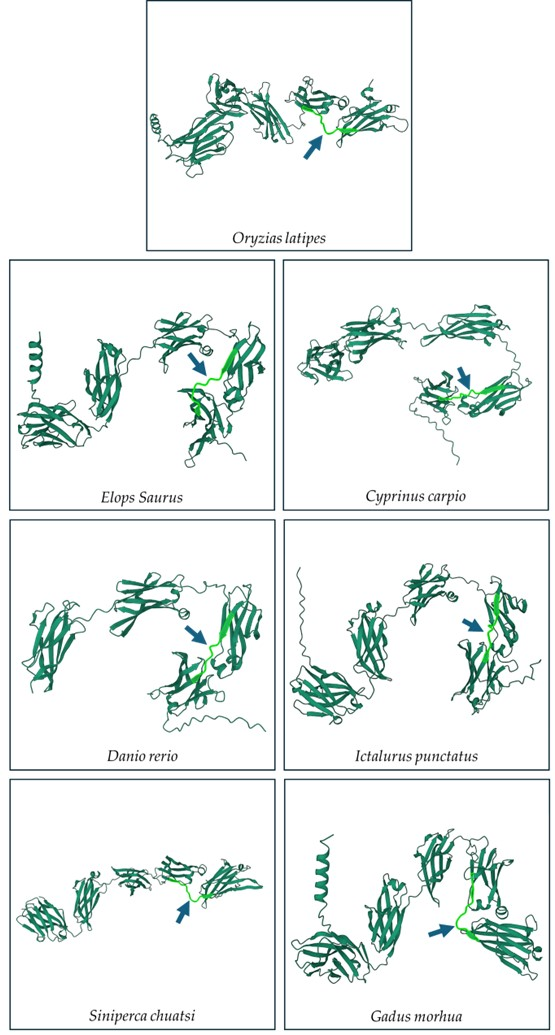
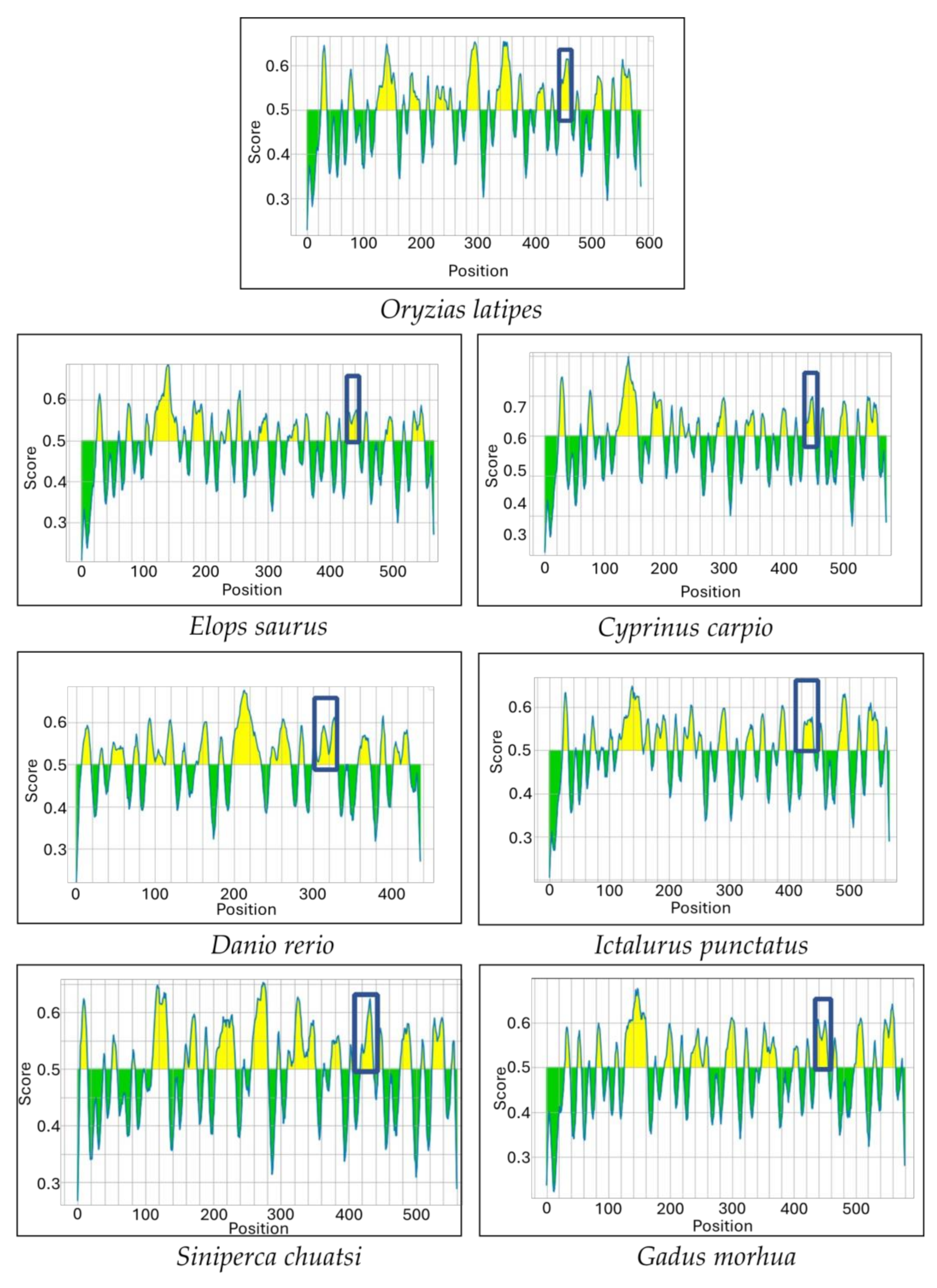
3.2. Prediction of Antigenic Sequences in Another Teleost IgM
3.3. Application of Anti-Medaka IgM Anti-Peptide Antibody for Evaluation of Adjuvant Effect in Bath Immunization
3.3.1. Preparation of BSA-Coated Microplastic Beads
3.3.2. Tracing MP in the Medaka Body After Bath Exposure
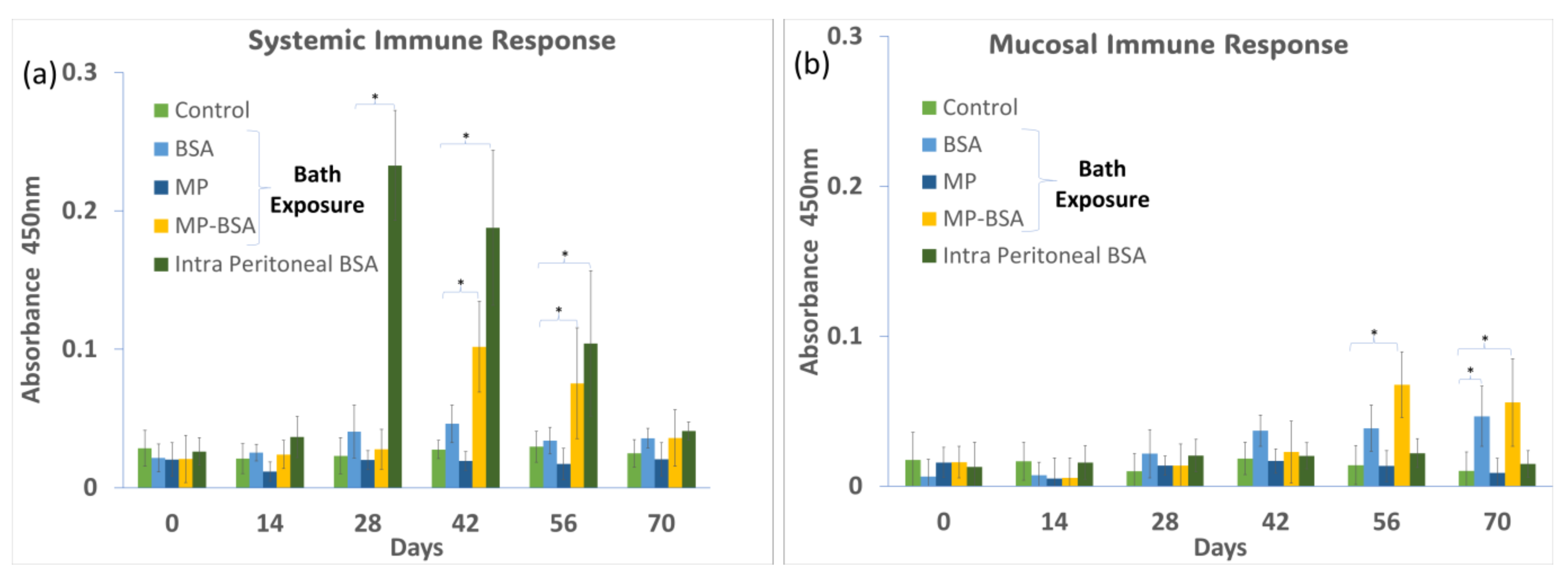
3.3.3. Bath Immunization Test for Antibody Response in Serum and Gut Mucus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IgM | Immunoglobulin M |
| KLH | Keyhole Limpet hemocyanin |
| PBS | Phosphate buffered saline |
| TPBS | 0.5% Tween 20-Phosphate buffered saline |
| BSA | Bovine Serum Albumin |
| MP | Microplastics |
References
- Solem, S.T.; Stenvik, J. Antibody repertoire development in teleosts—A review with emphasis on salmonids and Gadus morhua L. Dev. Comp. Immunol. 2006, 30, 57–76. [Google Scholar] [PubMed]
- Jones, E.M.; Oliver, L.P.; Ma, J.; Leeuwis, R.H.J.; Myrsell, V.; Arkoosh, M.R.; Dietrich, J.P.; Schuster, C.M.; Hawkyard, M.; Gamperl, A.K.; et al. Production of a monoclonal antibody specific to sablefish (Anoplopoma fimbria) IgM and its application in ELISA, western blotting, and immunofluorescent staining. Fish. Shellfish Immunol. 2022, 130, 479–489. [Google Scholar] [PubMed]
- Volff, J.N. Genome evolution and biodiversity in teleost fish. Heredity 2005, 94, 280–294. [Google Scholar]
- Velázquez, J.; Rodríguez, A.; Aragón, H.; Haidar, A.; González, M.; Valdés, R.; Garay, H.E.; Abreu, D.D.; Ramos, Y.; Cabrales, A.; et al. Monoclonal antibody against Nile tilapia (Oreochromis niloticus) IgM heavy chain: A valuable tool for detection and quantification of IgM and IgM+ cells. Fish. Shellfish Immunol. 2021, 110, 44–54. [Google Scholar]
- Trier, N.H.; Hansen, P.R.; Houen, G. Production and characterization of peptide antibodies. Methods 2012, 56, 136–144. [Google Scholar] [PubMed]
