A Reef’s High-Frequency Soundscape and the Effect on Telemetry Efforts: A Biotic and Abiotic Balance
Abstract
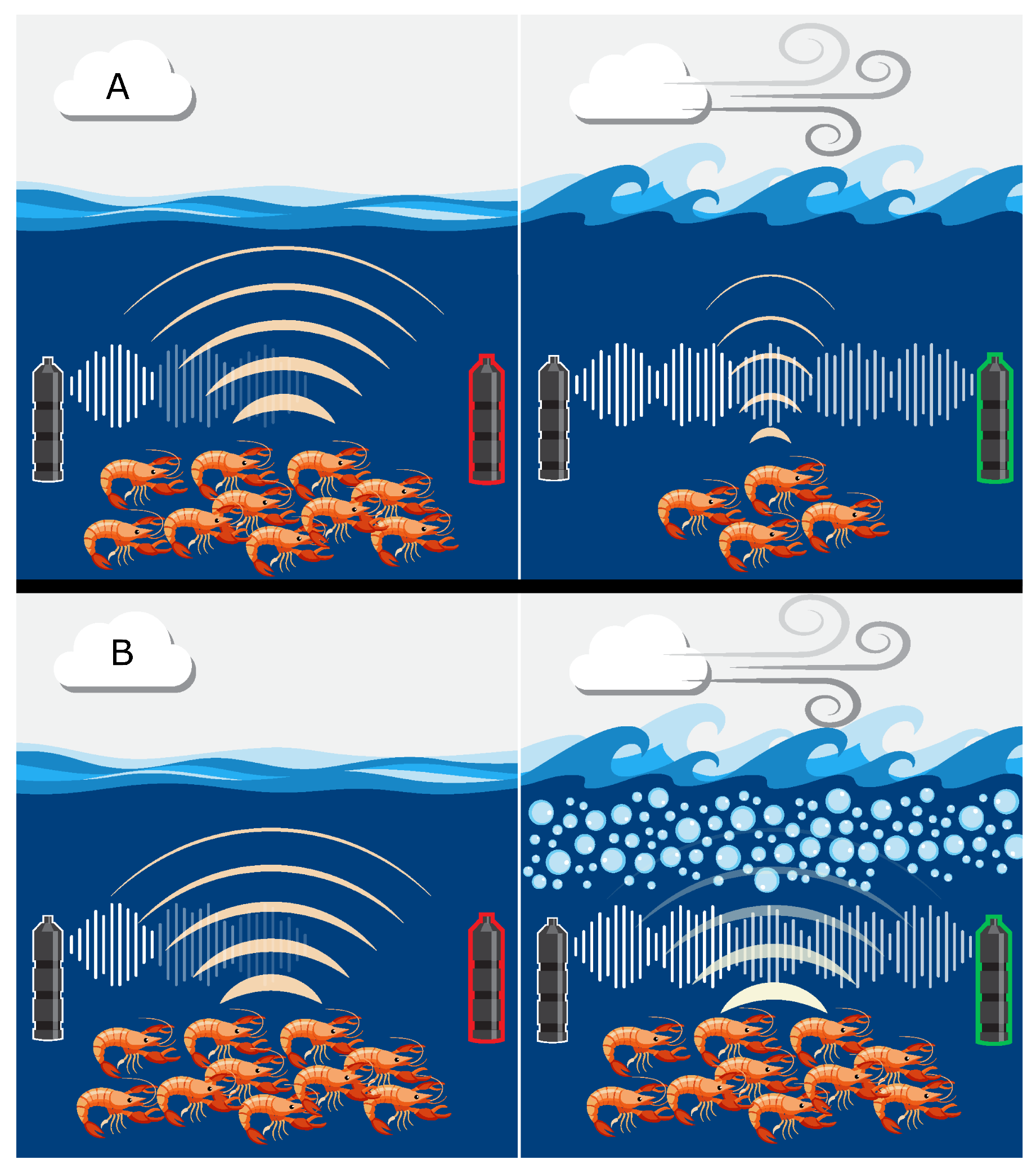
1. Introduction
2. Materials and Methods
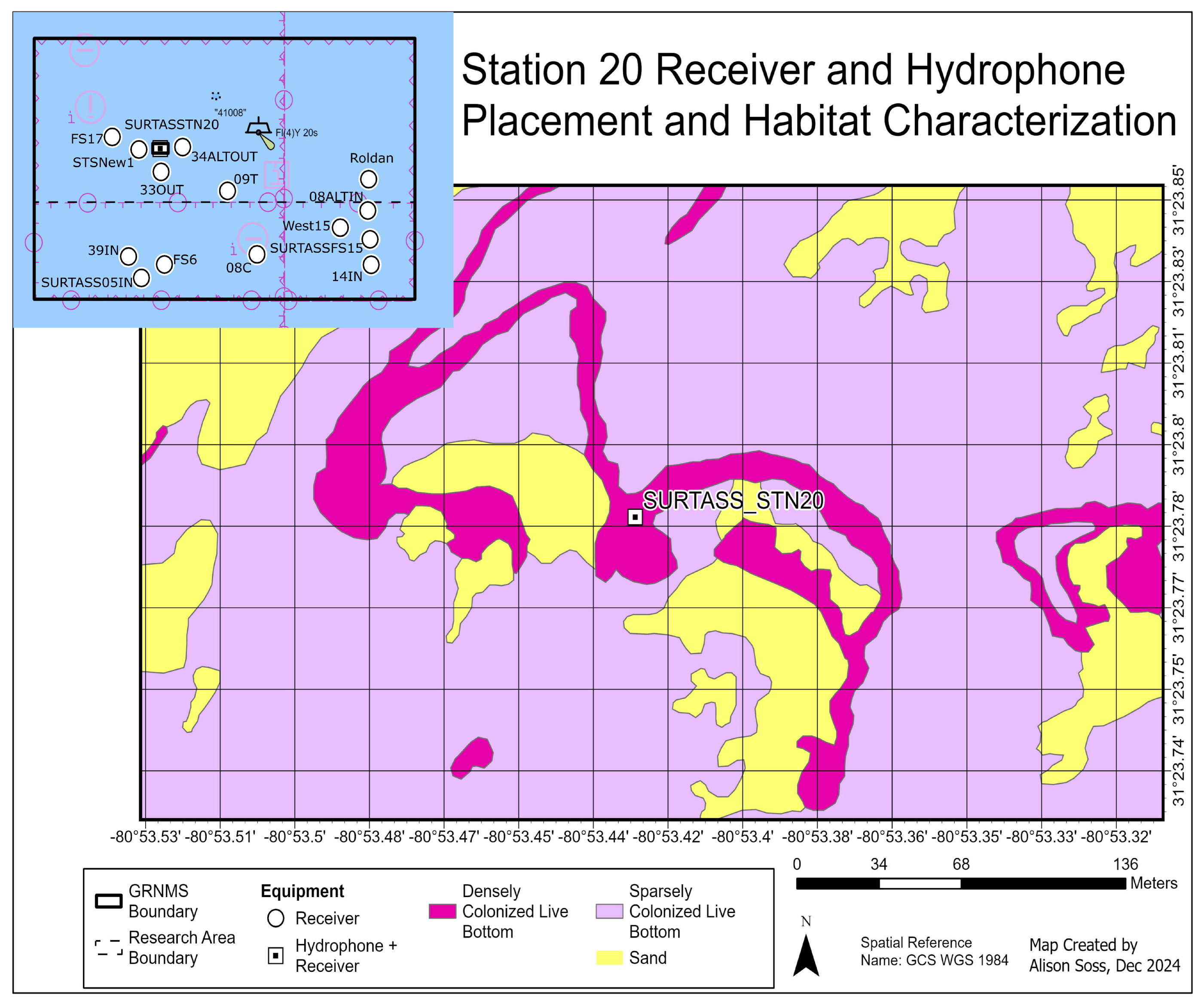
2.1. Environmental Context
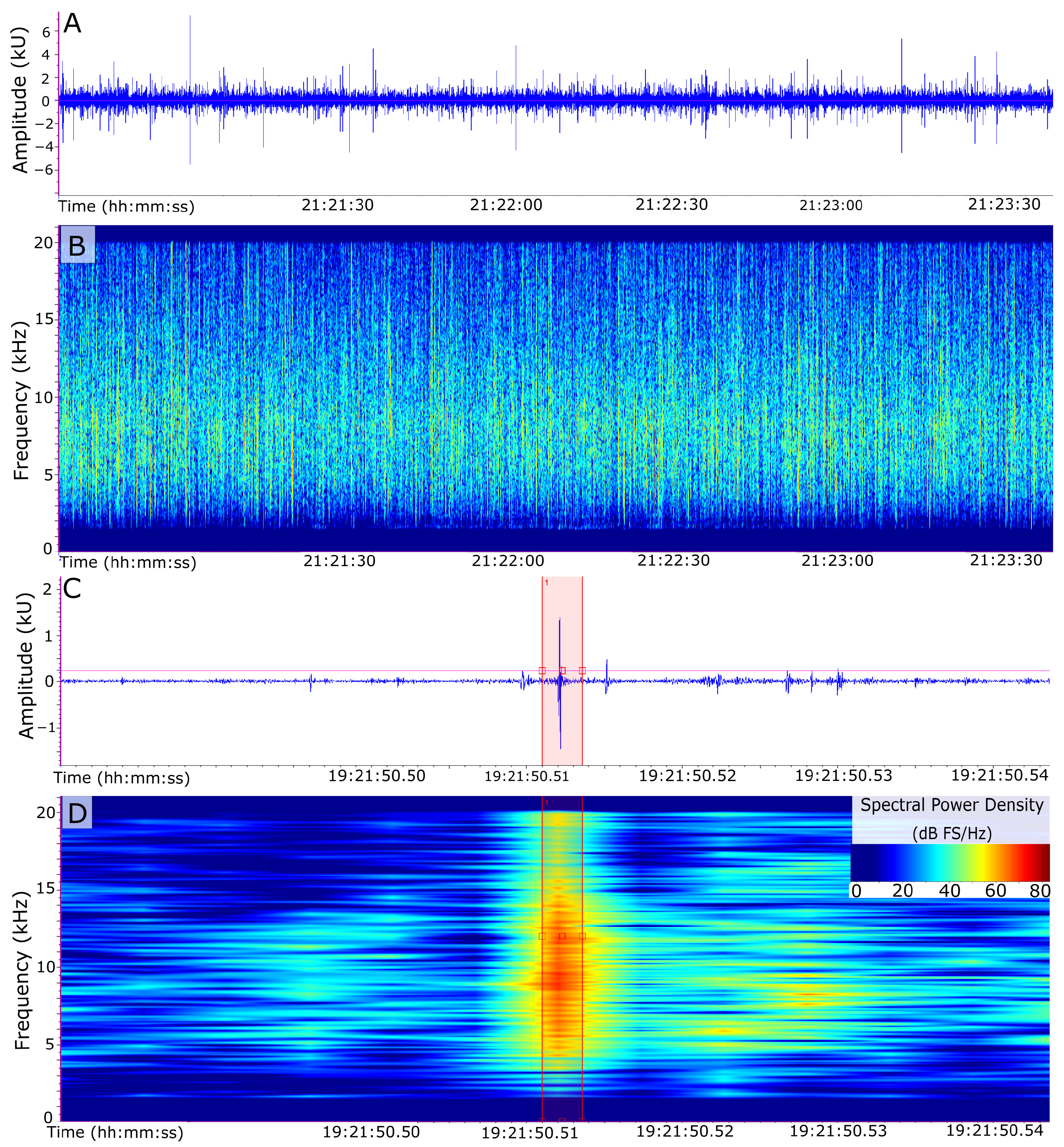
2.2. Telemetry Detections and Acoustic Measurements
2.3. Measuring Biotic Influence: Snap Rates
2.4. Calculating Abiotic Influence: Surface Bubble Loss
2.5. Filtering and Statistical Analysis
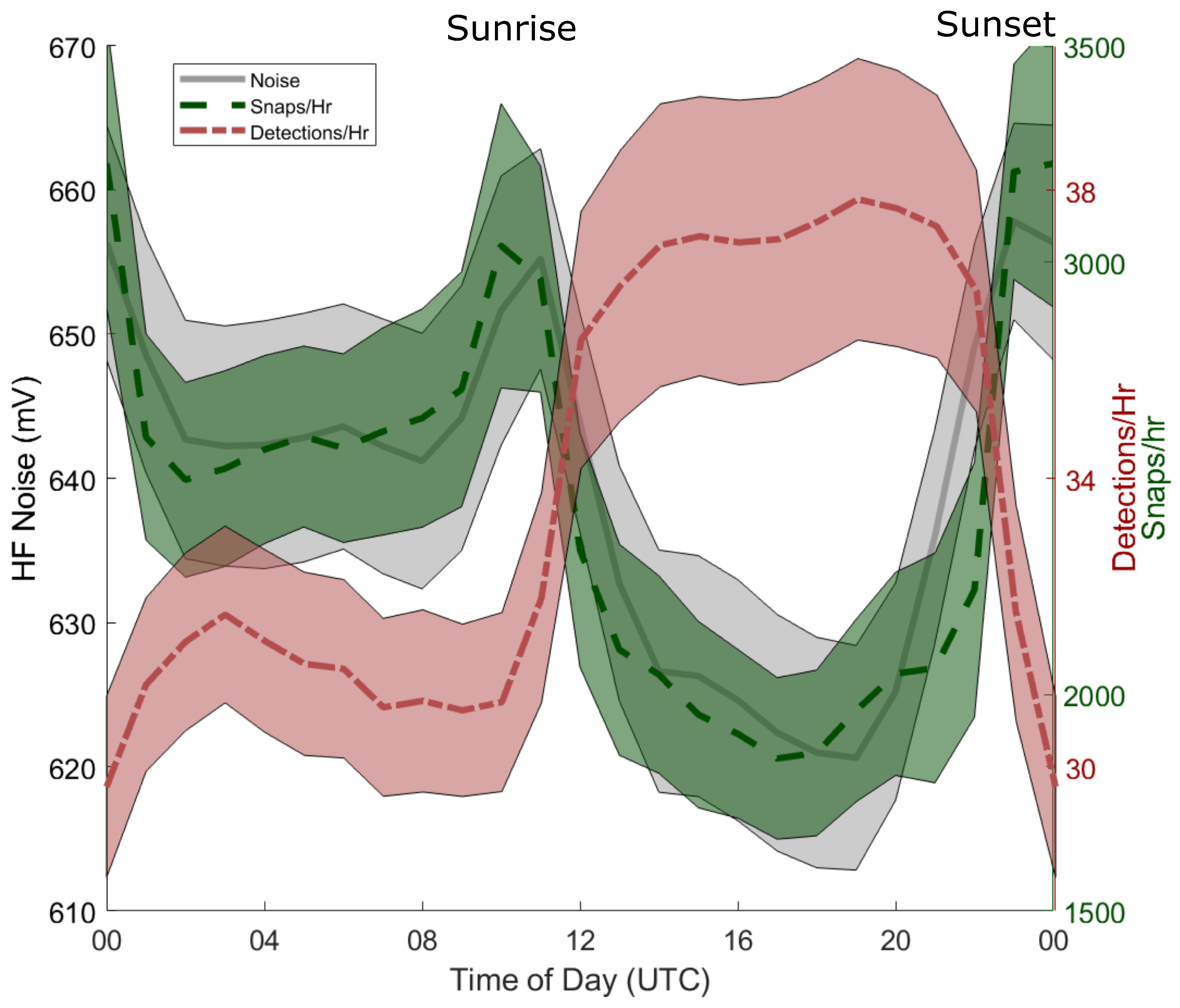
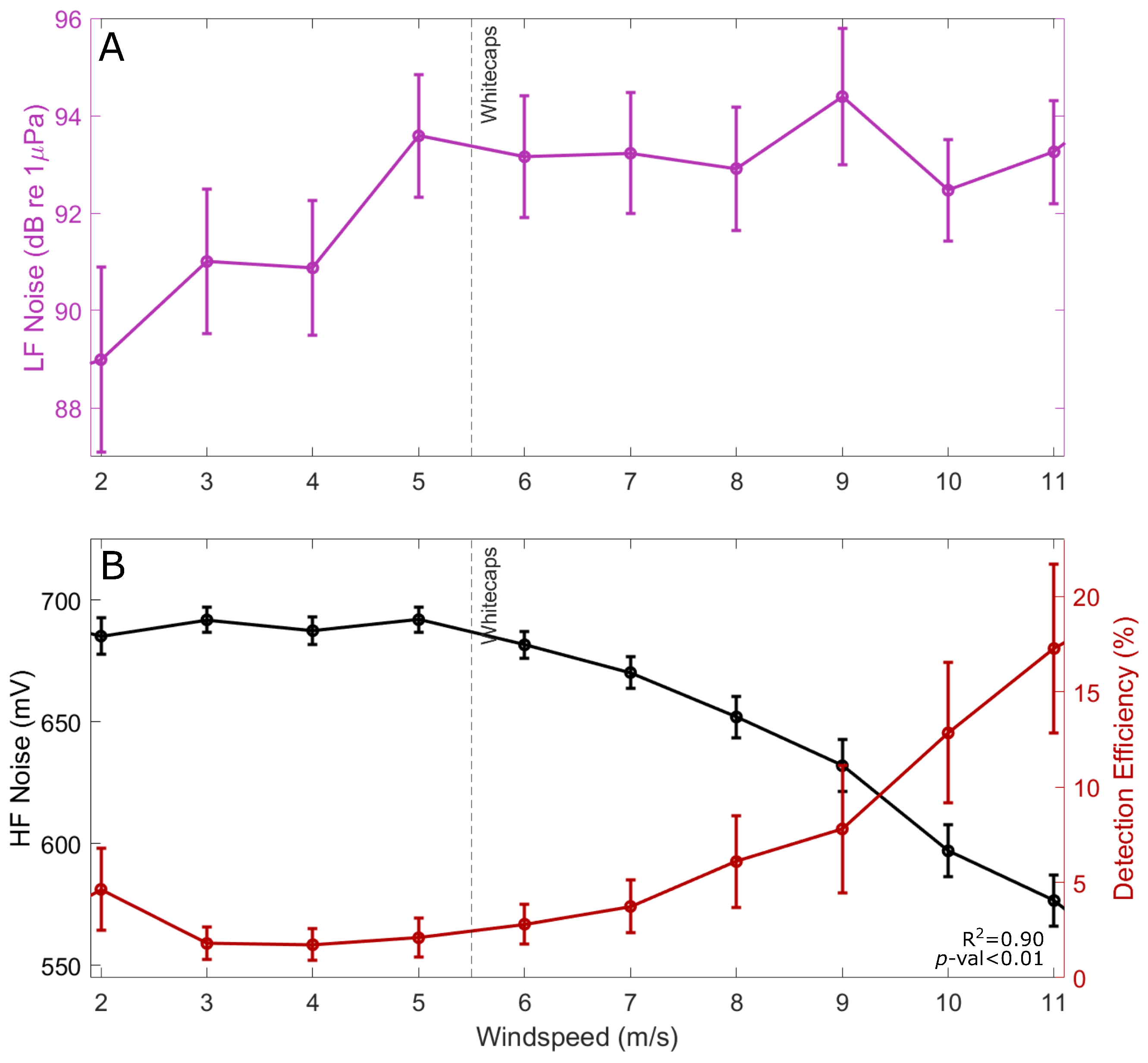
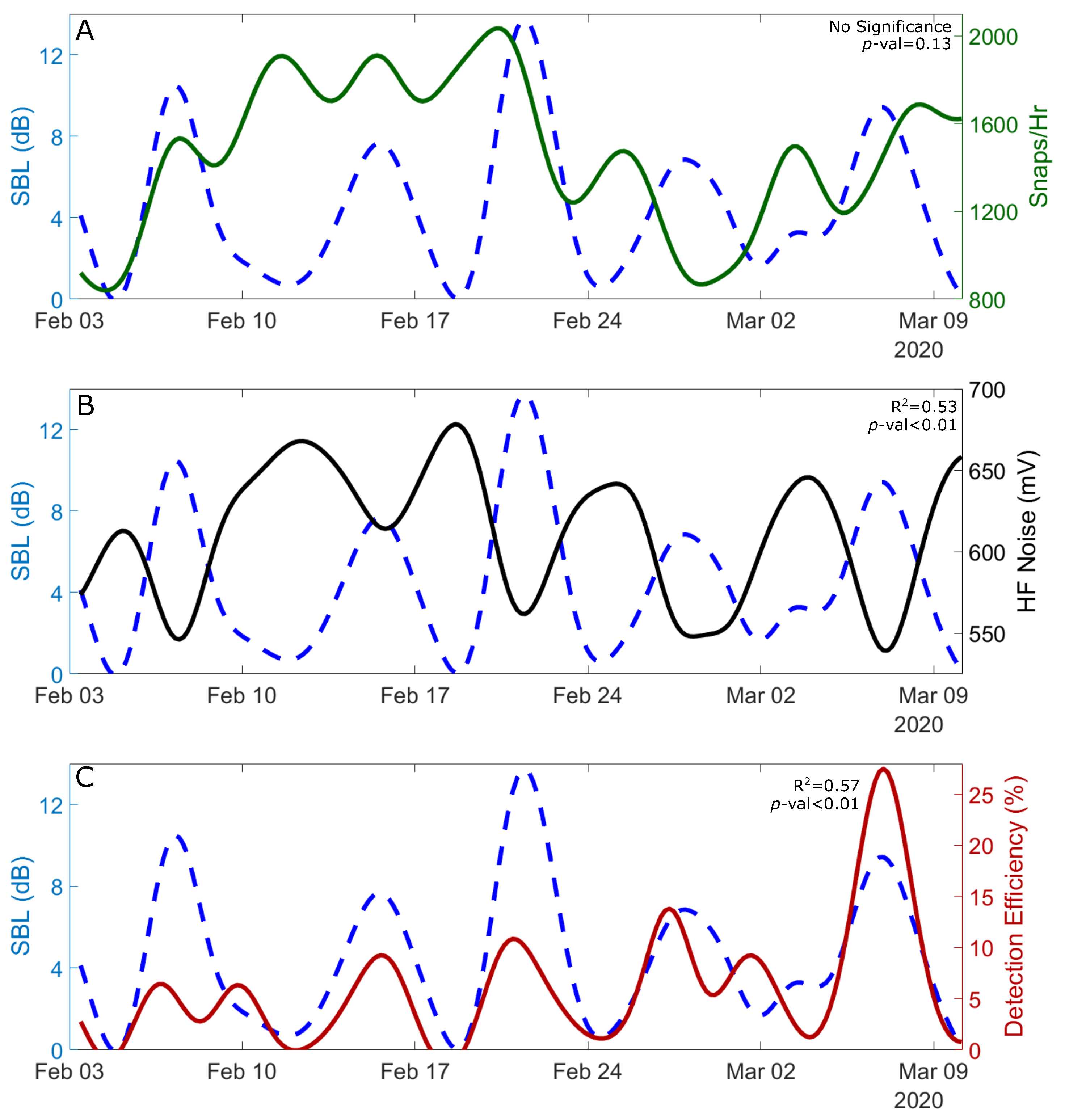
3. Results
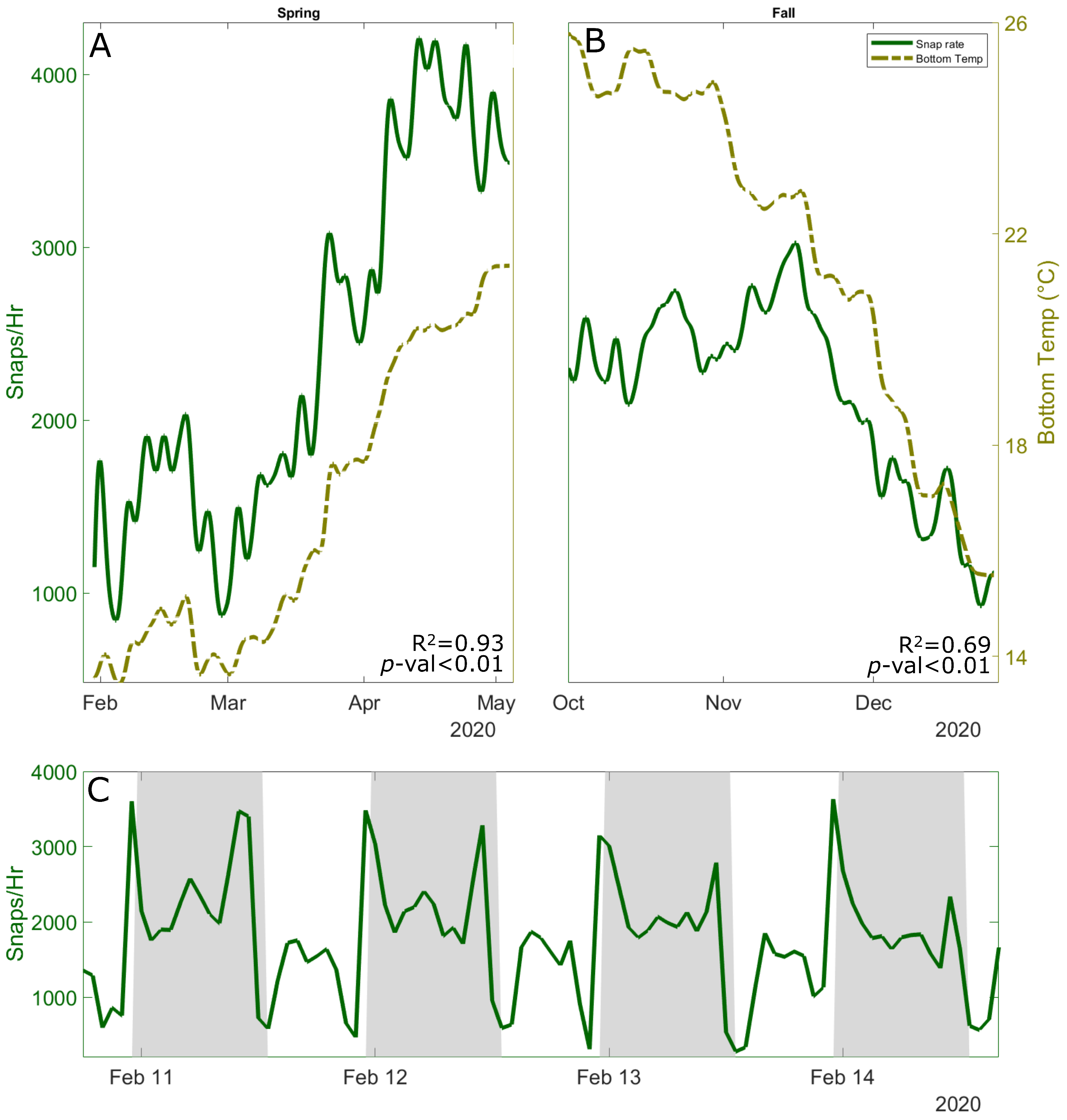
3.1. Variability in Snapping Shrimp Behavior
3.2. The Effect of Wind-Driven Attenuation on the Soundscape
4. Discussion
Further Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GRNMS | Gray’s Reef National Marine Sanctuary |
| HF | High-Frequency (50–90 kHz) |
| LF | Low-Frequency (0.17–0.36 kHz) |
| NDBC | National Data Buoy Center |
| SAB | South Atlantic Bight |
| SBL | Surface Bubble Loss |
References
- Payne, N.L.; Gillanders, B.M.; Webber, D.M.; Semmens, J.M. Interpreting diel activity patterns from acoustic telemetry: The need for controls. Mar. Ecol. Prog. Ser. 2010, 419, 295–301. [Google Scholar] [CrossRef]
