Observations on the Benthic Heterobranch “Sea Slugs” (Mollusca: Gastropoda) of Lampedusa, the Southernmost Island of Italy (MPA Isole Pelagie)
Abstract
1. Introduction
2. Materials and Methods
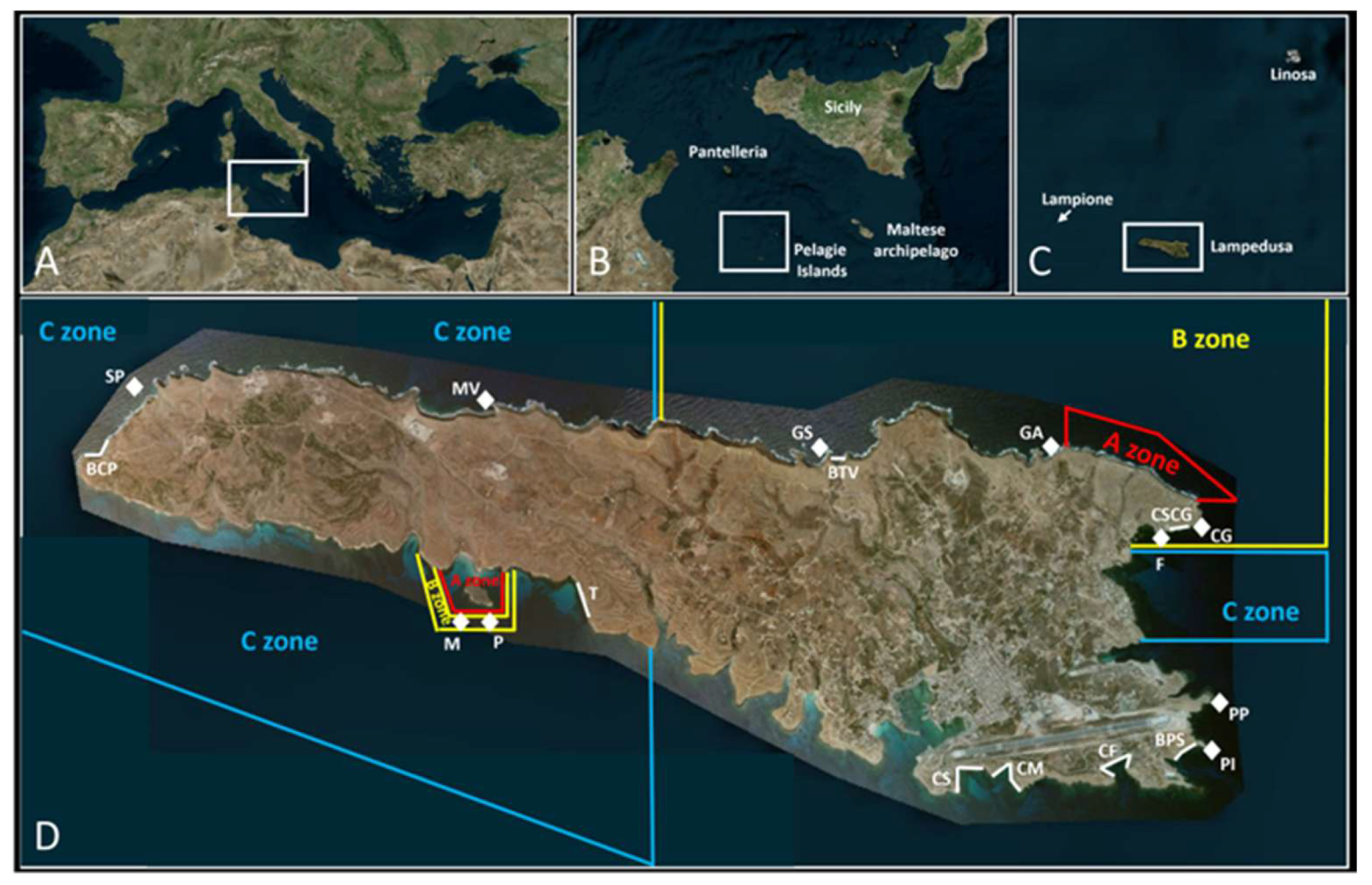
2.1. Study Area
2.2. Sampling and Data Analysis
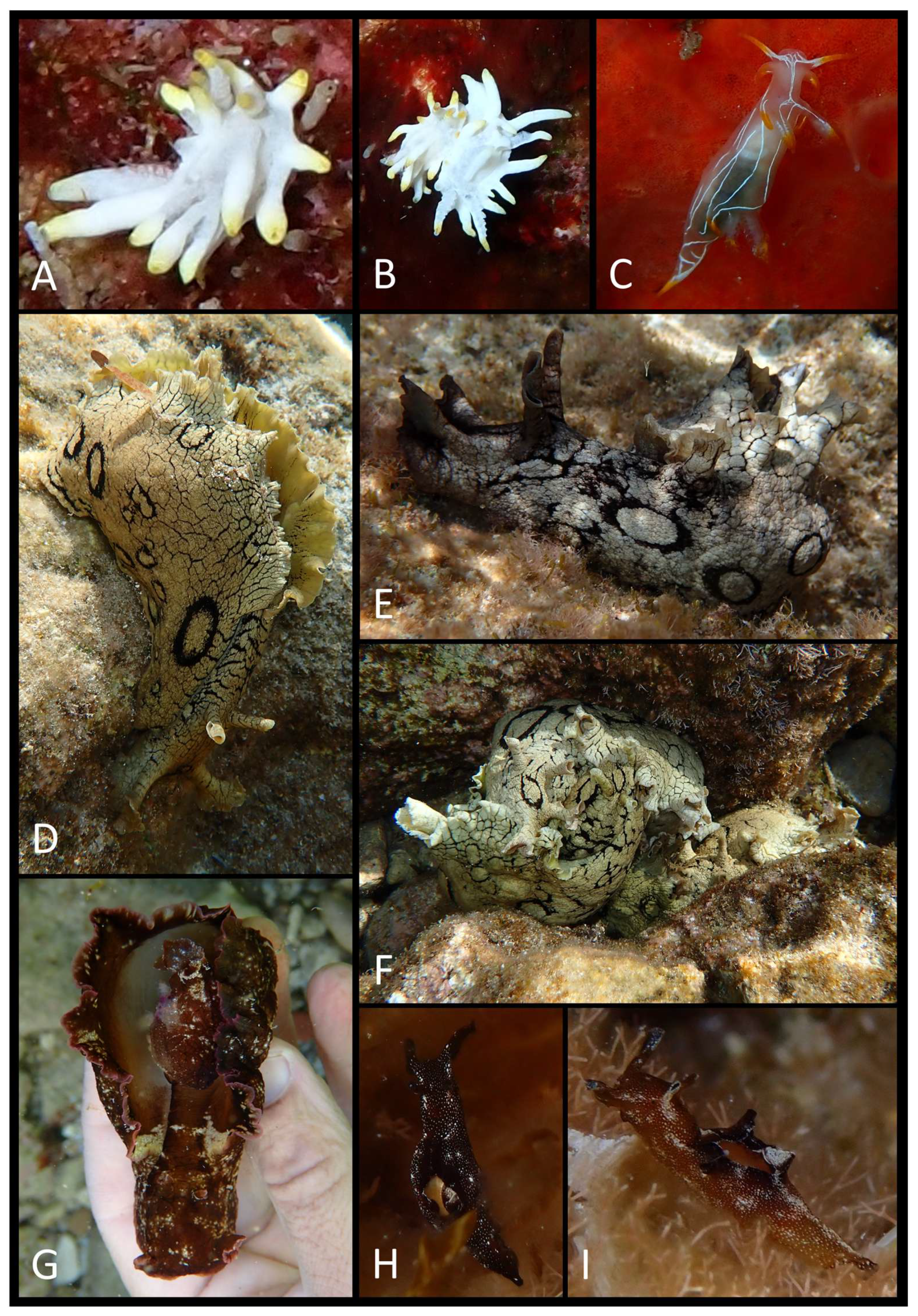
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treccani. Available online: https://www.treccani.it/enciclopedia/isole-pelagie_(Enciclopedia-Italiana)/ (accessed on 10 August 2025).
- Wikipedia. Available online: https://it.wikipedia.org/wiki/Isole_Pelagie (accessed on 10 August 2025).
- Wikipedia. Available online: https://it.wikipedia.org/wiki/Area_marina_protetta_Isole_Pelagie (accessed on 10 August 2025).
- Lasiciliainrete. Available online: https://www.lasiciliainrete.it/directory-tangibili/listing/fondali-delle-isole-pelagie-ita040014/ (accessed on 10 August 2025).
- Livingagrigento. Available online: https://livingagrigento.it/it_IT/Citta/main/citta?id=1041_Isole-Pelagie---Lampedusa-e-Linosa (accessed on 10 August 2025).
- Wikipedia. Available online: https://it.wikipedia.org/wiki/Isola_di_Lampedusa (accessed on 10 August 2025).
