AquaVib: Enabling the Separate Evaluation of Effects Induced by Acoustic Pressure and Particle Motion on Aquatic Organisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Sound Field Metrics
2.2. Sound Field Spectrum Computation
2.3. Description of the System Setup
2.3.1. Target Organisms Caring
2.3.2. Sound Exposure
2.3.3. System Control and Handling
2.3.4. Control of Kinetic-to-Potential Input Energy Ratio
- A homogeneously distributed sound field is recreated if the largest dimension of the acoustic chamber (d) is significantly smaller than the shortest wavelength () of the input excitation ().
- Through the pair of mechanical exciters placed at each end of the longitudinal section of the acoustic chamber, the whole bulk of the enclosed water can be compressed or shaken by playing with their relative phase in two configurations, i.e., 0 and 180 degrees, respectively. Thus, the kinetic-to-potential energy ratio can be significantly changed.
2.3.5. Physiological Assessment
2.3.6. Behavioral Tracking of the Target Organisms
2.4. Exposure Protocol
- Background noise levels.
- Accuracy of the corrected input signal to the target.
- Particle motion measurement location.
- Gain consistency of the system response between the acoustic pressure and particle motion components.
- Reproducibility of the target exposure across different kinetic-to-potential energy ratios.
- Accuracy in determining temperature and dissolved oxygen content inside the acoustic chamber.
3. Results and Discussion
3.1. Background Noise
3.2. Particle Motion Measurements Validation
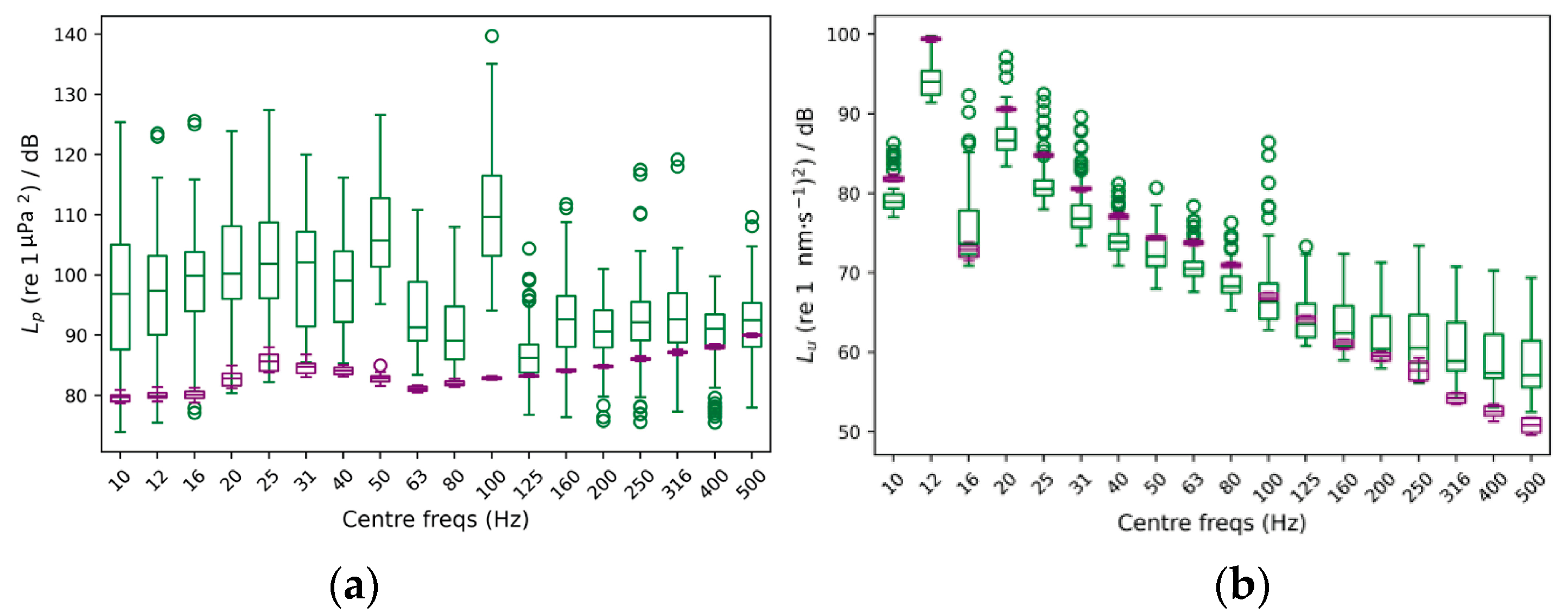
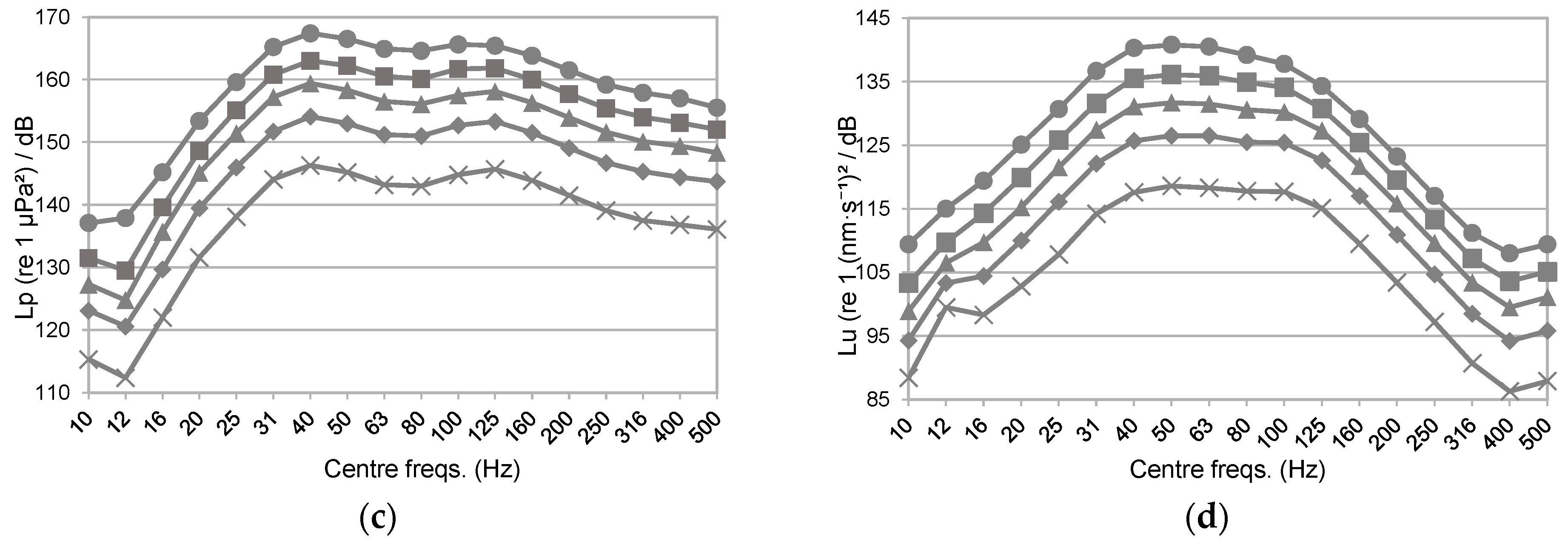
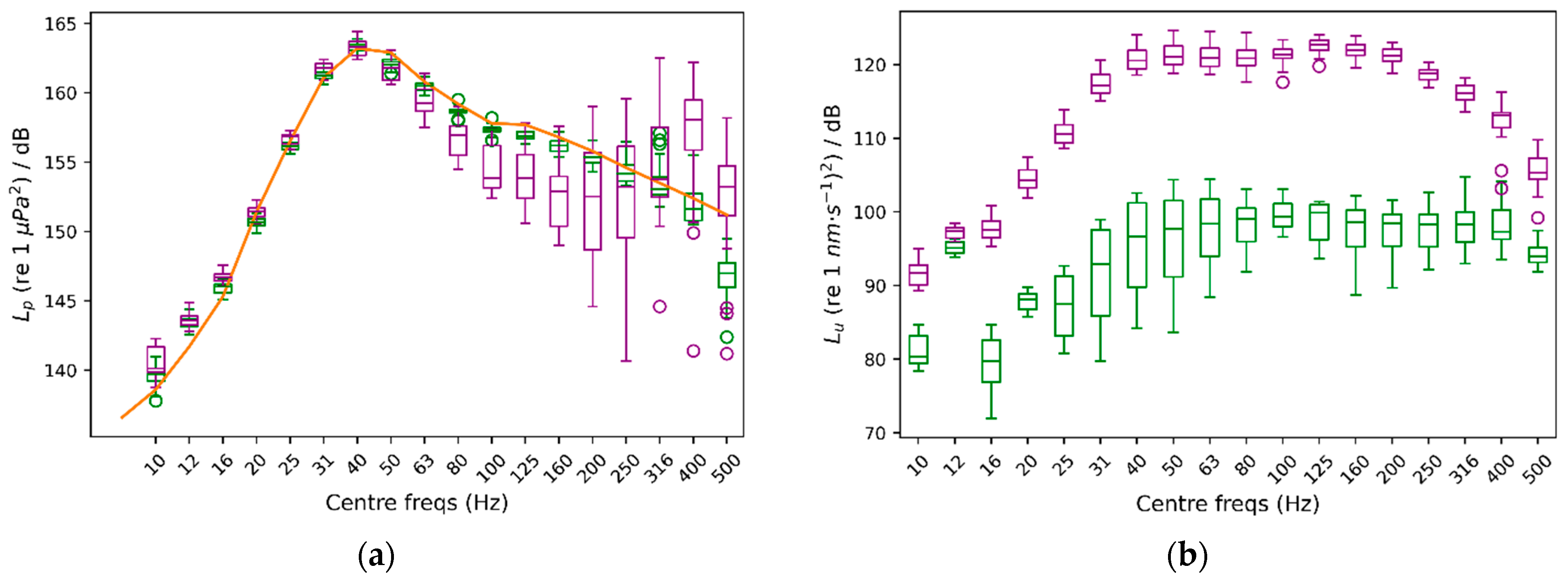
3.3. Gain’s Consistency Assessment
3.4. Addressing a Separate Evaluation of Acoustic Pressure- and Particle Motion-Elicited Responses on Aquatic Organisms
3.5. Physiological Assessment Procedure
4. Conclusions
Supplementary Materials
 Accelerometer at the current location for real exposures (i.e., rear face of the shaker’s moving head), used as a reference to be compared with the readings of the dummy accelerometer at six different measurement points
