Abstract
In this study, the potential use of corn-based crude bioethanol was investigated as an alternative energy source for marine fuel oil under increasingly stringent maritime emissions regulations. A small-scale combustion chamber with a capacity of approximately 1 ton was developed, and comparative combustion tests were conducted with various fuel types, including MGO, diesel, kerosene, and BE100. In addition, component analysis was performed and compared using the ISO-8217 method. Complete combustion of the fuel was performed under the same experimental conditions of stable atmospheric pressure and temperature. BE100 exhibited an 8.3% increase in the oxygen concentration and a 5.9% reduction in the carbon dioxide emissions compared to MGO. Despite the low nitrogen oxide (NOx) emissions of MGO at approximately 34.4 ppm, BE100 demonstrated superior reduction potential, with a reading of 1.9 ppm. Sulfur oxides (SOx) were not detected in any of the fuels tested, underscoring the high quality of the currently available low-sulfur MGO. The exhaust gas temperatures were reduced by approximately 44.6% when using BE100, from 367.1 °C for MGO to 203.2 °C for BE100. However, the combustion efficiency of BE100 was 8.3% lower than that of MGO. While crude bioethanol shows promise in reducing exhaust gas emissions, its limited thermal output poses a challenge for direct substitution. Future studies should investigate the development of blended fuels combining bioethanol and conventional marine fuels to improve the performance and sustainability.
1. Introduction
The increase in cargo transport driven by the expansion of ship sizes has led to severe environmental pollution due to rising fossil fuel consumption [1,2]. While fossil fuels currently account for approximately 80% of the world’s energy supply, their production is expected to decline sharply after peaking in 2020 [3,4]. This trend indicates an increasing dependence on alternative energy sources, especially as exhaust emissions from ships are a significant environmental problem [3]. According to reports from the International Maritime Organization (IMO), emissions from ships account for 14% of nitrogen oxides (NOx), 2% of carbon dioxide (CO2), and 5% of sulfur oxides (SOx) in the transportation sector [5]. In response, the IMO adopted the “Initial IMO Strategy on Reduction of GHG Emissions from Ships” at the 72nd Marine Environment Protection Committee (MEPC) meeting, with the aim of reducing greenhouse gas emissions by up to 50% by 2050 [6]. This goal was further affirmed at the 80th MEPC meeting, where it was decided to achieve net-zero emissions for ships [7]. Consequently, international shipping will require near-zero carbon dioxide (CO2) emissions from vessels in the future [1]. The IMO has also issued regulations mandating the use of low-sulfur fuels or engines that meet the designated NOx emission limits to control exhaust emissions from ships [8].
Marine fuel oils, which are usually of lower quality with higher viscosity and contain residual impurities, release more toxic gases compared to land-based fuels [9]. Therefore, research efforts are actively underway to improve the fuel quality or develop alternative energy sources to reduce emissions [10]. The IMO has identified five alternative fuels for low-carbon and zero-carbon applications: LNG, methanol, ammonia, hydrogen, and biofuels [11]. Among these, the Korean Register has highlighted the suitability of ethanol-based fuels, which share similar physical properties with methanol, as viable alternatives for marine use [2]. Bioethanol, which is biologically derived from renewable biomass, is recognized as a suitable petroleum substitute [12]. Despite policy-driven efforts to reduce greenhouse gas emissions and ongoing research into alternative fuels, the constraints of ship structures and limited space pose a challenge for modification or retrofitting [13]. Therefore, alternative energy sources that come close to the performance of conventional fuels while reducing atmospheric pollution warrant further investigation.
Research on alternative fuels that blend marine gas oil (MGO), commonly used for small vessels, with biomass-based fuels is actively underway [12]. However, most studies focus on biodiesel and blended fuels, while research on the use of bioethanol as a substitute fuel mixed with MGO remains relatively limited [2]. This scarcity is partly due to the physical characteristics of bioethanol, such as its water solubility, which makes it difficult to maintain a stable blend [1,14].
Our previous studies proposed an optimized process for the production of MGO–bioethanol blend fuels, with blending ratios of up to 30%, and conducted comparative combustion experiments using dedicated equipment [14].