- Surico, G. Lampedusa: Dall’agricoltura, Alla Pesca, al Turismo; Firenze University Press: Florence, Italy, 2020; pp. 1–247. [Google Scholar]
- Spada, G.; Sabelli, B.; Morandi, V. Contributo alla conoscenza della malacofauna dell’isola di Lampedusa. Conchiglie 1973, IX, 29–67. [Google Scholar]
- Chemello, R.; Russo, G.F. The molluscan taxocoene of photophilic algae from the island of Lampedusa (strait of Sicily, southern Mediterranean). Boll. Malacol. 1997, 33, 95–104. [Google Scholar]
- Micali, P.; Quadri, P. Su alcuni interessanti molluschi rinvenuti nell’isola di Lampedusa. Boll. Malacol. 2000, 36, 167–174. [Google Scholar]
- Trainito, E.; Doneddu, M. Nudibranchi del Mediterraneo, 2nd ed.; Il Castello: Cornaredo, Italy, 2014; pp. 1–192. [Google Scholar]
- Trainito, E. Arlecchini Mediterranei: Guida ai Molluschi Opistobranchi del Mediterraneo; Taphros: Olbia, Italy, 2003; pp. 1–59. [Google Scholar]
- Trainito, E.; Migliore, V.; Doneddu, M. How many seas must a nudibranch sail? Okenia picoensis (Mollusca: Nudibranchia: Goniodorididae) conquering the Mediterranean. Stud. Mar. 2022, 35, 15–25. [Google Scholar]
- Toma, M.; Betti, F.; Bavestrello, G.; Cattaneo-Vietti, R.; Canese, S.; Cau, A.; Andaloro, F.; Greco, S.; Bo, M. Diversity and abundance of heterobranchs (Mollusca, Gastropoda) from the mesophotic and bathyal zone of the Mediterranean Sea. Eur. Zool. J. 2022, 89, 160–182. [Google Scholar] [CrossRef]
- Lombardo, A.; Marletta, G. The biodiversity of the marine Heterobranchia fauna along the central-eastern coast of Sicily, Ionian Sea. Biodivers. J. 2020, 11, 861–870. [Google Scholar] [CrossRef]
- Lombardo, A.; Marletta, G. Diversity of the Marine Heterobranchia Fauna at the island of Pantelleria, Sicily Channel, Mediterranean Sea: First Contribution. Acta Zool. Bulg. 2023, 75, 37–48. [Google Scholar]
- Lombardo, A.; Marletta, G. The marine Heterobranchia (Mollusca: Gastropoda) fauna of the Aeolian archipelago (Tyrrhenian Sea). First contribution: Lipari and Vulcano. Int. J. Aquat. Biol. 2023, 11, 288–300. [Google Scholar]
- Lombardo, A.; Marletta, G. A First Approach to the marine Heterobranchia (Mollusca: Gastropoda) Fauna of Marettimo, Egadi Islands, MPA (Western Sicily, Mediterranean Sea). Coasts 2024, 4, 667–686. [Google Scholar] [CrossRef]
- Eales, N.B. Revision of the world species of Aplysia (Gastropoda, Opisthobranchia). Bull. Br. Mus. Nat. Hist. (Zool.) 1960, 5, 267–404. [Google Scholar]
- Schmekel, L.; Portmann, A. Opisthobranchia des Mittelmeeres: Nudibranchia und Saccoglossa; Springer: Berlin/Heidelberg, Germany, 1982; pp. 1–410. [Google Scholar]
- Jensen, K.R. Systematics, Phylogeny and Evolution of the Sacoglossa (Mollusca, Opisthobranchia); Vestjydsk Forlag: Bjert, Danmark, 1997; pp. 1–94. [Google Scholar]
