Harvest Recovery of a North Atlantic Intertidal Seaweed, Ascophyllum nodosum: Experimental Design Issues
Abstract
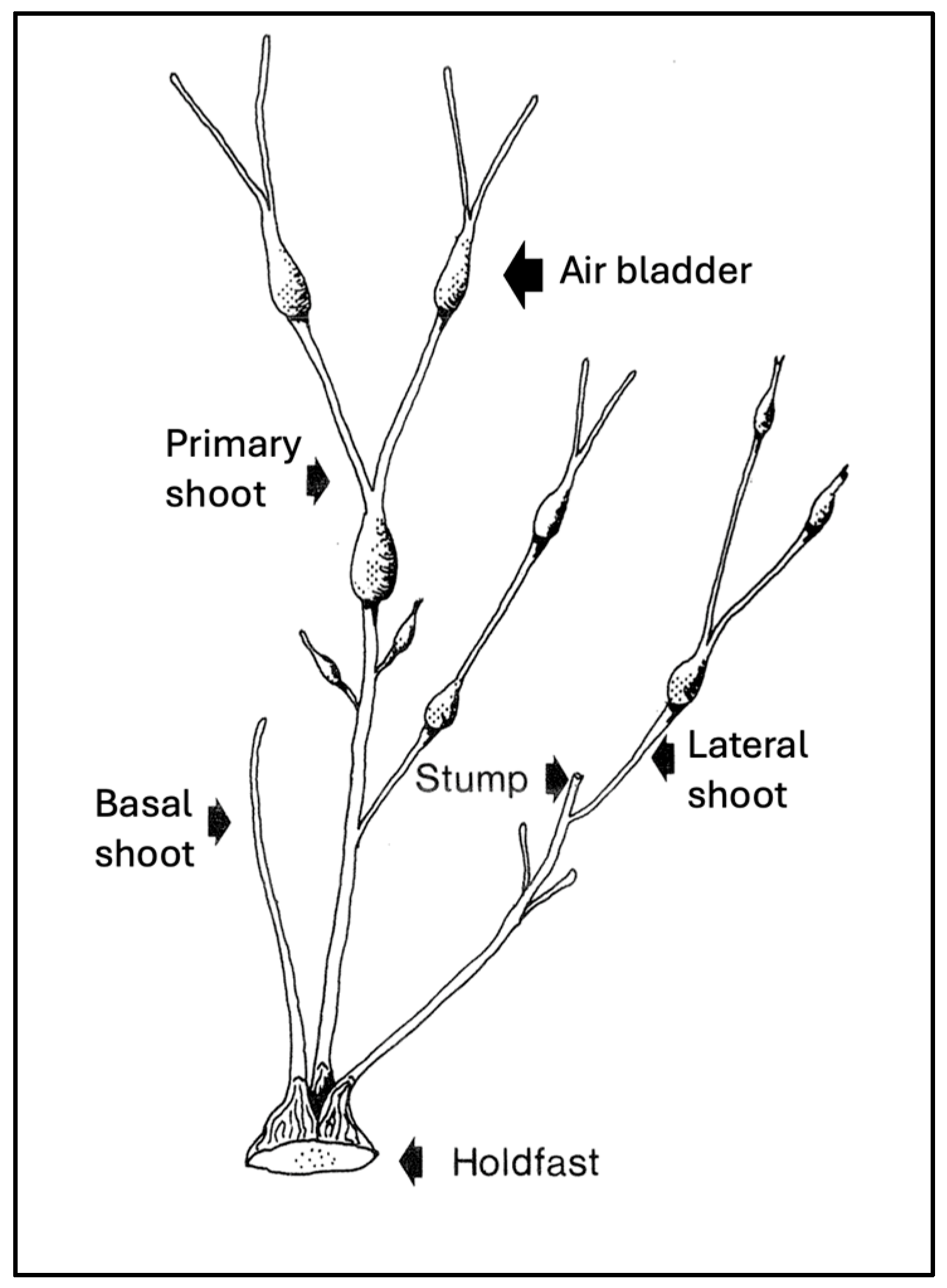
1. Introduction
2. Harvesting Methods and Regulations
“Historically then, from the first rake harvests through to mechanization, the management regime routinely allowed an intense harvest of Ascophyllum on many shores in southwest Nova Scotia which took years to recover. The evidence strongly indicates that this took place at bay-wide scales, suggesting that an undesirable level of habitat loss had occurred at a landscape scale”(see Appendix A for details).
3. Assessing Recovery of Rockweed After Harvesting
Recommended Protocols
- Quantify biomass and height as the dependent variables for documenting recovery.