While there are more studies on the use of bioethanol as an alternative fuel for land vehicles, research on its use as a marine fuel remains limited.
Bae et al. investigated the blending of bioethanol with diesel for land vehicle engines and found a reduction in particulate matter (PM) and NOx emissions but no decrease in hydrocarbon emissions [15,16]. However, this research pertains to land diesel and not marine fuels.
Ha and Yoon applied gasoline–bioethanol blends in both spark ignition (SI) and marine engines and reported reductions in carbon monoxide (CO) and emissions comparable to gasoline [17]. In other studies, the combustion characteristics of different ethanol samples were compared in four-cylinder engines [18]. An improvement in exhaust emissions and an increase in fuel efficiency of up to 26% were found when pure bioethanol was used in SI engines compared to gasoline [19]. Nevertheless, research on the substitution of MGO with bioethanol is still insufficient [14].
Therefore, the objective of this study was to analyze the emission reduction potential and feasibility of bioethanol as a substitute fuel through comparative combustion tests using a 1-ton combustion chamber developed in previous research. This study focused on MGO, which is commonly used for marine vessels, along with diesel oil and kerosene, which are widely used for land applications.
2. Materials and Methods
2.1. Marine Gas Oil (MGO) and Bioethanol (BE100)
MGO is widely used as a primary fuel for small vessel engines, as an auxiliary fuel for medium-sized ships, and as a generator fuel for large ships. According to the international standards for marine fuel (ISO-8217) [20], all the distillates categorized as DMX, DMA, DMZ, and DMB fall under MGO [21]. With a boiling point between 250 °C and 350 °C, MGO is similar to conventional diesel fuel but has a higher density and slightly better ignition and explosion properties [12,22]. It is produced during the crude oil refining process, lies between kerosene and heavy oil, and is referred to as “Gas Oil” to distinguish it from lower-grade heavy oils [14]. ISO-8217 classifies marine fuels into distillate and residual oils and specifies the minimum quality requirements [23]. Normally, MGO has a density of less than 0.89 (15/4 °C) and a kinematic viscosity of 1.5 to 6.0 cSt at 40 °C. The sulfur content is usually less than 1.5%. Recently, the sulfur content in MGO formulations produced to comply with Emission Control Area (ECA) regulations has been reduced to below 0.1%. This trend is expected to increase in the future [14].
Bioethanol is synthesized primarily from plant-based biomass, using raw materials such as starch, sugar, or lignocellulosic biomass. It is often blended with gasoline and used in gasoline engines, requiring only minimal adjustments to the existing petroleum refining and distribution infrastructure [24]. However, bioethanol production presents challenges due to the decentralized availability of resources, making large-scale production and transportation difficult [12,14]. Ships, in particular, require reliable and efficient fuel supply chains, which underscores the need for further technological advances tailored to marine applications [14]. Bioethanol has long been under active development in various fields and is categorized into three generations based on the edibility of its raw materials [25,26,27]. First-generation bioethanol is derived from edible resources such as corn and palm oil, the second generation uses non-edible lignocellulosic resources, and the third generation focuses on marine resources like algae and seaweed due to their potential carbon reduction capabilities [25,26,27]. In this study, bioethanol (Shicorp Co., Ltd., Seoul, Repubic of Korea) extracted from corn, a first-generation grain resource, was selected. Figure 1 compares examples of bioethanol for each generation [2,14].
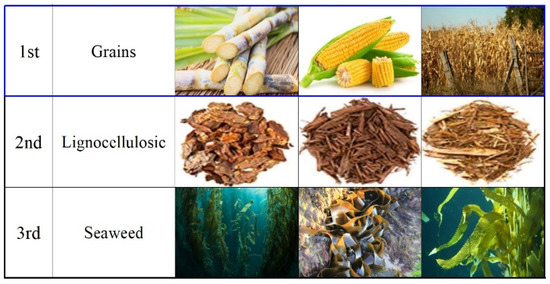
Figure 1.