- Edwards, C.R.; Cho, S.; Zhang, F.; Fangman, S. Field and Numerical Studies to Assess Performance of Acoustic Telemetry Collected by Autonomous Mobile Platforms, 2014. In Review of Scientific Research in and around the Designated Research Area of Gray’s Reef National Marine Sanctuary; National Marine Sanctuaries Conservation Series ONMS-20-08; U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Office of National Marine Sanctuaries: Silver Spring, MD, USA, 2020; pp. 157–177. [Google Scholar]
- McQuarrie, F.; Woodson, C.B.; Edwards, C.R. Analyzing Tidal, Diurnal, Synoptic, and Seasonal Drivers of Acoustic Telemetry Efficiency on a Coastal Reef. In Proceedings of the Marine Technology Society Soc’s 2023 OCEANS Conference 2023, Biloxi, MS, USA, 25–28 September 2023. [Google Scholar] [CrossRef]
- Cho, S.; Zhang, F.; Edwards, C. Tidal variability of acoustic detection. In Proceedings of the 2016 IEEE International Conferences on Big Data and Cloud Computing (BDCloud), Social Computing and Networking (SocialCom), Sustainable Computing and Communications (SustainCom) (BDCloud-SocialCom-SustainCom), Atlanta, GA, USA, 8–10 October 2016; pp. 431–436. [Google Scholar] [CrossRef]