- Biomass sampling should be carried out when fronds are at the same reproductive state in each year of the study.
- Unharvested rockweed beds often have a skewed or bimodal distribution of size classes, with a small proportion of very large fronds that cast shade on subcanopy and basal shoots [40,41]. Because most biomass is present in the tallest fronds, data on how size class distributions are affected by harvesting are useful (as in [36,40,41]).
- Height should be measured for the tallest fronds per quadrat as an index of canopy height. If only the average height per quadrat is reported, the height of the canopy may be underestimated due to the skewed or bimodal size distributions cited above.
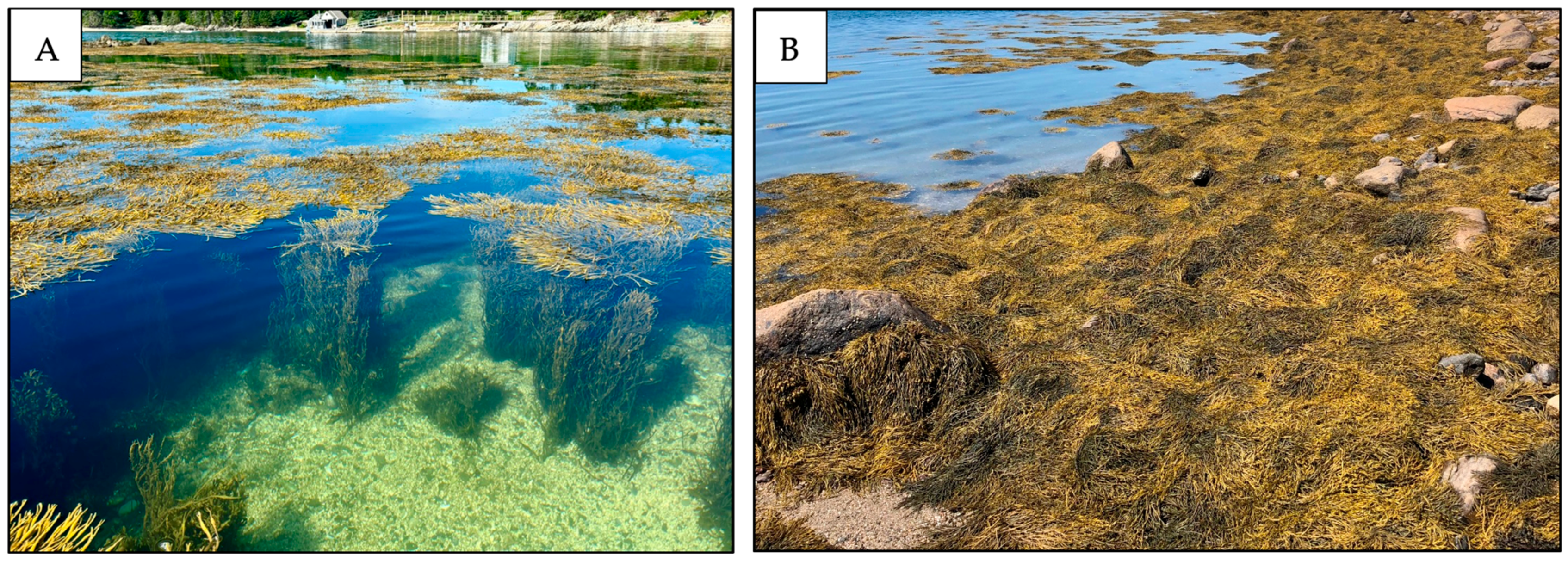
- Choose study sites where rockweed biomass and height are representative of commercially targeted areas, such as sheltered coves and bays or moderately exposed areas where rockweed is a minimum of ~1 m tall.
- Ideally, these should be sites that were not harvested previously or within the past decade. If the sites have been harvested recently or if their harvest history is unknown, this caveat should be explained in the paper’s abstract and conclusions.
- Site locations with GPS coordinates should be published to allow follow-up studies.
- To test for recovery of biomass and height from a single harvest event, compare harvested sites or plots with unharvested sites or plots.
- As noted above, an unharvested control treatment is essential because biomass and height can vary among years [36], confounding the effects of harvesting on recovery rates over time.
- For both treatments (control vs. harvested), obtain data on biomass and height immediately before the time of harvesting as well as immediately after harvesting, followed by repeated sampling in the same month over several years. This is often referred to as a BACI design, for Before-After and Control-Impact, where “impact” refers to experimental harvesting [30].