- Valdés, Á.; Alexander, J.; Crocetta, F.; Yokeş, M.B.; Giacobbe, S.; Poursanidis, D.; Zenetos, A.; Cervera, J.L.; Caballer, M.; Galil, B.S.; et al. The origin and dispersal pathway of the spotted sea hare Aplysia dactylomela (Mollusca: Opisthobranchia) in the Mediterranean Sea. Aquat. Invasions 2013, 8, 427–436. [Google Scholar] [CrossRef]
- Jouini, M.; Béranger, K.; Arsouze, T.; Beuvier, J.; Thiria, S.; Crépon, M.; Taupier-Letage, I. The Sicily Chennel surface circulation revisited using a neural clustering analysis of a high-resolution simulation. J. Geophys. Res. Ocean. 2016, 121, 4545–4567. [Google Scholar] [CrossRef]
- Salvador, X. Guía Práctica para Fotografiar Nudibranquios del Litoral Español; Marcombo: Barcelona, Spain, 2020; pp. 1–360. [Google Scholar]
- Paz-Sedano, S.; Ortigosa, D.; Pola, M. A new Okenia Menke, 1830 from the Azores Islands, Portugal. Spixiana 2017, 40, 13–22. [Google Scholar]
- Marletta, G.; Lombardo, A. New insights on the seasonal trend of Goniodoridella picoensis (Paz-Sedano, Ortigosa & Pola, 2017) along the central-eastern coast of Sicily: A possible warning of its expansion and establishment in the Mediterranean Sea? Int. J. Aquat. Biol. 2024, 12, 425–437. [Google Scholar]
- Orfanidis, S.; Alvito, A.; Azzurro, E.; Badreddine, A.; Ben Souissi, J.; Chamorro, C.; Crocetta, F.; Dalyan, C.; Fortič, A.; Galanti, L.; et al. New Alien Mediterranean Biodiversity Records (March 2021). Mediterr. Mar. Sci. 2021, 22, 180–198. [Google Scholar]
- Lombardo, A. A new Mediterranean record of the sacoglossan Thuridilla mazda (Mollusca, Gastropoda) with a review of its distribution, biology and ecology. Ann. Ser. Hist. Nat. 2023, 33, 1–6. [Google Scholar]
- Lombardo, A. The Marine Heterobranchs of the MPA Isole Ciclopi—A Seasonal and Faunistic Study with a Comparison with Adjacent Non-Protected Areas. Ph.D. Thesis, Università degli Studi di Catania, Catania, Italy, 31 March 2025. [Google Scholar]
- Malaquias, M.A.E.; Calado, G.; Da Cruz, J.F.; Jensen, K.R. On the occurrence of the Caribbean sea slug Thuridilla mazda in the eastern Atlantic Ocean. Mar. Biodivers. Rec. 2012, 5, e50. [Google Scholar] [CrossRef]
- Medrano, S.; Krug, P.J.; Gosliner, T.M.; Kumar, B.; Valdés, Á. Systematics of Polybranchia Pease, 1860 (Mollusca: Gastropoda: Sacoglossa) based on molecular and morphological data. Zool. J. Linn. Soc. 2019, 186, 76–115. [Google Scholar] [CrossRef]
- OPK-Opistobranquis. Available online: https://opistobranquis.info/en/?p=14401 (accessed on 30 September 2025).
- Trainito, E.; Doneddu, M. Contribution to the knowledge of the molluscan fauna in the Marine Protected Area Tavolara-Punta Coda Cavallo: Sacoglossa, Umbraculida, Pleurobranchomorpha, Anaspidea, Cephalaspidea, Thecosomata (Gastropoda; Heterobranchia). Boll. Malacol. 2016, 52, 79–89. [Google Scholar]


| Taxa | References |
|---|---|
| Acteonoidea | |
| Family Acteonidae A. d’Orbigny, 1842 | |
| Acteon tornatilis (Linnaeus, 1758) | [8] |
| Ringiculimorpha | |
| Family Ringiculidae R. A. Philippi, 1853 | |
| Ringicula auriculata (Ménard de la Groye, 1811) | [8,10] |