- Gjelland, K.; Hedger, R. Environmental influence on transmitter detection probability in biotelemetry: Developing a general model of acoustic transmission. Methods Ecol. Evol. 2013, 4, 665–674. [Google Scholar] [CrossRef]
- McQuarrie, F.; Woodson, C.B.; Edwards, C. Modeling Acoustic Telemetry Detection Ranges in a Shallow Coastal Environment. In Proceedings of the 15th International Conference on Underwater Networks & Systems, Guangdong, China, 22–24 November 2021. [Google Scholar] [CrossRef]
- Oliver, M.; Breece, M.; Haulsee, D.; Cimino, M.; Kohut, J.; Aragon, D.; Fox, D. Factors affecting detection efficiency of mobile telemetry. Anim. Biotelemetry 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Huveneers, C.; Simpfendorfer, C.; Kim, S.; Semmens, J.; Hobday, A.; Pederson, H.; Stieglitz, T.; Vallee, R.; Webber, D.; Heupel, M.; et al. The influence of environmental parameters on the performance and detection range of acoustic receivers. Methods Ecol. Evol. 2016, 7, 825–835. [Google Scholar] [CrossRef]
- Kendall, M.S.; Williams, B.L.; Ellis, R.D.; Flaherty-Walia, K.E.; Collins, A.B.; Roberson, K.W. Measuring and understanding receiver efficiency in your acoustic telemetry array. Fish. Res. 2021, 234, 105802. [Google Scholar] [CrossRef]