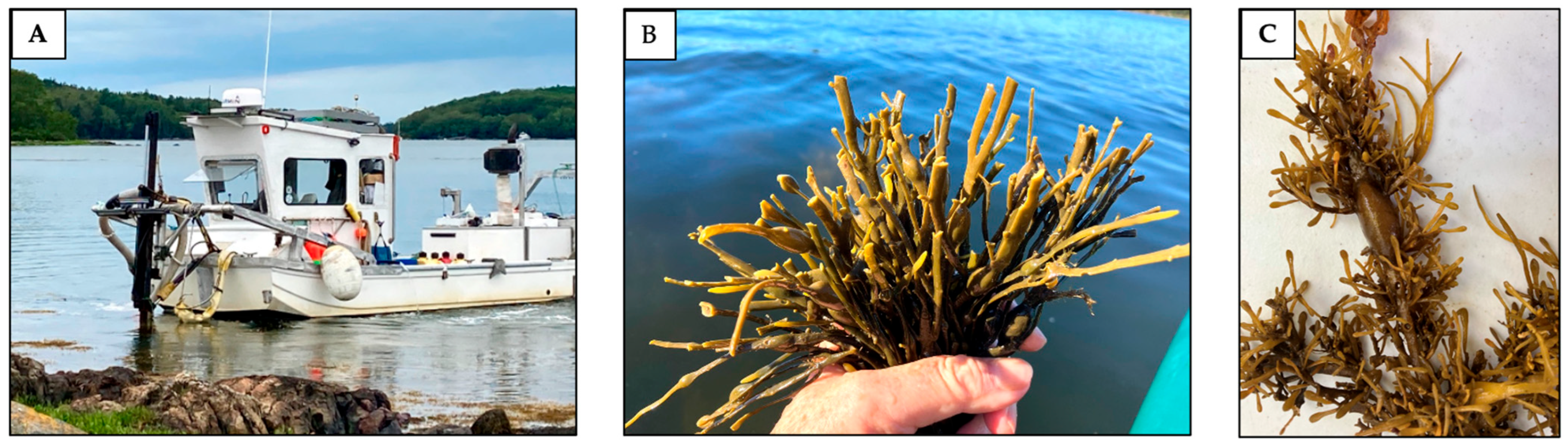
- Report how much biomass was removed from the site or experimental plot at the time of harvesting and explain the harvesting method at each site (e.g., hand-cut, rake-cut, or type of mechanical vessel).
- Use replicated treatments that take existing variability into account, ideally by performing power tests [39,42,43].
- The experimental design should allow inferential statistics and avoid pseudo-replication [44].
- For each experimental treatment (control vs. harvested), replication should be sufficient to ensure that biologically important differences between treatments (should they occur) are statistically significant.
- Use permanently marked plots, transects, and quadrats for documenting year to year changes at the same location.
- Inability to re-sample the same exact location adds to variability in the data, as occurred in [30].
- For long-term studies, include a treatment with repeated harvest events, such as every two or three years.
4. Review of Key Papers
4.1. Choice of Studies
4.2. Experiments with Hand-Cutting
4.2.1. Keser et al. (1981)—“Regrowth of Ascophyllum nodosum and Fucus vesiculosus Under Various Harvesting Regimes in Maine, USA” [24]
4.2.2. Fegley (2001)—“Ecological Implications of Rockweed, Ascophyllum nodosum (L.) Le Jolis, Harvesting” [36]
4.2.3. Gendron et al. (2018)—“Managing Disturbance: The Response of a Dominant Intertidal Seaweed Ascophyllum nodosum (L.) Le Jolis to Different Frequencies and Intensities of Harvesting” [45]
4.3. Experiments with Commercial Cutting
4.3.1. Ang et al. (1993)—“Changes in the Population Structure of Ascophyllum nodosum (L.) Le Jolis Due to Mechanical Harvesting” [41]
4.3.2. Lazo and Chapman (1996)—“Effects of Harvesting on Ascophyllum nodosum (L.) Le Jol. (Fucales, Phaeophyta): A Demographic Approach” [39]
4.3.3. Ugarte et al. (2006)—“Changes in the Brown Seaweed Ascophyllum nodosum (L.) Le Jol. Plant Morphology and Biomass Produced by Cutter Rake Harvests in Southern New Brunswick, Canada” [40]
4.3.4. Johnston et al. (2023)—“Bed-Scale Impact and Recovery of a Commercially Important Intertidal Seaweed” [30]
4.4. Comparative Studies
4.4.1. Lauzon-Guay et al. (2021)—“Biomass and Height of Ascophyllum nodosum After Two Decades of Continuous Commercial Harvesting in Eastern Canada” [35]
4.4.2. Lauzon-Guay et al. (2023)—“Morphology of Ascophyllum nodosum in Relation to Commercial Harvesting in New Brunswick, Canada” [46]
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- Goat Island and Vicinity—“...recently harvested and there was no weed left.”