- Jirapongpairoj, W.; Hirono, I.; Kondo, H. Development and evaluation of polyclonal antisera for detection of the IgM heavy chain of multiple fish species. J. Immunol. Methods 2017, 449, 71–75. [Google Scholar]
- Ozato, K.; Wakamatsu, Y. Developmental Genetics of Medaka: (medaka/mutants/transgenic/chimeras/pluripotent cell line). Dev. Growth Differ. 1994, 36, 437–443. [Google Scholar]
- Chen, X.; Li, L.; Cheng, J.; Chan, L.L.; Wang, D.Z.; Wang, K.J.; Baker, M.E.; Hardiman, G.; Schlenk, D.; Cheng, S.H. Molecular staging of marine medaka: A model organism for marine ecotoxicity study. Mar. Pollut. Bull. 2011, 63, 309–317. [Google Scholar]
- Sakaguchi, H.; Sato, Y.; Matsumoto, R.; Gomikawa, J.; Yoshida, N.; Suzuki, T.; Matsuda, M.; Iwanami, N. Maturation of the medaka immune system depends on reciprocal interactions between the microbiota and the intestinal tract. Front. Immunol. 2023, 14, 1259519. [Google Scholar]
- Jun, B.; Ling, C.; Jia-He, X.; Ke-Jian, W.; Doris, W.T.A. The marine medaka Oryzias melastigma—A potential marine fish model for innate immune study. Mar. Pollut. Bull. 2011, 63, 267–276. [Google Scholar]
- Alberghini, L.; Truant, A.; Santonicola, S.; Colavita, G.; Giaccone, V. Microplastics in Fish and Fishery Products and Risks for Human Health: A Review. Int. J. Environ. Res. Public Health 2022, 20, 789. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, X.; Xu, X.; Takai, Y.; Ogawa, H.; Shimasaki, Y.; Oshima, Y. Uptake and depuration kinetics of microplastics with different polymer types and particle sizes in Japanese medaka (Oryzias latipes). Ecotoxicol. Environ. Saf. 2021, 212, 112007. [Google Scholar] [PubMed]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017, 45, 24–29. [Google Scholar]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar]