- Ellis, R.D.; Flaherty-Walia, K.E.; Collins, A.B.; Bickford, J.W.; Boucek, R.; Burnsed, S.L.W.; Lowerre-Barbieri, S.K. Acoustic telemetry array evolution: From species- and project-specific designs to large-scale, multispecies, cooperative networks. Fish. Res. 2019, 209, 186–195. [Google Scholar] [CrossRef]
- Blanton, J.; Werner, F.; Kim, C.; Atkinson, L.; Lee, T.; Savidge, D. Transport and fate of low-density water in a coastal frontal zone. Cont. Shelf Res. 1994, 14, 401–427. [Google Scholar] [CrossRef]
- Mackenzie, K. Nine-term equation for sound speed in the oceans. J. Acoust. Soc. Am. 1981, 70, 807–812. [Google Scholar] [CrossRef]
- Stojanovic, M.; Preisig, J. Underwater Acoustic Communication Channels: Propagation Models and Statistical Characterization. IEEE Commun. Mag. 2009, 47, 84–89. [Google Scholar] [CrossRef]
- UWAPL. High-Frequency Ocean Environmental Acoustic Models Handbook; Technical Report; Applied Physics Laboratory University of Washington: Seattle, WA, USA, 1994. [Google Scholar]
- Hildebrand, J.; Frasier, K.; Baumann-Pickering, S.; Wiggins, S. An Empirical model for wind-generated ocean noise. J. Acoust. Soc. Am. 2021, 149, 4516–4533. [Google Scholar] [CrossRef]
- Huang, C.; Yeh, C.; Liu, K. Bubble entrainment and underwater noise caused by a single water drop falling on the surface of freshwater and saltwater. Phys. Fluids 2024, 36, 022109. [Google Scholar] [CrossRef]