Accelerometer at the current location for real exposures (i.e., rear face of the shaker’s moving head), used as a reference to be compared with the readings of the dummy accelerometer at six different measurement points  : [Shell 1–3] 25, 100 and 125 mm away to the reference accelerometer respectively; at the [Rail] of the sliding platform where the shaker sits on; at the support [Structure] close to the reference shaker at the same excitation orientation; and at the [Ground] right below the reference shaker perpendicular to the excitation; Figure S3: Shell-, structural- and ground-transmitted PVL (Lu re. 1 (nm·s−1)2), measured at three locations in the shell of the acoustic chamber [Shell 1–3]; at the [Rail] of the sliding platform where the shaker sits on; at the support [Structure] close to the reference shaker at the same excitation orientation; and at the [Ground] right below the reference shaker perpendicular to the excitation. The [Ref Acc] corresponds to the measured PVL spectrum at the accelerometer mounted in the rear face of the reference shaker moving element (exposure tests’ current location); Figure S4: Top. Measured SPL (Lp, dB re 1 µPa2) (left) and PVL (Lu, dB re 1 (nm−1)2) (right) spectra from playing a hydrophone-based corrected (HYD, blue lines) and non-corrected (NO eQ WN, black lines) band-filtered white noise (10 Hz to 500 Hz) in the AP configuration. Bottom. Measured SPL (Lp, dB re 1 µPa2) (left) and PVL (Lu, dB re 1 (nm−1)2) (right) spectrums from playing an accelerometer-based corrected (ACC, orange lines) and non-corrected (NO eQ WN, black lines) band-filtered white noise (10 Hz to 500 Hz) in the PM configuration. The spectra of the measured signals and the target (x-crossed continuous line) were scaled to ease comparison.; Figure S5: Accelerometers’ response curves A1 (continuous) and A2 (epoxy-encapsulated, dashed). (▲) and (■) refers to the calibration exciter measurement at 159.15 Hz with A1 and A2, respectively; Figure S6: Temperature (in °C; red line) and dissolved O2 content (in µMol/L; blue line) measured during a 20-min-long real exposure test carried out on a batch of 20 adult M. edulis, on 3 October 2022, using a container ship URN at 170 dB SPL (Lp, re. 1 µPa2) as input signal during the whole test; Figure S7: AquaVib GUI main page. Four different tests can be configured. The input signal correction routine (EQUALIZATION), the water flushing cycle (SWITCH RELAY), and the stored data clearing (CLEAR DATA) are also handled from it; Figure S8: Example of Ship Noise Experiment page. A wav file is loaded, and automatically corrected either HYD- or ACC-based, for the current system response at any of the two exposure