- Choi, Y.; Agarwal, S.; Deane, C.M. How long is a piece of loop? PeerJ 2013, 1, e1. [Google Scholar]
- Elcombe, B.M.; Chang, R.J.; Taves, C.J.; Winkelhake, J.L. Evolution of antibody structure and effector functions: Comparative hemolytic activities of monomeric and tetrameric IgM from rainbow trout, Salmo gairdnerii. Comp. Biochem. Physiol. B 1985, 80, 697–706. [Google Scholar]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 9, 827–835. [Google Scholar]
- Xiao, K.; Song, L.; Li, Y.; Li, C.; Zhang, S. Dietary intake of microplastics impairs digestive performance, induces hepatic dysfunction, and shortens lifespan in the annual fish Nothobranchius guentheri. Biogerontology 2023, 24, 207–223. [Google Scholar]
- Qiang, L.; Cheng, J. Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio). Chemosphere 2021, 263, 128161. [Google Scholar]
- Buwono, N.R.; Risjani, Y.; Soegianto, A. Oxidative stress responses of microplastic-contaminated Gambusia affinis obtained from the Brantas River in East Java, Indonesia. Chemosphere 2022, 293, 133543. [Google Scholar]
- Wei, G.K.; Da, C.Q.; Qing, J.M.; Zhao, R.D.; Yang, Z.L.; Tao, S.A.; Zhen, X. Mucosal immune responses and protective efficacy in yellow catfish after immersion vaccination with bivalent inactivated Aeromonas veronii and Edwardsiella ictaluri vaccine. Water Biol. Secur. 2022, 1, 100032. [Google Scholar]
- Marie, F.S.; Jean-Marie, V. Intestinal absorption of protein in teleost fish. Comp. Biochem. Physiol. A 1992, 103, 771–781. [Google Scholar]
- Mutoloki, S.; Munang’andu, H.M.; Evensen, Ø. Oral Vaccination of Fish—Antigen Preparations, Uptake, and Immune Induction. Front. Immunol. 2015, 6, 519. [Google Scholar] [CrossRef]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.L.; Bose, U.; Ni, D. Unravelling protein corona formation on pristine and leached microplastics. Micropl. Nanopl. 2024, 4, 9. [Google Scholar]