Examples of raw materials for bioethanol extraction by generation [2,14].
Kerosene and automotive diesel, which are extensively used in land-based applications and whose quality is strictly regulated, were also selected as experimental samples for comparison [28]. Kerosene typically has a density of between 780 and 810 kg/m3, is distilled from crude oil at 150 °C to 275 °C, and contains approximately 6 to 16 carbon atoms. Automotive diesel has a density between 820 and 845 kg/m3, shares a similar boiling range (150 °C to 275 °C), and contains about 16 to 30 carbon atoms [29]. Therefore, the objective of this study was to analyze the thermal output and exhaust emission reduction effects of corn-based first-generation bioethanol under identical combustion conditions, using automotive diesel and kerosene as terrestrial fuel benchmarks for comparison with MGO.
2.2. Analysis of Fuel Composition and Physical Properties
A comparative analysis of the four selected fuel samples was performed according to ISO-8217, the standard test method for marine fuels [30,31]. The comparison focused on five parameters: higher heating value (MJ/kg), lower heating value (J/g), density (kg/m3), flash point (°C), and kinematic viscosity (mm2/s). The summarized results are listed in Table 1. The lower heating value of MGO was approximately 43,030 J/g, similar to diesel and kerosene. However, BE100, a pure bioethanol, had a significantly lower value of 24,190 J/g, about 56.2% lower than MGO. The density of MGO was measured at 840 kg/m3, while BE100 was close at 811.5 kg/m3. Kerosene had the lowest density among the samples. The flash points were 67.5 °C for MGO, 47.5 °C for diesel, 44.0 °C for kerosene, and below 40 °C for BE100. The kinematic viscosity for MGO was registered at 3.011 mm2/s, while it was 2.557 mm2/s for diesel, 1.097 mm2/s for kerosene, and 1.210 mm2/s for BE100. These relatively low viscosity levels suggest that uniform atomization can be effectively promoted during combustion, allowing for stable combustion. Figure 2 provides a visual comparison between the samples and low-quality marine fuels.

Table 1.
Standard testing method for fuel materials.

Figure 2.
Comparison of samples with marine fuels and bioethanol: (a) heavy fuel oil, (b) marine diesel oil, (c) marine gas oil, (d) diesel, (e) kerosene, (f) bioethanol 100%, and (g) gasoline.
2.3. Design and Fabrication of a 1-Ton Combustion Chamber
To analyze the emission reduction characteristics of each fuel sample, a small combustion chamber with a capacity of approximately 1 ton was designed and fabricated. This chamber generates thermal energy through the combustion of fuel. The design was based on previous research by the study team and emphasized simplicity in order to be of an easily portable scale [22,32]. The internal volume of the chamber was approximately 900 L. One side featured an integrated gun-type burner with a fuel supply rate of 4–10 kg/h and a capacity of 99,000 kcal/h. The opposite side was fitted with a high-temperature, heat-resistant glass panel for monitoring and visualizing the flame condition [22,32]. The exhaust pipe was constructed using a 5K 200A-sized chimney(Gyeongnam Welding Co., Ltd., Tongyeong, Republic of Korea). A probe nipple for exhaust gas analysis, along with temperature and pressure sensors for data acquisition, was installed at the end of the pipe [14]. Table 2 outlines the detailed specifications of the chamber for the comparative combustion tests [22,32], and Figure 3 depicts the external appearance of the fabricated combustion chamber [14].

Table 2.
Specifications for the combustion chamber [22,32].
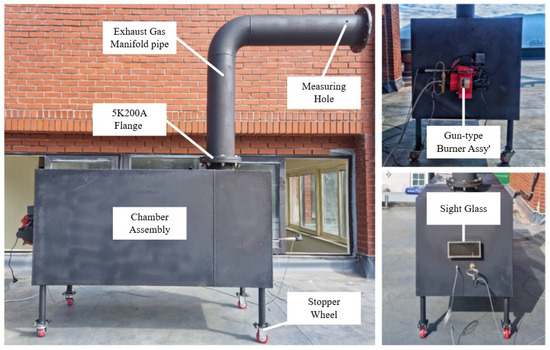
Figure 3.