configurations (i.e., AP, PM). Main input sound file parameters are listed (SELECTED FEATURES). The duration of the test can be manually set (TIMER). Valid data is transferred to LAB’s servers at the end of the test; Figure S9: Sketch of the current water piping circuit. A pair of electro valves are used to open/close the water recirculation between the maintenance tanks system and the AquaVib. The TOFTC2 respirometry cell and the little inline pump are connected in parallel between them; Figure S10: Sketch of the alternative water piping circuit. The TOFTC2 respirometry cell and the little inline pump are connected to AquaVib’s acoustic chamber through an independent inlet-outlet pair. This way, the TOFTC2 readings reflect more accurately the dissolved O2 content in the acoustic chamber, being much less affected by the incoming completely oxygenated water from the maintenance tanks; Figure S11: Detailed view of the respirometry module: the TOFTC2 cell with the two black optic cables (red circle), and the TSUB21 reference temperature sensor (blue circle) plugged into the FireSting®-O2 Optical Oxygen Meter. A low-power inline water pump (green circle) is used to continuously recirculate the water between the TOFCT2 cell and the acoustic chamber, preventing the stratification inside the respirometer sensor and ensuring a correct mixing of the dissolved oxygen content in the enclosed water volume; Figure S12: LAB-owned maintenance tank system. A pair of 2000-liter fiberglass-reinforced plastic tanks are interconnected with the AquaVib through a pair of 50-mm pipes that go into the ground. A pair of inline water pumps recirculate clean naturally oxygenated water between the maintenance tanks and the AquaVib. A physicochemical self-filtration system with activated carbon and sand (Granada 500, Kripsol, Spain), is used to keep water’s quality. A pair of temperature-control systems (TK 5K, Teco s.r.l, Italy), allows for setting a constant water temperature within the 5 to 35 °C range. Table S1: Computed broadband SPL (Lp, dB re 1 µPa2) and PVL (Lu, dB re 1 (nm s−1)2) at five different input levels of a band-filtered (10–500 Hz) HYD-based corrected container ship URN played in both AP and PM configurations. The relative difference between consecutive levels is included. (*) Computed PVL rejecting the decidecade bands below 31 Hz. References [32,33] are cited in the Supplementary Materials.