| Ringicula conformis Monterosato, 1877 | [10] |
| Pleurobranchida | |
| Family Pleurobranchidae Gray, 1827 | |
| Berthella plumula (Montagu, 1803) | [10] |
| Pleurobranchus testudinarius Cantraine, 1835 | [14] |
| Doridida | |
| Family Goniodorididae H. Adams & A. Adams, 1854 | |
| Goniodoridella picoensis (Paz-Sedano, Ortigosa & Pola, 2017) | [13] |
| Cephalaspidea | |
| Family Retusidae Thiele, 1925 | |
| Pyrunculus hoernesi (Weinkauff, 1866) | [10] |
| Retusa truncatula (Bruguière, 1792) | [8,10] |
| Retusa umbilicata (Montagu, 1803) | [10] |
| Family Rhizoridae Dell, 1952 | |
| Volvulella acuminata (Bruguière, 1792) | [10] |
| Family Bullidae Gray, 1827 | |
| Bulla striata Bruguière, 1792 | [8] |
| Family Haminoeidae Pilsbry, 1895 | |
| Haminoea hydatis (Linnaeus, 1758) | [8,9] |
| Haminoea navicula (da Costa, 1778) | [10] |
| Roxaniella jeffreysi (Weinkauff, 1866) | [10] |
| Weinkauffia turgidula (Forbes, 1844) | [10] |
| Family Scaphandridae G. O. Sars, 1878 | |
| Scaphander lignarius (Linnaeus, 1758) | [10] |
| Family Philinidae J. E. Gray, 1850 (1815) | |
| Hermania scabra (O. F. Müller, 1784) | [8] |
| Philine catena (Montagu, 1803) | [10] |
| Philine quadripartita Ascanius, 1772 | [8] |
| Family Laonidae Pruvot-Fol, 1954 | |
| Laona pruinosa (W. Clark, 1827) | [10] |
| Runcinida | |
| Family Runcinidae H. Adams & A. Adams, 1854 | |
| Runcina sp. | [9] |
| Pteropoda | |
| Euthecosomata | |
| Family Creseidae Rampal, 1973 | |
| Creseis acicula (Rang, 1828) | [10] |
| Aplysiida | |
| Family Aplysiidae Lamarck, 1809 | |
| Aplysia dactylomela Rang, 1828 | [12] |
| Aplysia fasciata Poiret, 1789 | [9] |
| Sacoglossa | |
| Family Plakobranchidae Gray, 1840 | |
| Elysia timida (Risso, 1818) | [9] |
| Elysia viridis (Montagu, 1804) | [9] |
| Thuridilla hopei (Vérany, 1853) | [9] |
| Family Hermaeidae H. Adams & A. Adams, 1854 | |
| Aplysiopsis elegans Deshayes, 1853 | [9] |
| Site | Date | Coordinates | Activity | Protection Zone | Depth Range | Bottom Type |
|---|---|---|---|---|---|---|
| Grotta dell’Acqua (GA) | 7 July 2025 | 35°31′20.0″ N 12°37′06.7″ E | Dive | B | Up to 25 m | Rocky wall on a sandy bottom |
| Grotta Santa (GS) | 7 July 2025 | 35°31′19.7″ N 12°35′39.6″ E | Dive | B | Up to 20 m | Massive promontory |
| Baia di Taccio Vecchio (BTV) | 7 July 2025 | from 35°31′18.6″ N 12°35′41.6″ E to 35°31′18.1″ N 12°35′46.8″ E | Snorkeling | B | 0–1 m | Rocky escarpment |
| Muro Vecchio (MV) | 8 July 2025 | 35°31′33.9″ N 12°33′35.2″ E | Dive | C | Up to 30 m | Huge promontory |
| Scoglio Pignata (SP) | 8 July 2025 | 35°31′37.9″ N 12°–31′24.3″ E | Dive | C | Up to 30 m | Rocky outcrops and boulders |
| Baia di Capo Ponente (BCP) | 8 July 2025 | from 35°31′22.5″ N 12°31′13.0″ E to 35°31′18.3″ N 12°31′07.2″ E | Snorkeling | C | 0–1 m | Large rocks |
| Capo Grecale (CG) | 9 July 2025 | 35°30′55.6″ N 12°37′58.6″ E | Dive | B | Up to 35 m | Rocky slope on a sandy bottom |