Appearance of the standard combustion chamber [14].
2.4. Experimental Conditions and Methods
The experimental conditions for analyzing the applicability of bioethanol as a marine fuel are summarized in Table 3. The external temperature during the experiment was between 11.1 °C and 23.5 °C, while the internal pressure of the combustion chamber was maintained at a slightly negative but stable level. The fuel injection pressure was between 9.02 and 9.27 bar, and the average fuel temperature was maintained between 12.03 °C and 15.33 °C. For the exhaust gas analysis, a T-340 model was used to measure the O2 (%), CO2 (%), NOx (ppm), and SO2 (ppm) [33]. The external and exhaust gas temperatures were measured simultaneously using thermocouples, the detailed specifications of which are summarized in Table 4 [34]. Data collection was standardized to continuous intervals of 300 s for all the samples. A 30 min warm-up period was performed between fuel changes to flush residual fuel from the pipes and stabilize the combustion chamber.

Table 3.
Experimental conditions.

Table 4.
Specification for the exhaust gas analyzer [34].
3. Results
3.1. Exhaust Gas Emissions
The characteristics of the exhaust emissions measured by the research team for the four fuel samples are illustrated in Figure 4. The oxygen concentration for MGO was approximately 8.0%. This compares to 10.5% for diesel, 12.0% for kerosene, and 16.3% for BE100, an increase of 8.3% over MGO. This increase can be attributed to the oxygen content and moisture in the bioethanol as well as the residual unburned oxygen from the burner’s air supply.
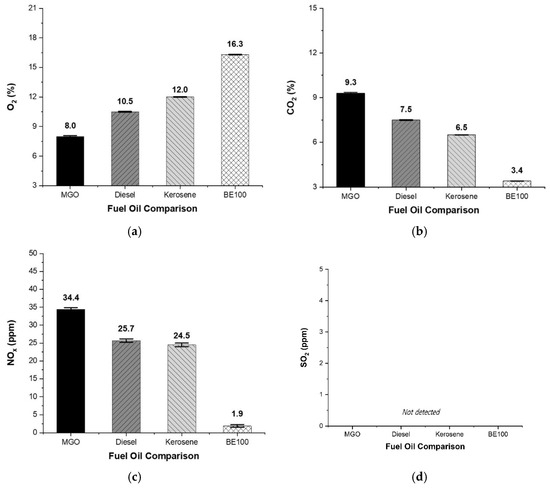
Figure 4.
Comparison of exhaust gas emissions: (a) oxygen, (b) carbon dioxide, (c) nitrogen oxide, and (d) sulfur dioxide.
MGO recorded a CO₂ concentration of 9.3%, diesel 7.5%, kerosene 6.5%, and BE100 approximately 3.4%, which corresponds to a reduction of 5.9% compared to MGO. This indicates that carbon neutrality in terms of marine emissions can be achieved through the partial substitution of conventional marine fuels with bioethanol.
The NOx emissions were 34.4 ppm for MGO, 25.7 ppm for diesel, and 24.5 ppm for kerosene. BE100 clearly outperformed these fuels, recording just 1.9 ppm—a reduction of up to 94% compared to MGO. This highlights the potential of bioethanol as a marine fuel alternative to reduce nitrogen oxide emissions.
Sulfur dioxide (SO2) was not detected in any sample, including MGO. The low sulfur content (<0.01%) of MGO indicates superior fuel quality compared to heavy fuel oil or lower-grade marine diesel oil (MDO). The absence of SO2 in land-based fuels and bioethanol suggests that these fuels could enable net-zero sulfur oxide operation.