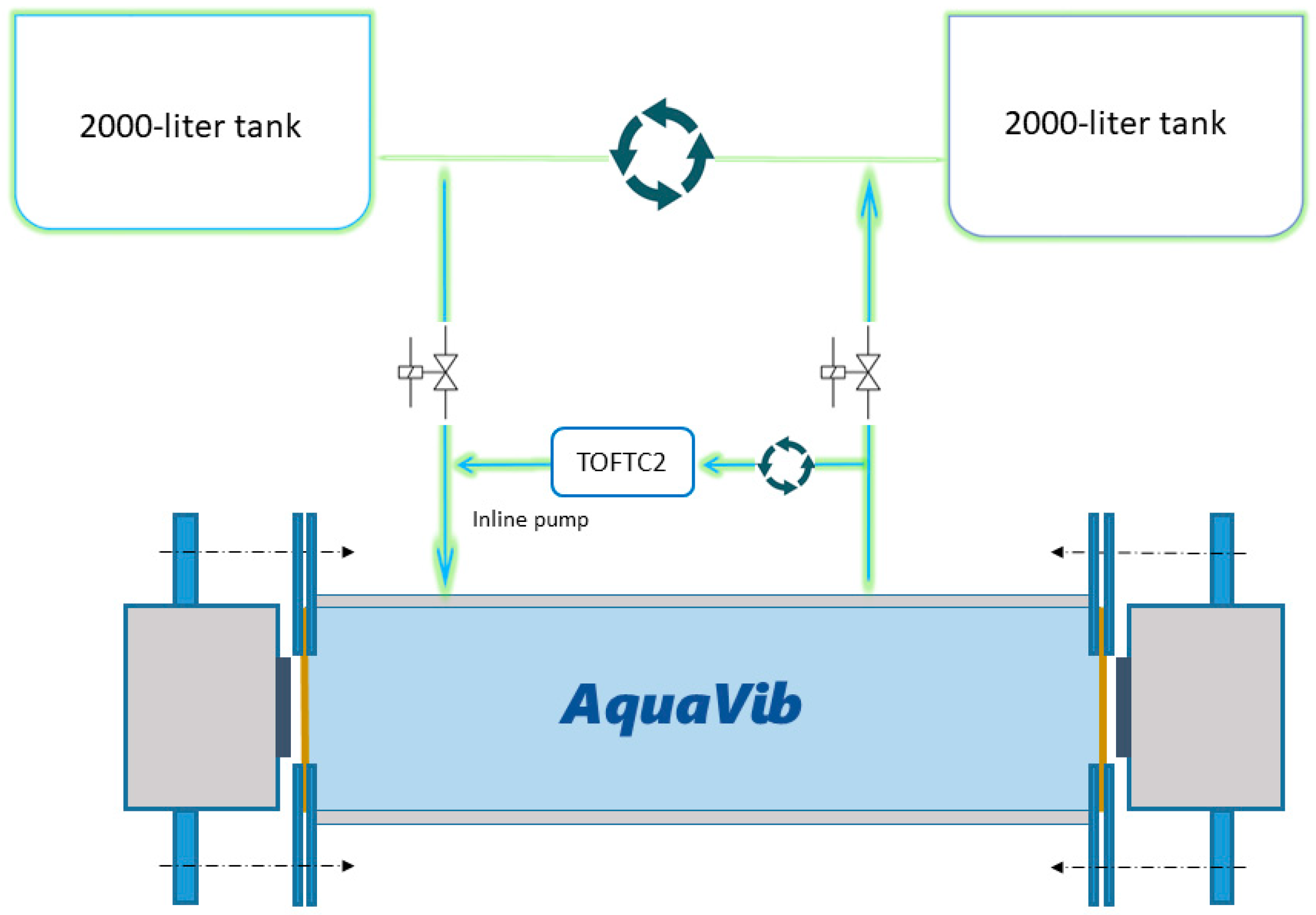
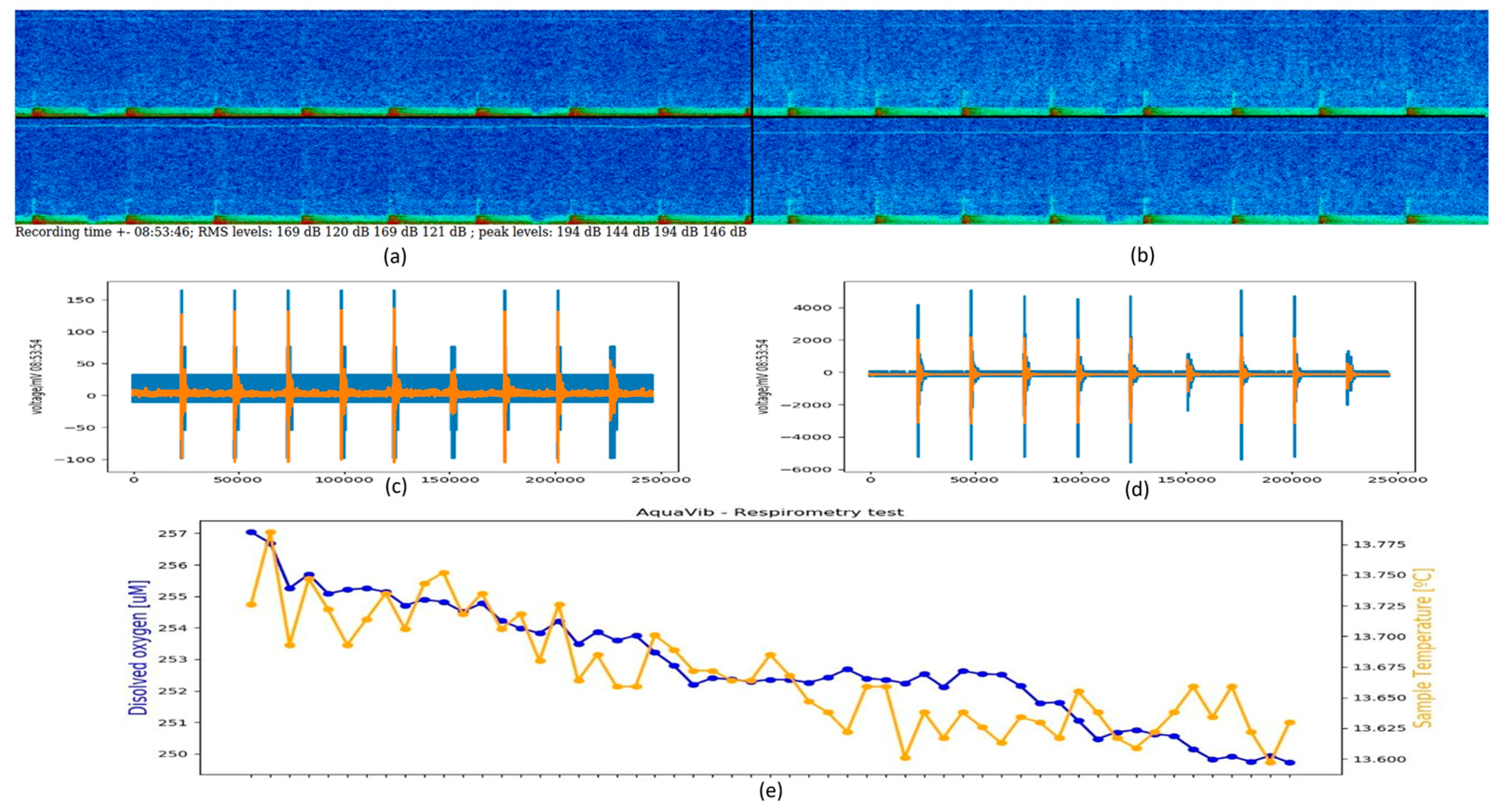
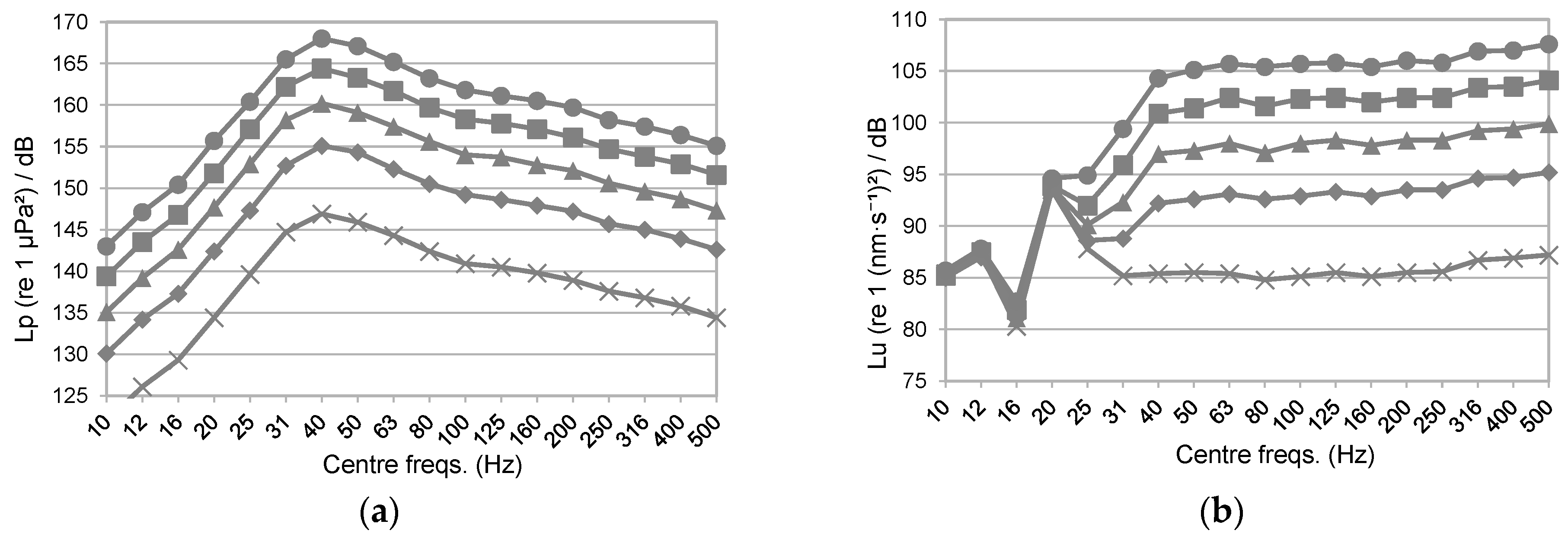
: [Shell 1–3] 25, 100 and 125 mm away to the reference accelerometer respectively; at the [Rail] of the sliding platform where the shaker sits on; at the support [Structure] close to the reference shaker at the same excitation orientation; and at the [Ground] right below the reference shaker perpendicular to the excitation; Figure S3: Shell-, structural- and ground-transmitted PVL (Lu re. 1 (nm·s−1)2), measured at three locations in the shell of the acoustic chamber [Shell 1–3]; at the [Rail] of the sliding platform where the shaker sits on; at the support [Structure] close to the reference shaker at the same excitation orientation; and at the [Ground] right below the reference shaker perpendicular to the excitation. The [Ref Acc] corresponds to the measured PVL spectrum at the accelerometer mounted in the rear face of the reference shaker moving element (exposure tests’ current location); Figure S4: Top. Measured SPL (Lp, dB re 1 µPa2) (left) and PVL (Lu, dB re 1 (nm−1)2) (right) spectra from playing a hydrophone-based corrected (HYD, blue lines) and non-corrected (NO eQ WN, black lines) band-filtered white noise (10 Hz to 500 Hz) in the AP configuration. Bottom. Measured SPL (Lp, dB re 1 µPa2) (left) and PVL (Lu, dB re 1 (nm−1)2) (right) spectrums from playing an accelerometer-based corrected (ACC, orange lines) and non-corrected (NO eQ WN, black lines) band-filtered white noise (10 Hz to 500 Hz) in the PM configuration. The spectra of the measured signals and the target (x-crossed continuous line) were scaled to ease comparison.; Figure S5: Accelerometers’ response curves A1 (continuous) and A2 (epoxy-encapsulated, dashed). (▲) and (■) refers to the calibration exciter measurement at 159.15 Hz with A1 and A2, respectively; Figure S6: Temperature (in °C; red line) and dissolved O2 content (in µMol/L; blue line) measured during a 20-min-long real exposure test carried out on a batch of 20 adult M. edulis, on 3 October 2022, using a container ship URN at 170 dB SPL (Lp, re. 1 µPa2) as input signal during the whole test; Figure S7: AquaVib GUI main page. Four different tests can be configured. The input signal correction routine (EQUALIZATION), the water flushing cycle (SWITCH RELAY), and the stored data clearing (CLEAR DATA) are also handled from it; Figure S8: Example of Ship Noise Experiment page. A wav file is loaded, and automatically corrected either HYD- or ACC-based, for the current system