| Immunization Group | Fish Weight at 0th Day | Fish Weight at 70th Day |
|---|---|---|
| Negative Control | 41.3 ± 8.2 mg | 64.3 ± 9.1 mg |
| BSA | 35.1 ± 5.2 mg | 53.8 ± 4.8 mg |
| MP | 46.2 ± 7.2 mg | 62.2 ± 5.3 mg |
| MP-BSA | 39.1 ± 5.9 mg | 56.7 ± 8.8 mg |
| Intra Peritoneal BSA | 40.8 ± 3.2 mg | 55.2 ± 7.7 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kizhakkumpat, A.; Mako, I.; Prakash, H.; Oshima, Y.; Nagasawa, T.; Somamoto, T.; Nakao, M. Development of Anti-Peptide Antibody Specific for IgM Heavy Chain of Oryzias latipes and Its Application to Assay of Immune Response Triggered by BSA-Coated Microplastics. J. Mar. Sci. Eng. 2025, 13, 659. https://doi.org/10.3390/jmse13040659
Kizhakkumpat A, Mako I, Prakash H, Oshima Y, Nagasawa T, Somamoto T, Nakao M. Development of Anti-Peptide Antibody Specific for IgM Heavy Chain of Oryzias latipes and Its Application to Assay of Immune Response Triggered by BSA-Coated Microplastics. Journal of Marine Science and Engineering. 2025; 13(4):659. https://doi.org/10.3390/jmse13040659
Chicago/Turabian StyleKizhakkumpat, Akhil, Izumi Mako, Harsha Prakash, Yuji Oshima, Takahiro Nagasawa, Tomonori Somamoto, and Miki Nakao. 2025. "Development of Anti-Peptide Antibody Specific for IgM Heavy Chain of Oryzias latipes and Its Application to Assay of Immune Response Triggered by BSA-Coated Microplastics" Journal of Marine Science and Engineering 13, no. 4: 659. https://doi.org/10.3390/jmse13040659
APA StyleKizhakkumpat, A., Mako, I., Prakash, H., Oshima, Y., Nagasawa, T., Somamoto, T., & Nakao, M. (2025). Development of Anti-Peptide Antibody Specific for IgM Heavy Chain of Oryzias latipes and Its Application to Assay of Immune Response Triggered by BSA-Coated Microplastics. Journal of Marine Science and Engineering, 13(4), 659. https://doi.org/10.3390/jmse13040659