- Thornes Cove—“The beds at this cove and nearby were completely depleted.”
- Bear Island—“Examining several beds in the Deep Brook area we found them all harvested with the exception of a few small patches. Very little biomass is left behind, perhaps less than 2%”.
- Pinkney’s Point—“At present it would be very difficult to harvest any Asco in an economical manner.”
- Inner Spectacle Island—“...has been really overharvested.”
- Murder Island—“...has been severely harvested...”
- East side of Goose Bay—“...very heavily harvested...”
- Tusket River, western shore—“The whole area has been heavily harvested during the past several years...”
- The Tittle—“Most of the usual places were so harvested that the weed was too short to bother with.”
- Rocko Point and Abram’s River—“There is little of value to count as available weed at this point.”
- Etoile Island—“The island has been heavily harvested...”
- Pubnico Harbour western shore—“Very little Asco available.”
- Goodwins Island, Solomons Island, Egg Island, Vigneau Island—“The harvest has been heavy and complete...”
- Port Latour—“...heavily harvested...”
References
- Schmidt, A.L.; Coll, M.; Romanuk, T.N.; Lotze, H.K. Ecosystem structure and services in eelgrass Zostera marina and rockweed Ascophyllum nodosum habitats. Mar. Ecol. Prog. Ser. 2011, 437, 51–68. [Google Scholar] [CrossRef]
- Seeley, R.H.; Schlesinger, W.H. Sustainable seaweed cutting? The rockweed (Ascophyllum nodosum) industry of Maine and the Maritime Provinces. Ann. New York Acad. Sci. 2012, 1249, 84–103. [Google Scholar] [CrossRef]
- Maine Dept of Marine Resources (Department of Marine Resources, Rockweed Plan Development Team). Fishery Management Plan for Rockweed (Ascophyllum nodosum). 2014. Available online: https://www.maine.gov/dmr/sites/maine.gov.dmr/files/docs/DMRRockweedFMPJan2014.pdf (accessed on 15 November 2025).
- Kay, L.M.; Eddy, T.D.; Schmidt, A.L.; Lotze, H.K. Regional differences and linkage between canopy structure and community composition of rockweed habitats in Atlantic Canada. Mar. Biol. 2016, 163, 251. [Google Scholar] [CrossRef]
- Lotze, H.K.; Milewski, I.; Fast, J.; Kay, L.; Worm, B. Ecosystem-based management of seaweed harvesting. Bot. Mar. 2019, 62, 395–409. [Google Scholar] [CrossRef]
- Dudgeon, S.R.; Petraitis, P.S. Experimental evidence for resilience of rockweeds on rocky shores in the Gulf of Maine, USA. Limnol. Oceanogr. 2021, 67, S211–S223. [Google Scholar] [CrossRef]
- Guiry, M.D.; Morrison, L. The sustainable harvesting of Ascophyllum nodosum (Fucaceae, Phaeophyceae) in Ireland, with notes on the collection and use of some other brown algae. J. Appl. Phycol. 2013, 25, 1823–1830. [Google Scholar] [CrossRef]
- Kvanneid, A.J.; Sundnes, F. Nature’s values in marine resource governance: An ethnographic case study of rockweed in Norway. Socio-Ecol. Practice Res. 2024, 6, 177–189. [Google Scholar] [CrossRef]
- Baardseth, E. Synopsis of Biological Data on Knobbed Wrack Ascophyllum nodosum (Linnaeus) Le Jolis; FAO: Rome, Italy, 1970; p. 56. Available online: https://openknowledge.fao.org/handle/20.500.14283/b0672e (accessed on 15 November 2025).