response at any of the two exposure configurations (i.e., AP, PM). Main input sound file parameters are listed (SELECTED FEATURES). The duration of the test can be manually set (TIMER). Valid data is transferred to LAB’s servers at the end of the test; Figure S9: Sketch of the current water piping circuit. A pair of electro valves are used to open/close the water recirculation between the maintenance tanks system and the AquaVib. The TOFTC2 respirometry cell and the little inline pump are connected in parallel between them; Figure S10: Sketch of the alternative water piping circuit. The TOFTC2 respirometry cell and the little inline pump are connected to AquaVib’s acoustic chamber through an independent inlet-outlet pair. This way, the TOFTC2 readings reflect more accurately the dissolved O2 content in the acoustic chamber, being much less affected by the incoming completely oxygenated water from the maintenance tanks; Figure S11: Detailed view of the respirometry module: the TOFTC2 cell with the two black optic cables (red circle), and the TSUB21 reference temperature sensor (blue circle) plugged into the FireSting®-O2 Optical Oxygen Meter. A low-power inline water pump (green circle) is used to continuously recirculate the water between the TOFCT2 cell and the acoustic chamber, preventing the stratification inside the respirometer sensor and ensuring a correct mixing of the dissolved oxygen content in the enclosed water volume; Figure S12: LAB-owned maintenance tank system. A pair of 2000-liter fiberglass-reinforced plastic tanks are interconnected with the AquaVib through a pair of 50-mm pipes that go into the ground. A pair of inline water pumps recirculate clean naturally oxygenated water between the maintenance tanks and the AquaVib. A physicochemical self-filtration system with activated carbon and sand (Granada 500, Kripsol, Spain), is used to keep water’s quality. A pair of temperature-control systems (TK 5K, Teco s.r.l, Italy), allows for setting a constant water temperature within the 5 to 35 °C range. Table S1: Computed broadband SPL (Lp, dB re 1 µPa2) and PVL (Lu, dB re 1 (nm s−1)2) at five different input levels of a band-filtered (10–500 Hz) HYD-based corrected container ship URN played in both AP and PM configurations. The relative difference between consecutive levels is included. (*) Computed PVL rejecting the decidecade bands below 31 Hz. References [32,33] are cited in the Supplementary Materials.Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Accelerometer |
| EPDM | Ethylene Propylene Diene Monomer |