3.2. Exhaust Gas Temperature
The comparison of the exhaust gas temperatures for the four fuel samples is shown in Figure 5. The temperature for MGO was approximately 367.1 °C, while diesel for land use was measured at 335.4 °C, and kerosene, which was used for heating, was recorded at 317.7 °C. For pure bioethanol, BE100 exhibited a much lower exhaust temperature of about 203.2 °C, which is approximately 55.3% of the temperature of MGO. This reduction can be attributed to factors such as the latent heat of evaporation due to moisture in the bioethanol, the presence of unburned oxygen from the fixed supply air conditions, and the relatively lower calorific value of bioethanol compared to fossil fuels. While bioethanol shows excellent performance in reducing exhaust emissions compared to MGO, its limited heat output makes it less suitable as a standalone fuel for marine use. Consequently, the use of a blended fuel combining MGO with bioethanol at specific ratios seems more appropriate. Previous studies by this research team have found that blending MGO with up to 30% bioethanol results in a significant reduction in exhaust emissions while limiting the temperature reduction to around 5.8% [14]. This indicates the need for further studies investigating different blending ratios to optimize the performance.
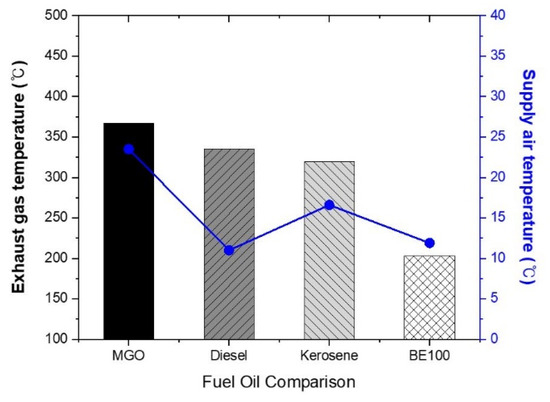
Figure 5.
Comparison of the exhaust gas temperature.
3.3. Combustion Efficiency
Combustion efficiency is defined as the ratio between the heat produced during actual combustion and the calorific value of the fuel [22]. In this comparative study, MGO was used as a control to calculate the combustion efficiency of land-use diesel, heating kerosene, and bioethanol under identical experimental conditions. Although there are multiple formulas for determining the combustion efficiency, a method using constant reference values for each fuel type was used in this study, as derived from the exhaust gas analyzer T-340 [33]. In Equation (1), qA represents the combustion loss, FT is the exhaust gas temperature, and AT is the supply air temperature. A2 is a fuel-specific parameter specified by the manufacturer, with dimensionless constants of 0.686 for MGO and 0.007 for B1 [34]. The reference oxygen concentration O2ref is 21%, and Kk is a correction constant applied when exhaust temperatures fall below the dew point. The final combustion loss rate (qA) was used to calculate the efficiency Effc (%) shown in Figure 6.
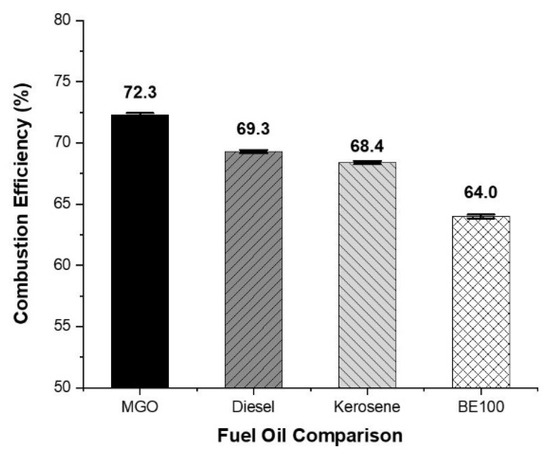
Figure 6.
Comparison of the combustion efficiency.
Under identical experimental conditions, the combustion efficiency for MGO was approximately 72.3%. For diesel, it was 69.3%, and for kerosene, 68.4%, which is a slight decrease compared to MGO. BE100 achieved a combustion efficiency of 64.0%, a decrease of 8.3% compared to MGO.
4. Statistical Analysis
One-Way Analysis of Variance (ANOVA)
A one-way analysis of variance (ANOVA) was performed to investigate whether there were statistically significant differences in the average exhaust gas temperatures, oxygen concentrations, and carbon dioxide and nitrogen oxide emissions depending on the fuel samples used. Given that the sample sizes were uniformly controlled, the Tukey post hoc test was selected to determine the significance level [35].