- Prosperetti, A. Bubble-related ambient noise in the ocean. J. Acoust. Soc. Am. 1988, 84, 1042–1054. [Google Scholar] [CrossRef]
- Pelaez-Zapata, D.; Pakrashi, V.; Dias, F. Dynamics of bubble plumes produced by breaking waves. J. Phys. Oceanogr. 2024, 54, 2059–2071. [Google Scholar] [CrossRef]
- Swadling, D.S.; Knott, N.A.; Rees, M.J.; Pederson, H.; Adams, K.R.; Taylor, M.D.; Davis, A.R. Seagrass canopies and the performance of acoustic telemetry: Implications for the interpretation of fish movements. Anim. Biotelemetry 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Delory, E.; Toma, D.M.; Rio, J.D.; Molina, P.R.; Toma, D.; Rio, J.D.; Ruiz, P.; Corradino, L.; Brault, P.; Fiquet, F. Nexos Objectives in Multi-Platform Underwater Passive Acoustics. In Proceedings of the 2nd International Conference and Exhibition on Underwater Acoustics, Rhodes, Greece, 22–27 June 2014. [Google Scholar]
- Stanley, J.; Parijs, S.V.; Davis, G.; Sullivan, M.; Hatch, L. Monitoring spatial and temporal soundscape features within ecologically significant U.S. National Marine Sanctuaries. Ecol. Appl. 2021, 31, e02439. [Google Scholar] [CrossRef]
- Pincock, D.G. Understanding the Performance of VEMCO 69 kHz Single Frequency Acoustic Telemetry; Technical Report; Amirix: Halifax, NS, Canada, 2008. [Google Scholar]