| GUI | Graphical User Interface |
| HYD | Hydrophone |
| IEPE | Integrated Electronics Piezo-Electric |
| PTFE-FEP | Polytetrafluoroethylene-Fluorinated Ethylene Propylene |
| PVL | Particle Velocity Level |
| SPL | Sound Pressure Level |
| URN | Underwater Radiated Noise |
References
- Day, R.D.; McCauley, R.D.; Fitzgibbon, Q.P.; Hartmann, K.; Semmens, J.M. Seismic air guns damage rock lobster mechanosensory organs and impair righting reflex. Proc. R. Soc. B 2019, 286, 20191424. [Google Scholar] [CrossRef]
- Solé, M.; Kaifu, K.; Mooney, T.A.; Nedelec, S.L.; Olivier, F.; Radford, A.N.; Vazzana, M.; Wale, M.A.; Semmens, J.M.; Simpson, S.D.; et al. Marine Invertebrates and Noise. Front. Mar. Sci. 2023, 10, 185. [Google Scholar] [CrossRef]
- Hu, M.Y.; Yan, H.Y.; Chung, W.S.; Shiao, J.C.; Hwang, P.P. Acoustically evoked potentials in two cephalopods inferred using the auditory brainstem response (ABR) approach. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 278–283. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Bouton, N.; van Opzeeland, I.; Coers, A.; ten Cate, C.; Popper, A.N. A noisy spring: The impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 2010, 25, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, S.L.; Campbell, J.; Radford, A.N.; Simpson, S.D.; Merchant, N.D. Particle motion: The missing link in underwater acoustic ecology. Methods Ecol. Evol. 2016, 7, 836–842. [Google Scholar] [CrossRef]
- Popper, A.N.; Hawkins, A.D. The importance of particle motion to fishes and invertebrates. J. Acoust. Soc. Am. 2018, 143, 470–488. [Google Scholar] [CrossRef] [PubMed]
- Solé, M.; Lenoir, M.; Durfort, M.; Fortuño, J.M.; van der Schaar, M.; De Vreese, S.; André, M. Seagrass Posidonia is impaired by human-generated noise. Commun. Biol. 2021, 4, 743. [Google Scholar] [CrossRef]
- McCauley, R.D.; Meekan, M.G.; Parsons, M.J.G. Acoustic Pressure, Particle Motion, and Induced Ground Motion Signals from a Commercial Seismic Survey Array and Potential Implications for Environmental Monitoring. J. Mar. Sci. Eng. 2021, 9, 571. [Google Scholar] [CrossRef]
- Jézéquel, Y.; Cones, S.; Jensen, F.H.; Brewer, H.; Collins, J.; Mooney, T.A. Pile driving repeatedly impacts the giant scallop (Placopecten magellanicus). Sci. Rep. 2022, 12, 15380. [Google Scholar] [CrossRef]
- Kaifu, K.; Akamatsu, T.; Segawa, S. Underwater sound detection by cephalopod statocyst. Fish. Sci. 2008, 74, 781–786. [Google Scholar] [CrossRef]