| Fortino (F) | 9 July 2025 | 35°30′50.3″ N 12°37′44.3″ E | Dive | B | Up to 25 m | Rocky walls and canyons on a sandy bottom |
| Costa Sud di Capo Grecale (CSCG) | 9 July 2025 | from 35°30′55.5″ N 12°37′55.0″ E to 35°30′53.8″ N 12°37′43.8″ E | Snorkeling | B | 0–1 m | Rocky vertical wall |
| Panettone (P) | 10 July 2025 | 35°30′26.0″ N 12°33′33.2″ E | Dive | B | Up to 20 m | Rocky outcrop on a sandy bottom |
| Madonnina (M) | 10 July 2025 | 35°30′28.1″ N 12°33′24.2″ E | Dive | B | Up to 18 m | Large rocky outcrop with a cave |
| Tabaccara (T) | 10 July 2025 | from 35°30′39.8″ N 12°34′07.3″ E to 35°30′27.6″ N 12°34′11.5″ E | Snorkeling | C | 0–1 m | Vertical rocky wall intermingled with large, medium, and small rocks |
| Punta Parrino (PP) | 11 July 2025 | 35°30′02.6″ N 12°38′04.5″ E | Dive | Non-protected | Up to 40 m | Rocky outcrops on a sand bed |
| Punta Iavuta (PI) | 11 July 2025 | 35°29′49.0″ N 12°38′02.2″ E | Dive | Non-protected | Up to 20 m | Rocky outcrops on a sand bed |
| Baia di Punta Sottile (BPS) | 11 July 2025 | from 35°29′50.0″ N 12°37′58.2″ E to 35°29′46.2″ N 12°37′51.6″ E | Snorkeling | Non-protected | 0–1 m | Continuous horizontal crevice |
| Cala Spugne (CS) | 12 July 2025 | from 35°29′35.3″ N 12°36′29.9″ E to 35°29′43.5″ N 12°36′38.3″ E | Snorkeling | Non-protected | 0–1 m | Cove with many jagged inlets forming small grottos |
| Cala Maluk (CM) | 12 July 2025 | from 35°29′41.2″ N 12°36′42.5″ E to 35°29′36.1″ N 12°36′52.0″ E | Snorkeling | Non-protected | 0–1 m | Cove with many jagged inlets forming small grottos |
| Cala Francese (CF) | 12 July 2025 | from 35°29′40.6″ N 12°37′26.6″ E to 35°29′43.6″ N 12°37′30.7″ E | Snorkeling | Non-protected | 0–1 m | Cove characterized by two inlets |
| Western Coast | Northern Coast | Eastern Coast | Southern Coast | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C Zone | B Zone | Non-Protected Area | C Zone | B Zone | |||||||||||||||
| Taxa | BCP | SP | MV | GS | BTV | GA | CG | CSCG | F | PP | PI | BPS | CF | CM | CS | T | P | M | Depth Range |
| Superfamily Rhodopoidea | |||||||||||||||||||
| Family Rhodopidae Ihering, 1876 | |||||||||||||||||||
| Rhodopesp. | 1 | 12.1 m | |||||||||||||||||
| Order Pleurobranchida | |||||||||||||||||||
| Family Pleurobranchidae Gray, 1827 | |||||||||||||||||||
| Berthellasp. | * | 13.3 m | |||||||||||||||||
| Berthellinacf. edwardsii (Vayssière, 1897) | 1 | 16.6 m | |||||||||||||||||
| Order Nudibranchia | |||||||||||||||||||
| Family Facelinidae Bergh, 1889 | |||||||||||||||||||
| Caloria quatrefagesi(Vayssière, 1888) | 1 | <1 m | |||||||||||||||||
| Family Flabellinidae Bergh, 1889 | |||||||||||||||||||
| Edmundsella pedata(Montagu, 1816) | 5 * | 1 | 1 | 2 | 10–22.7 m | ||||||||||||||
| Paraflabellina ischitana(Hirano & T. E. Thompson, 1990) | 2 | 11.7–16.4 m | |||||||||||||||||
| Family Trinchesiidae F. Nordsieck, 1972 | |||||||||||||||||||
| Tenelliasp. | 1 | <1 m | |||||||||||||||||