The ANOVA results revealed a statistically significant difference, with F = 2,342,163.674 and p = 0.000 at a significance level of 0.01. The post hoc analysis revealed differences in the mean exhaust gas temperatures for each fuel sample: 367.113 °C for MGO, 335.366 °C for diesel, 319.703 °C for kerosene, and 203.239 °C for BE100. The order of the exhaust gas temperature was M > D > K > BE, showing that MGO, diesel, and kerosene had higher exhaust gas temperatures than BE100.
In terms of the oxygen concentration, the ANOVA results showed F = 3,026,252.487, p = 0.000, indicating a statistically significant difference at a 0.01 significance level. Post hoc analysis showed mean differences in the oxygen concentration for each fuel sample: 8.034% for MGO, 10.538% for diesel, 11.976% for kerosene, and 16.251% for BE100. The order was M < D < K < BE, indicating that BE100 had a higher oxygen concentration than MGO.
For carbon dioxide, the ANOVA results revealed F = 2,939,641.218, p = 0.000, indicating a statistically significant difference at a 0.01 significance level. Post hoc analysis showed mean differences in the carbon dioxide concentrations for each fuel sample: 9.322% for MGO, 7.524% for diesel, 6.531% for kerosene, and 3.420% for BE100. The order of the carbon dioxide concentration was M > D > K > BE, indicating that MGO, diesel, and kerosene had higher concentrations than BE100.
For the nitrogen oxide emissions, the ANOVA yielded F = 359,719.804, p = 0.000, indicating a statistically significant difference at a 0.01 significance level. Post hoc analysis revealed mean differences in the nitrogen oxide levels for each fuel sample: 34.353 ppm for MGO, 25.670 ppm for diesel, 24.477 ppm for kerosene, and 1.850 ppm for BE100. The order was M > D > K > BE, showing that the nitrogen oxide emissions were lower for BE100 compared to the other fuels.
Table 5 lists the results of the one-way ANOVA for the exhaust gas temperature.

Table 5.
Results of the one-way ANOVA for the exhaust gas emissions.
5. Conclusions
In this study, pure bioethanol was compared with MGO and similar land-based fuels to evaluate its suitability as an environmentally friendly alternative energy source. The following conclusions were drawn.
The fuel composition analysis revealed that BE100 had a lower heating value of 24,190 J/g, approximately 56.2% lower than that of MGO at 43,030 J/g. While the density was nearly identical, the kinematic viscosity of BE100 of 1.210 mm2/s was significantly lower than that of MGO at 3.011 mm2/s, indicating better atomization during fuel injection and possibly leading to more stable combustion.
Under identical experimental conditions using a 1-ton combustion chamber, BE100 had an approximately 8.3% higher oxygen concentration compared to MGO. The carbon dioxide emissions were reduced by approximately 5.9%, indicating the potential contribution of bioethanol as a partial replacement for marine fuels to achieve carbon neutrality.
Despite the already low nitrogen oxide emissions of MGO at 34.4 ppm, diesel and kerosene were between 24.5 and 25.7 ppm, while BE100 was measured at just 1.9 ppm, confirming its superior emission reduction potential. Sulfur oxides were not detected in any sample, which demonstrates the high quality of MGO as a low-sulfur fuel.
The exhaust gas temperature of BE100 was approximately 203.2 °C, a reduction of 44.6% compared to the 367.1 °C for MGO. The combustion efficiency fell by approximately 8.3% compared to MGO. While bioethanol offers excellent environmental benefits in terms of reducing exhaust emissions, its limited thermal output indicates challenges for direct substitution as a marine fuel. Future research on blended fuel formulations is necessary to determine the optimal mixing ratio to maximize the performance and environmental benefits.
This study is limited to a basic simulation test for replacing marine fuel oil with bioethanol, and it is planned to be directly applied to marine engines in the future.