- Åberg, P. Size-based demography of the seaweed Ascophyllum nodosum in stochastic environments. Ecology 1992, 73, 1488–1501. [Google Scholar] [CrossRef]
- Menge, B.A. Predation intensity in a rocky intertidal community. Effects of an algal canopy, wave action, and desiccation on predator feeding rates. Oecologia 1978, 34, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Scheibling, R.E. Structure and dynamics of epifaunal assemblages on intertidal macroalgae Ascophyllum nodosum and Fucus vesiculosus in Nova Scotia, Canada. Mar. Ecol. Prog. Ser. 1987, 27, 209–227. Available online: https://www.jstor.org/stable/24824696 (accessed on 15 November 2025). [CrossRef]
- Vadas, R.L.; Wright, W.A.; Beal, B.F. Biomass and productivity of intertidal rockweeds (Ascophyllum nodosum LeJolis) in Cobscook Bay. Northeastern Nat. 2004, 11, 123–142. Available online: https://www.jstor.org/stable/60225652 (accessed on 15 November 2025). [CrossRef]
- Vercaemer, B.; Wong, M.C.; Bravo, M.A. Fish assemblages in rockweed (Ascophyllum nodosum (L.) Le Jolis) beds on the Atlantic Coast of Nova Scotia, Canada. Can. Tech. Rep. Fish. Aquat. Sci. 2018, 3249, 34. [Google Scholar]
- Garbary, D.J.; Jamieson, M.M.; Taylor, B.R. Population ecology of the marine insect Halocladius variabilis (Diptera: Chironomidae) in the rocky intertidal zone of Nova Scotia, Canada. Mar. Ecol. Prog. Ser. 2009, 376, 193–202. [Google Scholar] [CrossRef]
- Garbary, D.J.; Brown, N.E.; MacDonell, H.J.; Toxopeus, J. Ascophyllum and its symbionts—A complex symbiotic community on North Atlantic shores. In Algal and Cyanobacterial Symbioses; Grube, M., Seckbach, J., Muggia, L., Eds.; World Scientific: Hackensack, NJ, USA, 2017; pp. 547–572. [Google Scholar]
- Garbary, D.J.; London, J. The Ascophyllum/Polysiphonia/Mycosphaerella symbiosis. V. Mycosphaerella protects A. nodosum from desiccation. Bot. Mar. 1995, 38, 529–533. [Google Scholar] [CrossRef]
- Rangeley, R.W.; Kramer, D.L. Tidal effects on habitat selection and aggregation by juvenile pollock Pollachius virens in the rocky intertidal zone. Mar. Ecol. Prog. Series 1995, 126, 19–29. [Google Scholar] [CrossRef]
- Rangeley, R.W.; Kramer, D.L. Use of rocky habitats by juvenile pollock Pollachius virens. Mar. Ecol. Prog. Series 1995, 126, 9–17. [Google Scholar] [CrossRef]
- Hamilton, D.J. Feeding behavior of common Eider ducklings in relation to availability of rockweed habitat and duckling age. Waterbirds 2021, 24, 233–241. [Google Scholar] [CrossRef]
- Johnston, E.; Klemmer, A.; Blomberg, E.; Baron, A.; Watson, V.; Tudor, L.; Welch, L.; Olsen, B. Macroalgae composition alters occupancy of multiple bird guilds in rocky intertidal communities. Mar. Ecol. Prog. Ser. 2021, 659, 29–47. [Google Scholar] [CrossRef]
- Sharp, G.J. An Assessment of Ascophyllum nodosum Harvesting Methods in Southwestern Nova Scotia; Fisheries and Oceans Canada, Invertebrates and Marine Plants Division: St. Andrews, NB, Canada, 1981; p. 1012.
- MacFarlane, C. Observations on the annual growth of Ascophyllum nodosum. Trans. Nova Scotian Inst. Sci. 1932, 18, 27–33. [Google Scholar]
- Keser, M.; Vadas, R.L.; Larson, B.R. Regrowth of Ascophyllum nodosum and Fucus vesiculosus under various harvesting regimes in Maine, USA. Bot. Mar. 1981, 24, 29–38. [Google Scholar] [CrossRef]
- Vadas, R.L.; Wright, W.A.; Miller, S.L. Recruitment of Ascophyllum nodosum: Wave action as a source of mortality. Mar. Ecol. Prog. Ser. 1990, 61, 263–272. [Google Scholar] [CrossRef]
- Halat, L.; Galway, M.E.; Gitto, S.; Garbary, D.J. Epidermal shedding in Ascophyllum nodosum (Phaeophyceae): Seasonality, productivity and relationship to harvesting. Phycologia 2015, 54, 599–608. [Google Scholar] [CrossRef]
- Webber, H.M.; Claesson, S.; Erhart, S.; Schmitt, C.V.; Muhlin, J.F. Maine’s potential to be a global leader in sustainable seaweed harvesting and management. Maine Policy Rev. 2023, 32, 70–77. [Google Scholar] [CrossRef]
- Ugarte, R.A.; Sharp, G. Management and production of the brown algae Ascophyllum nodosum in the Canadian maritimes. J. Appl. Phycol. 2012, 24, 409–416. [Google Scholar] [CrossRef]
- Ugarte, R.A.; Sharp, G. A new approach to seaweed management in eastern Canada: The case of Ascophyllum nodosum. Can. Biol. Mar. 2001, 42, 63–70. [Google Scholar]
- Johnston, E.M.; Mittlestadt, H.N.; Braun, L.A.; Muhlin, J.F.; Olsen, B.J.; Webber, H.M.; Klemmer, A.J. Bed-scale impact and recovery of a commercially important intertidal seaweed. J. Exp. Mar. Biol. Ecol. 2023, 561, 151869. [Google Scholar] [CrossRef]