| Tenellia genovae(O’Donoghue, 1926) | 1 | 5.4 m | |||||||||||||||||
| Order Doridida | |||||||||||||||||||
| Family Chromodorididae Bergh, 1891 | |||||||||||||||||||
| Felimare picta(R. A. Philippi, 1836) | 1 | 18.2 m | |||||||||||||||||
| Felimare tricolor(Cantraine, 1835) | 1 | 1 | 15.5–28 m | ||||||||||||||||
| Family Goniodorididae H. Adams & A. Adams, 1854 | |||||||||||||||||||
| Goniodoridella picoensis(Paz-Sedano, Ortigosa & Pola, 2017) | 2 | 2 | 3 | 4 | 1 | 3 ‡ | 5 | 11.6–32.6 m | |||||||||||
| Trapania lineataHaefelfinger, 1960 | 1 | 11.7 m | |||||||||||||||||
| Order Aplysiida | |||||||||||||||||||
| Family Aplysiidae Lamarck, 1809 | |||||||||||||||||||
| Aplysia dactylomelaRang, 1828 | 9 | 9 | 14 ‡ | 1 < m | |||||||||||||||
| Aplysiacf. fasciata Poiret, 1789 | 1 | 1 < m | |||||||||||||||||
| Aplysia punctata(Cuvier, 1803) | 1 | 2 | 2 | 1 | 1 < m | ||||||||||||||
| Petalifera petalifera(Rang, 1828) | 1 | 7.5 m | |||||||||||||||||
| Superorder Sacoglossa | |||||||||||||||||||
| Family Plakobranchidae Gray, 1840 | |||||||||||||||||||
| Bosellia mimeticaTrinchese, 1891 | 1 | 1 < m | |||||||||||||||||
| Elysia timida(Risso, 1818) | 5 | 2 | 3 | 4 | 9 | 1 | 5 | 24 | 39 ‡ | 20 ‡ | 6 | 1 | 1 < −11.9 m | ||||||
| Thuridilla hopei(Vérany, 1853) | 2 | 6 | 1 | 2 | 1 | 5 | 2 | 3 | 1 < −20 m | ||||||||||
| Thuridilla mazdaOrtea & Espinosa, 2000 | 1 | 1 | 1 | 1 | 1 < m | ||||||||||||||
| Family Caliphyllidae Tiberi, 1881 | |||||||||||||||||||
| Cyerce graecaT. E. Thompson, 1988 | 1 | 1 < m | |||||||||||||||||
| Polybranchiasp. | 2 * | 1 < m | |||||||||||||||||
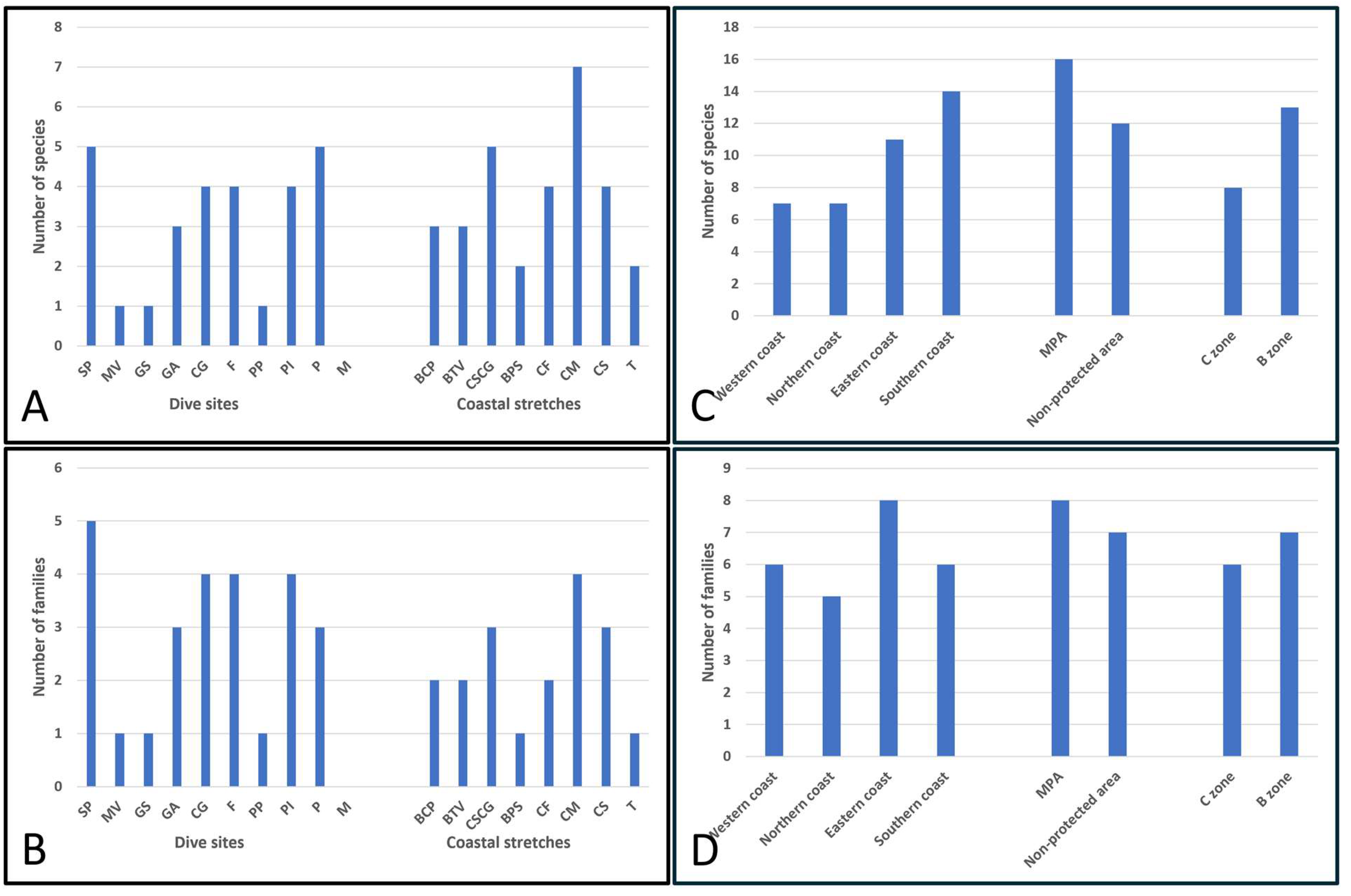
| Total number of species | 22 | ||||||||||||||||||
| Total number of species per site | 3 | 5 | 1 | 1 | 3 | 3 | 4 | 5 | 4 | 1 | 4 | 2 | 4 | 7 | 4 | 2 | 5 | 0 | |
| Total number of families | 10 | ||||||||||||||||||
| Total number of families per site | 2 | 5 | 1 | 1 | 2 | 3 | 4 | 3 | 4 | 1 | 4 | 1 | 2 | 4 | 3 | 1 | 3 | 0 | |
| Taxa | |
|---|---|
| Rhodopoidea | Bulla striata Bruguière, 1792 |
| Family Rhodopidae Ihering, 1876 | Family Haminoeidae Pilsbry, 1895 |
| Rhodope sp. | Haminoea hydatis (Linnaeus, 1758) |
| Acteonoidea | Haminoea navicula (da Costa, 1778) |
| Family Acteonidae A. d’Orbigny, 1842 | Roxaniella jeffreysi (Weinkauff, 1866) |
| Acteon tornatilis (Linnaeus, 1758) | Weinkauffia turgidula (Forbes, 1844) |
| Ringiculimorpha | Family Scaphandridae G. O. Sars, 1878 |
| Family Ringiculidae R. A. Philippi, 1853 | Scaphander lignarius (Linnaeus, 1758) |
| Ringicula auriculata (Ménard de la Groye, 1811) | Family Philinidae J. E. Gray, 1850 (1815) |
| Ringicula conformis Monterosato, 1877 | Hermania scabra (O. F. Müller, 1784) |
| Pleurobranchida | Philine catena (Montagu, 1803) |
| Family Pleurobranchidae Gray, 1827 | Philine quadripartita Ascanius, 1772 |
| Berthella plumula (Montagu, 1803) | Family Laonidae Pruvot-Fol, 1954 |
| Berthella sp. | Laona pruinosa (W. Clark, 1827) |