- Bohnenstiehl, D.R.; Lillis, A.; Eggleston, D.B. The curious acoustic behavior of estuarine snapping shrimp: Temporal patterns of snapping shrimp sound in sub-tidal oyster reef habitat. PLoS ONE 2016, 11, 3691. [Google Scholar] [CrossRef]
- Song, Z.; Ou, W.; Su, Y.; Li, H.; Fan, W.; Sun, S.; Wang, T.; Xu, X.; Zhang, Y. Sounds of snapping shrimp (Alpheidae) as important input to the soundscape in the southeast China coastal sea. Front. Mar. Sci. 2023, 10, 9003. [Google Scholar] [CrossRef]
- VEMCO. Receiver Noise Measurements; Technical Report; Innovasea-VEMCO: Boston, MA, USA, 2021. [Google Scholar]
- Jung, S.K.; Choi, B.K.; Kim, B.C.; Kim, B.N.; Kim, S.H.; Park, Y.; Lee, Y.K. Seawater temperature and wind speed dependences and diurnal variation of ambient noise at the snapping shrimp colony in shallow water of southern sea of Korea. Jpn. J. Appl. Phys. 2012, 51, 07GG09. [Google Scholar] [CrossRef]
- Edwards, J.E.; Buijse, H.V.W.; Bijleveld, A.I. Gone with the wind: Environmental variation influences detection efficiency in a coastal acoustic telemetry array. Anim. Biotelemetry 2024, 12, 21. [Google Scholar] [CrossRef]