- Vandermeulen, H. Information to support assessment of stock status of commercially harvested species of marine plants in Nova Scotia: Irish moss, rockweed, and kelp. DFO Can. Sci. Advis. Sec. Res. Doc. 2013, 42, 50. [Google Scholar]
- Fass, M.P. The current rockweed, Ascophyllum nodosum, harvesting regime on the shores of Nova Scotia–A review. Proc. Nova Scotian Inst. Sci. 2021, 51, 443–454. [Google Scholar] [CrossRef]
- Sharp, G.J.; Sharp, J.T. Ascophyllum nodosum (L.) Le Jolis harvesting impacts and management options using GPS tracking of mechanical harvesters in Nova Scotia, Canada. J. Appl. Phycol. 2023, 36, 605–610. [Google Scholar] [CrossRef]
- Reiter, S.M.; Post, D.; Wedding, L.; Strong, A. The future of the public trust: The muddied waters of rockweed management in Maine. Ocean Coastal Law J. 2020, 25, 235. [Google Scholar]
- Lauzon-Guay, J.-S.; Ugarte, R.A.; Morse, B.L.; Robertson, C.A. Biomass and height of Ascophyllum nodosum after two decades of continuous commercial harvesting in eastern Canada. J. Appl. Ecol. 2021, 33, 1695–1708. [Google Scholar] [CrossRef]
- Fegley, J. Ecological Implications of Rockweed, Ascophyllum nodosum (L.) Le Jolis, Harvesting. Ph.D. Thesis, University of Maine, Orono, ME, USA, 2001. Available online: https://digitalcommons.library.umaine.edu/etd/397/ (accessed on 15 November 2025).
- Cousens, R. Quantitative reproduction and reproductive effort by stands of the brown alga Ascophyllum nodosum (L.) Le Jolis in southeastern Canada. Estuar. Coast. Shelf Sci. 1985, 22, 495–507. [Google Scholar] [CrossRef]
- Albuquerque, C.; Olesen, B.; Marbà, N.; Krause-Jensen, D. Reproductive allocation of the habitat-forming intertidal macroalga Ascophyllum nodosum decreases at its northern distribution edge. Ecol. Evol. 2025, 15, e72141. [Google Scholar] [CrossRef] [PubMed]
- Lazo, L.; Chapman, A.R.O. Effects of harvesting on Ascophyllum nodosum (L.) Le Jol. (Fucales, Phaeophyta): A demographic approach. J. Appl. Phycol. 1996, 8, 87–103. [Google Scholar] [CrossRef]
- Ugarte, R.A.; Sharp, G.; Moore, B. Changes in the brown seaweed Ascophyllum nodosum (L.) Le Jol. plant morphology and biomass produced by cutter rake harvests in southern New Brunswick, Canada. J. Appl. Phycol. 2006, 18, 351–359. [Google Scholar] [CrossRef]
- Ang, P.O.; Sharp, G.J.; Semple, R.E. Changes in the population structure of Ascophyllum nodosum (L.) Le Jolis due to mechanical harvesting. Hydrobiologia 1993, 260, 321–326. [Google Scholar] [CrossRef]
- Seeley, R.H.; Hardy, S.; Prentiss, N.K.; Adey, W.H. Comment: A reexamination of bed-scale impact and recovery of a commercially important intertidal seaweed. J. Exp. Mar. Biol. Ecol. 2024, 574, 151984. [Google Scholar] [CrossRef]
- Johnston, E.M.; Mittlestadt, H.N.; Braun, L.A.; Muhlin, J.F.; Olsen, B.J.; Webber, H.M.; Klemmer, A.J. Bed-scale rockweed harvest findings are not altered by study critiques, a response to Seeley et al. J. Exp. Mar. Biol. Ecol. 2024, 578, 152039. [Google Scholar] [CrossRef]
- Hurlbert, S. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Gendron, L.; Merzouk, A.; Bergeron, P.; Johnson, L.E. Managing disturbance: The response of a dominant intertidal seaweed Ascophyllum nodosum (L.) Le Jolis to different frequencies and intensities of harvesting. J. Appl. Phycol. 2018, 30, 1877–1892. [Google Scholar] [CrossRef]
- Lauzon-Guay, J.-S.; Feibel, A.I.; Morse, B.L.; Ugarte, R.A. Morphology of Ascophyllum nodosum in relation to commercial harvesting in New Brunswick, Canada. J. Appl. Phycol. 2023, 35, 2371–2381. [Google Scholar] [CrossRef]
- Moss, B.L. Meristems and growth control in Ascophyllum nodosum (L.) Le Jol. New Phytol. 1970, 69, 253–260. [Google Scholar] [CrossRef]
- Cousens, R. Frond size distributions and the effects of algal canopy on the behaviour of Ascophyllum nodosum (L.) Le Jolis. J. Exp. Mar. Biol. Ecol. 1985, 92, 231–249. [Google Scholar] [CrossRef]
- Vadas, R.L.; Wright, W.A. Recruitment, growth and management of Ascophyllum nodosum. Actas II Congr. Algas Mar. Chilenas 1986, 2, 101–113. [Google Scholar]
- Braun, L. Understanding the Impacts of Anthropogenic Effects and Habitat Variability Interactions on Maine’s Rocky Intertidal Ecosystem. Master’s Thesis, University of Maine, Orono, ME, USA, 2022. Available online: https://digitalcommons.library.umaine.edu/etd/3709 (accessed on 15 November 2025).