| Berthellina cf. edwardsii (Vayssière, 1897) | Runcinida |
| Pleurobranchus testudinarius Cantraine, 1835 | Family Runcinidae H. Adams & A. Adams, 1854 |
| Nudibranchia | Runcina sp. |
| Family Facelinidae Bergh, 1889 | Pteropoda |
| Caloria quatrefagesi (Vayssière, 1888) | Euthecosomata |
| Family Flabellinidae Bergh, 1889 | Family Creseidae Rampal, 1973 |
| Edmundsella pedata (Montagu, 1816) | Creseis acicula (Rang, 1828) |
| Paraflabellina ischitana (Hirano & T. E. Thompson, 1990) | Aplysiida |
| Family Trinchesiidae F. Nordsieck, 1972 | Family Aplysiidae Lamarck, 1809 |
| Tenellia sp. | Aplysia dactylomela Rang, 1828 |
| Tenellia genovae (O’Donoghue, 1926) | Aplysia fasciata Poiret, 1789 |
| Doridida | Aplysia cf. fasciata Poiret, 1789 |
| Family Chromodorididae Bergh, 1891 | Aplysia punctata (Cuvier, 1803) |
| Felimare picta (R. A. Philippi, 1836) | Petalifera petalifera (Rang, 1828) |
| Felimare tricolor (Cantraine, 1835) | Sacoglossa |
| Family Goniodorididae H. Adams & A. Adams, 1854 | Family Plakobranchidae Gray, 1840 |
| Goniodoridella picoensis (Paz-Sedano, Ortigosa & Pola, 2017) | Bosellia mimetica Trinchese, 1891 |
| Trapania lineata Haefelfinger, 1960 | Elysia timida (Risso, 1818) |
| Cephalaspidea | Elysia viridis (Montagu, 1804) |
| Family Retusidae Thiele, 1925 | Thuridilla hopei (Vérany, 1853) |
| Pyrunculus hoernesi (Weinkauff, 1866) | Thuridilla mazda Ortea & Espinosa, 2000 |
| Retusa truncatula (Bruguière, 1792) | Family Hermaeidae H. Adams & A. Adams, 1854 |
| Retusa umbilicata (Montagu, 1803) | Aplysiopsis elegans Deshayes, 1853 |
| Family Rhizoridae Dell, 1952 | Family Caliphyllidae Tiberi, 1881 |
| Volvulella acuminata (Bruguière, 1792) | Cyerce graeca T. E. Thompson, 1988 |
| Family Bullidae Gray, 1827 | Polybranchia sp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, A.; Marletta, G. Observations on the Benthic Heterobranch “Sea Slugs” (Mollusca: Gastropoda) of Lampedusa, the Southernmost Island of Italy (MPA Isole Pelagie). J. Mar. Sci. Eng. 2025, 13, 2150. https://doi.org/10.3390/jmse13112150
Lombardo A, Marletta G. Observations on the Benthic Heterobranch “Sea Slugs” (Mollusca: Gastropoda) of Lampedusa, the Southernmost Island of Italy (MPA Isole Pelagie). Journal of Marine Science and Engineering. 2025; 13(11):2150. https://doi.org/10.3390/jmse13112150
Chicago/Turabian StyleLombardo, Andrea, and Giuliana Marletta. 2025. "Observations on the Benthic Heterobranch “Sea Slugs” (Mollusca: Gastropoda) of Lampedusa, the Southernmost Island of Italy (MPA Isole Pelagie)" Journal of Marine Science and Engineering 13, no. 11: 2150. https://doi.org/10.3390/jmse13112150
APA StyleLombardo, A., & Marletta, G. (2025). Observations on the Benthic Heterobranch “Sea Slugs” (Mollusca: Gastropoda) of Lampedusa, the Southernmost Island of Italy (MPA Isole Pelagie). Journal of Marine Science and Engineering, 13(11), 2150. https://doi.org/10.3390/jmse13112150