- ONMS. Gray’s Reef Condition Report 2012 Addendum: Gray’s Reef; NOAA Condition Report; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2012. [Google Scholar]
- Blanton, J.; Atkinson, L.P. Transport and fate of river discharge on the continental shelf of the southeastern United States. J. Geophys. Res. Oceans 1983, 88, 4730–4738. [Google Scholar] [CrossRef]
- Scherrer, S.; Rideout, B.; Giorli, G.; Nosal, E.; Weng, K. Depth-and range-dependent variation in the perfomance of aquatic telemetry systems: Understanding and predicting the susceptibility of acoustic tag-receiver pairs to close proximity detection interference. PeerJ 2018, 6, e4249. [Google Scholar] [CrossRef] [PubMed]
- Binder, T.; Holbrook, C.; Hayden, T.; Krueger, C. Spatial and temporal variation in positioning probability of acoustic telemetry arrays: Fine-scale variability and complex interactions. Anim. Biotelemetry 2016, 4, 1–15. [Google Scholar] [CrossRef]
- K. Lisa Yang Center for Conservation Bioacoustics. Raven Pro: Interactive Sound Analysis Software (Version 1.6.5). 2024. Available online: https://www.ravensoundsoftware.com/software/raven-pro/ (accessed on 14 August 2024).
- Ocean Acoustics Toolbox, Office of Naval Research, USA, Published 1999/Updated 3/2023. Available online: http://oalib.hlsresearch.com/AcousticsToolbox/ (accessed on 30 April 2024).