- Johnston, E.M.; Klemmer, A.J.; Braun, L.A.; Mittelstaedt, H.N.; Muhlin, J.F.; Webber, H.M.; Olsen, B.J. Evaluating bottom-up forcing of a rocky intertidal resource harvest on a high trophic- level consumer group. Estuar. Coastal Shelf Sci. 2024, 298, 10827. [Google Scholar] [CrossRef]
- Mittelstaedt, H.N. Untangling Influences of Community Dynamics at the Coastal Interface. Ph.D. Thesis, University of Maine, Orono, ME, USA, 2023. Available online: https://digitalcommons.library.umaine.edu/etd/3860 (accessed on 15 November 2025).
- Webber, H.M. The Foundation Roles of Rockweed, Ascophyllum nodosum (Linneaus) Le Jolis, in Maine’s Rocky Intertidal Zone. Ph.D. Thesis, University of Maine, Orono, ME, USA, 2024. Available online: https://digitalcommons.library.umaine.edu/etd/3954 (accessed on 15 November 2025).
- Johnston, E.M. Cross-Trophic-Level Dynamics in Aquatic Ecosystems and Their Application Across Ecological Contexts. Ph.D. Thesis, University of Maine, Orono, ME, USA, 2023. Available online: https://digitalcommons.library.umaine.edu/etd/3764 (accessed on 15 November 2025).
- David, H.M. Studies in the autecology of Ascophyllum nodosum Le Jol. J. Ecol. 1943, 31, 178–198. Available online: https://www.jstor.org/stable/2256547 (accessed on 15 November 2025). [CrossRef]
- Printz, H. Recuperation and recolonization in Ascophyllum. Int. Seaweed Symp. 1956, 2, 194–197. [Google Scholar]
- Printz, H. Investigations of the failure of recuperation and repopulation in cropped Ascophyllum nodosum. Norske Vidensk. Akad. K. Mat. Nat. Kl. 1959, 3, 1–15. [Google Scholar]
- Garbary, D.J.; Fass, M.P.; Vandermeulen, H. Invasive Fucus serratus (Fucaceae, Phaeophyceae) responds to climate change along the Atlantic coast of Nova Scotia. Bot. Mar. 2021, 64, 407–417. [Google Scholar] [CrossRef]
- MacFarlane, C. A survey of certain seaweeds of commercial importance in southwest Nova Scotia. Can. J. Bot. 1952, 30, 8–97. [Google Scholar] [CrossRef]
- Canadian Atlantic Fisheries Scientific Advisory Committee. Rockweed in Southwestern New Brunswick; Canadian Atlantic Fisheries Scientific Advisory Committee: Dartmouth, NS, Canada, 1993.
- Chopin, T. The seaweed resources of eastern Canada. In Seaweed Resources of the World; Critchley, A., Ohno, M., Eds.; Japan International Cooperation Agency: Yokosuka, Japan, 1998; pp. 273–302. [Google Scholar]
- Cunningham, W. An assessment of Ascophyllum nodosum Resources in Selected Areas of Annapolis, Digby, Yarmouth and Shelburne Counties from a Commercial Perspective. Consultant Report to Nova Scotia Department of Fisheries. 1990. Available online: https://publications.gc.ca/pub?id=9.577630&sl=0 (accessed on 15 November 2025).