Author Contributions
Conceptualization, J.-W.K. and T.-H.L.; methodology, T.-H.L.; software, T.-H.L.; validation, J.-W.K. and T.-H.L.; formal analysis, J.-W.K.; investigation, J.-W.K. and T.-H.L.; resources, J.-W.K.; data curation, J.-W.K.; writing—original draft preparation, T.-H.L.; writing—review and editing, J.-W.K. and T.-H.L.; visualization, J.-W.K. and T.-H.L.; supervision, T.-H.L.; project administration, T.-H.L.; funding acquisition, T.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant from Gyeongsang National University in 2024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, J.W.; Lee, T.H.; Ryu, Y.H. Thermo-Gravimetric Analysis of MGO-Bioethanol Blended Fuel Oil. J. Korean Soc. Mech. Technol. 2024, 26, 1187–1192. [Google Scholar]
- Kim, J.W. The Effects of MGO-Bioethanol Blended Oil Using Standard Combustion Chamber on Exhaust Emissions Characteristics; Pukyoung National University: Busan, Republic of Korea, 2024. [Google Scholar]
- Lee, T.H.; Lee, S.H.; Lee, J.K. Exhaust gas emission improvements of water/bunker C oil-emulsified fuel applied to marine boiler. J. Mar. Sci. Eng. 2021, 9, 477. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel: A Realistic Fuel Alternative for Diesel Engines; Springer: London, UK, 2008. [Google Scholar]
- Force, C.A.T. Prevention of Air Pollution from Ships: Reducing Shipping Emissions of Air Pollution-Feasible and Cost-Effective Options Submitted by Friends of the Earth International to the Marine Environment Protection Committee; IMO: London, UK, 2005. [Google Scholar]
- Gritsenko, D. Regulating GHG Emissions from shipping: Local, global or polycentric approach? Mar. Policy 2017, 84, 130–133. [Google Scholar] [CrossRef]
- Lee, S.B.; Cho, M.I.; Kang, S.G.; Huh, C. Necessity and Research Challenges of Onboard Carbon Capture Technology in Achieving the IMO’s Goal of Reducing GHG. J. Korean Soc. Mar. Environ. Energy 2023, 26, 336–348. [Google Scholar] [CrossRef]
- Choi, J.S.; Han, S.G.; Choi, J.H.; Park, S.K.; Park, R.S.; Kim, D.H. A Study on Characteristics of Exhaust Gas Emissions of Water-Bunker Oil Mixed by Homogenizer. J. Korean Soc. Mar. Environ. Saf. 2013, 19, 518–524. [Google Scholar] [CrossRef]
- Lee, T.H.; Ryu, Y.H. A Study on mixing properties of coffee ground-fuel for improvement of air pollution from ships. J. Korean Soc. Mech. Technol. 2021, 23, 181–186. [Google Scholar]
- Ryu, Y.H.; Dan, T. Investigation on the Effects of Dimethyl Ether Blending to Bunker Oil for Marine Diesel Engine Use; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- IMO. Alternative Fuels-Scalability and Sustainability, Future Fuels and Technology for Lowand Zero-Carbon Shipping Project in Website; IMO: London, UK, 2024. [Google Scholar]
- Kim, J.W.; Lee, T.H.; Park, J.U. Manufacturing Method for MGO-Bioethanol Mixed Fuel Oil. J. Korean Soc. Mech. Technol. 2024, 26, 293–298. [Google Scholar]
- Lee, T.H.; Kang, I.S. Small Combustion Chamber for Marine Fuel Oil and Analysis of Exhaust Gas Characteristics of Marine Gas Oil. J. Mar. Sci. Eng. 2023, 11, 609. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, J.U.; Lee, T.H.; Kang, I.S. Characteristics of exhaust emissions from MGO–bioethanol fuel blend using a combustion chamber. Adv. Mech. Eng. 2024, 16, 16878132241298338. [Google Scholar] [CrossRef]
- Bae, S.J. Combustion and emission characteristics of a diesel engine fueled with diesel-bioethanol blends according to engine speed. J. Korean. Soc. Mech. Technol. 2022, 24, 572–577. [Google Scholar]
- Bae, S.J. A study on the combustion and emission characteristics of diesel-bioethanol blends in compressionignition engines according to idle operating conditions. J. Korean. Soc. Mech. Technol. 2022, 24, 706–711. [Google Scholar]
- Ha, S.Y. A Study on Combustion and Exhaust Emissions Characteristics in a Spark Ignition (SI) Engine with Bioethanol Fuel; Hanyang University: Busan, Republic of Korea, 2010. [Google Scholar]
- Poulopoulos, S.G.; Philippopoulos, C.J. The effect of adding oxygenated compounds to gasoline on automotive exhaust emissions. J. Eng. Gas Turbines Power 2003, 125, 344–350. [Google Scholar] [CrossRef]
- Jeuland, N.; Montagne, X.; Gautrot, X. Potentiality of ethanol as a fuel for dedicated engine. Oil Gas Sci. Technol. 2004, 59, 559–570. [Google Scholar] [CrossRef]
- ISO 8217:2017; ISO 8217—Petroleum Products—Fuels (Class F)—Specifications of Marine Fuels. ISO: Geneva, Switzerland, 2017.