- Spiga, I. The acoustic response of snapping shrimp to synthetic impulsive acoustic stimuli between 50 and 600 Hz. Mar. Pollut. Bull. 2022, 185, 4238. [Google Scholar] [CrossRef]
- Huang, J.; Barbeau, M.; Blouin, S.; Hamm, C.; Taillefer, M. Simulation and modeling of hydro acoustic communication channels with wide band attenuation and ambient noise. Int. J. Parallel Emergent Distrib. Syst. 2017, 32, 466–485. [Google Scholar] [CrossRef]
- Reubens, J.; Verhelst, P.; van der Knaap, I.; Deneudt, K.; Moens, T.; Hernandez, F. Environmental factors influence the detection probability in acoustic telemetry in a marine environment: Results from a new setup. Hydrobiologia 2019, 845, 81–94. [Google Scholar] [CrossRef]
- Chua, G.; Chitre, M.; Deane, G. Impact of Persistent Bubbles on Underwater Acoustic Communication. In Proceedings of the 2018 Fourth Underwater Communications and Networking Conference (UComms), Lerici, Italy, 28–30 August 2018. [Google Scholar]
- Kessel, S.T.; Hussey, N.E.; Webber, D.M.; Gruber, S.H.; Young, J.M.; Smale, M.J.; Fisk, A.T. Close proximity detection interference with acoustic telemetry: The importance of considering tag power output in low ambient noise environments. Anim. Biotelemetry 2015, 3, 1–14. [Google Scholar] [CrossRef]
- Pederson, H.; Bowen, E.; McDonald, J.; Smedbol, S.; Jaine, F. Field Testing the New NexTrak R1 Acoustic Receiver in a High Noise Environment; Technical Report; Innovasea: Boston, MA, USA, 2024. [Google Scholar]





| Units | Source | Range | Usage | |
|---|---|---|---|---|
| Bottom Temp. | °C | VR2Tx | 13.5–21.5 | Env. context |
| Detections | detections/h | VR2Tx | 0–8 | Telemetry efficiency |
| High-Freq. Noise | mV | VR2Tx | 400–769 | Background noise (50–90 kHz) |
| Low-Freq. Noise | dB re 1 Pa | SoundTrap 500 | 74–116 | Background noise (0.17–0.36 kHz) |
| Snaps | snaps/h | SoundTrap 500 | 171–6758 | Noise creation |
| SST | °C | NDBC Station 41008 | 12.6–23.7 | Env. context |
| Surface Bubble Loss (SBL) | dB | Calculated | 0.2–15 | Noise attenuation |
| Thermal Stratification | °C | VR2Tx, NDBC | 0–6.5 | Env. context |
| Wave Height | m | NDBC Station 41008 | 0–2.87 | Env. context |
| Wind Speed | m/s | NDBC Station 41008 | 0.2–14.3 | Env. context |
| Avg. HF Noise (mV) | Total Dets | Transceiver | Tagged Fish | |
|---|---|---|---|---|
| SURTASSTN20 | 749 ± 54 | 4398 | 3121 (71%) | 1277 (29%) |
| SURTASS05IN | 642 ± 83 | 312,101 | 19,492 (6%) | 292,609 (94%) |
| Month | Bottom Temp (°C) | Wind Speed (m/s) | Hourly Snaps | HF Noise (mV) | Avg. Detections |
|---|---|---|---|---|---|
| February 2020 | 14.0 ± 0.5 | 6.45 ± 3.03 | 1479 ± 736 | 655 ± 49 | 2.7 ± 4.2 |
| March 2020 | 15.4 ± 1.3 | 4.92 ± 2.57 | 2012 ± 886 | 697 ± 45 | 1.0 ± 1.7 |
| April 2020 | 19.8 ± 0.9 | 5.64 ± 2.64 | 3664 ± 897 | 746 ± 30 | 0.4 ± 1.1 |
| May 2020 | 22.4 ± 0.9 | 4.97 ± 2.26 | 3604 ± 1105 | 760 ± 25 | 0.3 ± 0.9 |
| October 2020 | 24.7 ± 0.4 | 6.12 ± 2.88 | 2427 ± 767 | 781 ± 27 | 0.04 ± 0.3 |
| November 2020 | 21.8 ± 1.0 | 6.54 ± 2.77 | 2503 ± 783 | 762 ± 30 | 0.05 ± 0.4 |
| December 2020 | 17.6 ± 1.2 | 6.14 ± 3.43 | 1294 ± 623 | 697 ± 42 | 0.40 ± 1.0 |
| Dates | Duration (h) | Wind Speed (m/s) | Snap Rate vs. SBL () | Noise vs. SBL () | Detections vs. SBL () |
|---|---|---|---|---|---|
| 5–10 February | 133 | 1.4–14.2 | - | 0.89 | - |
| 12–18 February | 161 | 0.73–11.0 | - | 0.97 | 0.95 |
| 30 March–3 April | 109 | 0.8–12.8 | - | 0.92 | 0.76 |
| 14–18 April | 90 | 2.31–10.8 | - | 0.91 | 0.98 |
| 28 April–2 May | 93 | 1.3–11.3 | 0.80 | 0.91 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McQuarrie, F., Jr.; Woodson, C.B.; Edwards, C.R. A Reef’s High-Frequency Soundscape and the Effect on Telemetry Efforts: A Biotic and Abiotic Balance. J. Mar. Sci. Eng. 2025, 13, 517. https://doi.org/10.3390/jmse13030517
McQuarrie F Jr., Woodson CB, Edwards CR. A Reef’s High-Frequency Soundscape and the Effect on Telemetry Efforts: A Biotic and Abiotic Balance. Journal of Marine Science and Engineering. 2025; 13(3):517. https://doi.org/10.3390/jmse13030517
Chicago/Turabian StyleMcQuarrie, Frank, Jr., C. Brock Woodson, and Catherine R. Edwards. 2025. "A Reef’s High-Frequency Soundscape and the Effect on Telemetry Efforts: A Biotic and Abiotic Balance" Journal of Marine Science and Engineering 13, no. 3: 517. https://doi.org/10.3390/jmse13030517
APA StyleMcQuarrie, F., Jr., Woodson, C. B., & Edwards, C. R. (2025). A Reef’s High-Frequency Soundscape and the Effect on Telemetry Efforts: A Biotic and Abiotic Balance. Journal of Marine Science and Engineering, 13(3), 517. https://doi.org/10.3390/jmse13030517