- Bolle, L.J.; De Jong, C.A.; Bierman, S.M.; Van Beek, P.J.; Van Keeken, O.A.; Wessels, P.W.; Van Damme, C.J.; Winter, H.V.; De Haan, D.; Dekeling, R.P. Common sole larvae survive high levels of pile-driving sound in controlled exposure experiments. PLoS ONE 2012, 7, e33052. [Google Scholar] [CrossRef]
- André, M.; Kaifu, K.; Solé, M.; van der Schaar, M.; Akamatsu, T.; Balastegui, A.; Sánchez, A.M.; Castell, J.V. Contribution to the understanding of particle motion perception in marine invertebrates. In The Effects of Noise on Aquatic Life II; Springer: Berlin/Heidelberg, Germany, 2016; pp. 47–55. [Google Scholar]
- Roberts, L.; Harding, H.R.; Voellmy, I.; Bruintjes, R.; Simpson, S.D.; Radford, A.N.; Breithaupt, T.; Elliott, M. Exposure of benthic invertebrates to sediment vibration: From laboratory experiments to outdoor simulated pile-driving. Proc. Meet. Acoust. 2016, 27, 010029. [Google Scholar] [CrossRef]
- Wale, M.A.; Briers, R.A.; Hartl, M.G.; Bryson, D.; Diele, K. From DNA to ecological performance: Effects of anthropogenic noise on a reef-building mussel. Sci. Total Environ. 2019, 689, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Shafiei Sabet, S.; Slabbekoorn, H. Particle motion and sound pressure in fish tanks: A behavioural exploration of acoustic sensitivity in the zebrafish. Behav. Processes 2019, 164, 38–47. [Google Scholar] [CrossRef]
- Guan, S.; Popper, A.N.; Haxel, J.; Martin, J.; Miller, J.H.; Nedelec, S.; Potty, G.; Roberts, L.; Sisneros, J.A.; Dangerfield, A. Research Methodologies to Study Behavioral and Physiological Effects on Fishes and Aquatic Invertebrates from Particle Motion and Substrate-Borne Vibration Exposure: Study and Workshop; U.S. Department of the Interior, Bureau of Ocean Energy Management: Washington, DC, USA, 2024; p. 102. Available online: https://www.researchgate.net/publication/383525697_Research_Methodologies_to_Study_Behavioral_and_Physiological_Effects_on_Fishes_and_Aquatic_Invertebrates_from_Particle_Motion_and_Substrate-Borne_Vibration_Exposure_Study_and_Workshop (accessed on 24 September 2025).
- Akamatsu, T.; Okumura, T.; Novarini, N.; Yan, H.Y. Empirical refinements applicable to the recording of fish sounds in small tanks. J. Acoust. Soc. Am. 2002, 112, 3073–3082. [Google Scholar] [CrossRef]
- Nedelec, S.L.; Ainslie, M.A.; Andersson, M.; Cheong, S.; Halvorsen, M.; Linné, M.; Martin, B.; Nöjd, A.; Robinson, S.; Simpson, S.; et al. Best Practice Guide for Underwater Particle Motion Measurement for Biological Applications 2021. Available online: https://repository.oceanbestpractices.org/handle/11329/1884?show=full (accessed on 24 September 2025).