- Legislative Bureau. Oil and Alternative Fuel Business Act; National Legal Information Center: Sejong, Republic of Korea, 2019.
- Kim, J.W.; Lee, T.H.; Park, J.U. Development of a Lap scale 1-ton Standard Combustion Chamber. J. Korean Soc. Mech. Technol. 2024, 26, 371–376. [Google Scholar]
- Kang, M.K. A Study on the Exhaust Characteristics of Diesel Engine Emissions and the Feasibility of Using Waste Vinyl Pyrolysis Oil as Marine Fuel; National Korea Maritime & Ocean University: Busan, Republic of Korea, 2024. [Google Scholar]
- Lee, J.H.; Lee, S.Y. Current status and prospects of bioalcohol. News Inf. Chem. Eng. 2018, 36, 673–679. [Google Scholar]
- Ra, C.H.; Sunwoo, I.S.; Kim, S.K. Bioethanol Production from Macroalgal Biomass. J. Life. Sci. 2016, 26, 976–982. [Google Scholar] [CrossRef]
- Lee, S.M.; Choi, I.S.; Kim, S.K.; Lee, J.H. Production for bio-ethanol from brown algae by enzymic hydrolysis. J. KSBB 2009, 24, 483–488. [Google Scholar]
- Badal, C.S.; Michael, C.A. Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzym. Microb. Technol. 2007, 41, 528–532. [Google Scholar]
- Hwang, I.H.; Doe, J.W.; Kang, H.K.; Sung, S.R.; Ha, J.H.; Na, B.K. Study on the Density and Volume Change Property of Petroleum Products according to Temperature Variation. J. Oil Appl. Sci. 2017, 34, 1112–1120. [Google Scholar]
- Sattarin, M.; Modarresi, H.; Bayat, M.; Teymori, M. New Viscosity Correlations for Dead Crude Oils. Pet. Coal 2007, 49, 33–39. [Google Scholar]
- K-petro. Composion Analysis Test Report of TSC2024-0118E; K-petro Co., Ltd.: Sungnam, Republic of Korea, 2024. [Google Scholar]
- K-petro. Composion Analysis Test Report of TSC2025-0024E; K-petro Co., Ltd.: Sungnam, Republic of Korea, 2025. [Google Scholar]
- Lee, T.H.; Kim, J.W.; Ryu, Y.H. Exhaust Emission Characterisitics of MGO-Biodiesel Mixed Oil. J. Korean Soc. Mech. Technol. 2024, 26, 660–667. [Google Scholar]
- Lee, T.H.; Kang, I.S. Development of 30 liter small boiler for testing marine fuel oil. J. Korean Soc. Mech. Technol. 2023, 25, 223–228. [Google Scholar]
- TESTO. Flue Gas Analyzer Instruction Manual on Testo-340; Testo Co., Ltd.: West Chester, PA, USA, 2016. [Google Scholar]
- No, K.S. The Proper Methods of Statistical Analysis for Dissertation; Hanbit Academy Inc.: Seoul, Repubic of Korea, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).