- Sharp, G.; Semple, R. An Assessment of Ascophyllum nodosum Resources in Scotia/Fundy 1990; Canadian Atlantic Fisheries Scientific Advisory Committee: Dartmouth, NS, Canada, 1991.
- DFO (Department of Fisheries and Oceans). Rockweed in the Maritimes; DFO Science Stock Status Report; DFO: Ottawa, ON, USA, 1998.

| REQUIREMENTS | USA | CANADA | ||
|---|---|---|---|---|
| Maine 1 | Cobscook Bay Mgmt Area, ME | New Brunswick | Nova Scotia | |
| Minimum Cutting Height | 41.0 cm | 41.0 cm | 12.7 cm | 12.7 cm |
| Assigned Harvesting Sectors | No | Yes | Yes 2 | Yes 2 |
| Assigned Sector Biomass Reported Pre-Harvest | Not Applicable | Yes | Yes | Yes |
| Max Annual Exploitation Rate/Sector | No limit | 17% | 17% | 20–25% |
| Prohibited Cutting Methods | None | None | Mechanical Vessel | Mechanical Vessel 3 |
| Max Holdfast Removal Allowed per Sector | 0% 4 | 0% 4 | 10% | 15% |
| Landowner Permission Required | Yes | Yes | No | No |
| Areas Permanently Closed to Cutting | Yes 5 | Yes 6 | Yes 7 | No |
| Study | Harvest Method | Reported Biomass Recovery | Reported Height Recovery | Number of Control Replicates | Number of Harvested Replicates | Total Number of Sites | Number of Harvest Events | Years Allowed for Recovery | Harvest History of Study Area |
|---|---|---|---|---|---|---|---|---|---|
| (1) Hand Cut Expmt | |||||||||
| Keser et al., 1981 [24] | hand cut: 0 vs. 15 vs. 25 cm | yes | no | 0 | not clear | 8 | 1 to 3 | 1 to 3 | unknown |
| * Fegley 2001 [36] | hand cut: 18 vs. 36 cm | yes | yes, by size class | 12 plots | 12 plots | 4 | 1 | 2 | unknown |
| Gendron et al., 2018 [45] | hand cut: 15 vs. 30 cm | yes | no | 0 | 0 | 1 | 6 to 18 | 1 to 5 | unharvested |
| (2) Commercial Cut Expmt | |||||||||
| Ang et al., 1993 [41] | mechanical vessel | no | no | 2 transects | 4 transects | 1 | 1 | 0 | unharvested |
| Lazo & Chapman 1996 [39] | mechanical vessel | no | no | 3 plots | 3 plots | 1 | 1 | 2 | cut ~2 yrs ago |
| * Ugarte et al., 2006 [40] | rake | yes | yes | 2 plots | 3 plots | 1 | 1 | 2 | closed area |
| * Johnston et al., 2023 [30] | rake or mechanical vessel | yes | yes | 19 sites | 19 sites | 38 (in 4 regions) | 1 | 1 | unknown |
| (3) Comparative Study | |||||||||
| Lauzon-Guay et al., 2021 [35] | rake | NA | NA | 0 | many sectors | 4 regions | NA | NA | repeatedly |
| Lauzon-Guay et al., 2023 [46] | rake | NA | NA | 3 sites | 3 sites | 6 | NA | NA | closed vs. repeatedly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snow, A.A.; Porter, D.; Garbary, D.J.; Vandermeulen, H. Harvest Recovery of a North Atlantic Intertidal Seaweed, Ascophyllum nodosum: Experimental Design Issues. J. Mar. Sci. Eng. 2025, 13, 2207. https://doi.org/10.3390/jmse13112207
Snow AA, Porter D, Garbary DJ, Vandermeulen H. Harvest Recovery of a North Atlantic Intertidal Seaweed, Ascophyllum nodosum: Experimental Design Issues. Journal of Marine Science and Engineering. 2025; 13(11):2207. https://doi.org/10.3390/jmse13112207
Chicago/Turabian StyleSnow, Allison A., David Porter, David J. Garbary, and Herb Vandermeulen. 2025. "Harvest Recovery of a North Atlantic Intertidal Seaweed, Ascophyllum nodosum: Experimental Design Issues" Journal of Marine Science and Engineering 13, no. 11: 2207. https://doi.org/10.3390/jmse13112207
APA StyleSnow, A. A., Porter, D., Garbary, D. J., & Vandermeulen, H. (2025). Harvest Recovery of a North Atlantic Intertidal Seaweed, Ascophyllum nodosum: Experimental Design Issues. Journal of Marine Science and Engineering, 13(11), 2207. https://doi.org/10.3390/jmse13112207