- Jézéquel, Y.; Bonnel, J.; Aoki, N.; Mooney, T.A. Tank acoustics substantially distort broadband sounds produced by marine crustaceans. J. Acoust. Soc. Am. 2022, 152, 3747–3755. [Google Scholar] [CrossRef]
- Duarte, C.M.; Chapuis, L.; Collin, S.P.; Costa, D.P.; Devassy, R.P.; Eguiluz, V.M.; Erbe, C.; Gordon, T.A.C.; Halpern, B.S.; Harding, H.R.; et al. The soundscape of the Anthropocene ocean. Science 2021, 371, eaba4658. [Google Scholar] [CrossRef]
- Halvorsen, M.B.; Carlson, T.J.; Popper, A.N. Hydroacoustic Impacts on Fish from Pile Installation; Transportation Research Board: Washington, DC, USA, 2011; Volume 363. [Google Scholar]
- Halvorsen, M.B.; Casper, B.M.; Woodley, C.M.; Carlson, T.J.; Popper, A.N. Threshold for Onset of Injury in Chinook Salmon from Exposure to Impulsive Pile Driving Sounds. PLoS ONE 2012, 7, e38968. [Google Scholar] [CrossRef]
- Sand, O.; Karlsen, H.E. Detection of infrasound by the Atlantic cod. J. Exp. Biol. 1986, 125, 197–204. [Google Scholar] [CrossRef]
- ISO 18405:2017; Underwater Acoustics—Terminology. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/62406.html (accessed on 24 September 2025).
- Flamant, J.; Bonnel, J. Broadband properties of potential and kinetic energies in an oceanic waveguide. J. Acoust. Soc. Am. 2023, 153, 3012. [Google Scholar] [CrossRef]
- Dahl, P.H.; MacGillivray, A.; Racca, R. Vector acoustic properties of underwater noise from impact pile driving measured within the water column. Front. Mar. Sci. 2023, 10, 1146095. [Google Scholar] [CrossRef]
- Dahl, P.H.; Dall’Osto, D.R. Potential and kinetic energy of underwater noise measured below a passing ship and response to sub-bottom layering. J. Acoust. Soc. Am. 2022, 152, 3648–3658. [Google Scholar] [CrossRef]
- Oppeneer, V.O.; de Jong, C.A.; Binnerts, B.; Wood, M.A.; Ainslie, M.A. Modelling sound particle motion in shallow water. J. Acoust. Soc. Am. 2023, 154, 4004–4015. [Google Scholar] [CrossRef]
- André, M.; Van Der Schaar, M.; Zaugg, S.; Houégnigan, L.; Sánchez, A.; Castell, J. Listening to the deep: Live monitoring of ocean noise and cetacean acoustic signals. Mar. Pollut. Bull. 2011, 63, 18–26. [Google Scholar] [CrossRef]
- Rodgers, G.; Tenzing, P.; Clark, T. Experimental methods in aquatic respirometry: The importance of mixing devices and accounting for background respiration. J. Fish. Biol. 2016, 88, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Killen, S.S.; Christensen, E.A.F.; Cortese, D.; Závorka, L.; Norin, T.; Cotgrove, L.; Crespel, A.; Munson, A.; Nati, J.J.H.; Papatheodoulou, M.; et al. Guidelines for reporting methods to estimate metabolic rates by aquatic intermittent-flow respirometry. J. Exp. Biol. 2021, 224, jeb242522. [Google Scholar] [CrossRef] [PubMed]
- De Jong, C.A.F. Harmonized Shipping Sound Test Signals to Assess Effects on Aquatic Animals. In The Effects of Noise on Aquatic Life; Springer: Cham, Switzerland, 2023; Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-031-10417-6_38-1#citeas (accessed on 24 September 2025).
- Solé, M.; Lenoir, M.; Durfort, M.; López-Bejar, M.; Lombarte, A.; Van Der Schaar, M.; André, M. Does exposure to noise from human activities compromise sensory information from cephalopod statocysts? Deep Sea Res. Part II Top Stud. Oceanogr. 2013, 95, 160–181. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pla, P.; de Jong, C.A.F.; van der Schaar, M.; Solé, M.; André, M. AquaVib: Enabling the Separate Evaluation of Effects Induced by Acoustic Pressure and Particle Motion on Aquatic Organisms. J. Mar. Sci. Eng. 2025, 13, 1885. https://doi.org/10.3390/jmse13101885
Pla P, de Jong CAF, van der Schaar M, Solé M, André M. AquaVib: Enabling the Separate Evaluation of Effects Induced by Acoustic Pressure and Particle Motion on Aquatic Organisms. Journal of Marine Science and Engineering. 2025; 13(10):1885. https://doi.org/10.3390/jmse13101885
Chicago/Turabian StylePla, Pablo, Christ A. F. de Jong, Mike van der Schaar, Marta Solé, and Michel André. 2025. "AquaVib: Enabling the Separate Evaluation of Effects Induced by Acoustic Pressure and Particle Motion on Aquatic Organisms" Journal of Marine Science and Engineering 13, no. 10: 1885. https://doi.org/10.3390/jmse13101885
APA StylePla, P., de Jong, C. A. F., van der Schaar, M., Solé, M., & André, M. (2025). AquaVib: Enabling the Separate Evaluation of Effects Induced by Acoustic Pressure and Particle Motion on Aquatic Organisms. Journal of Marine Science and Engineering, 13(10), 1885. https://doi.org/10.3390/jmse13101885






