Towards Sustainable Aquafeeds: Valorization of Codium sp. and Osmundea sp. as Functional Ingredients to Enhance Nutrient and Bioactive Compounds in European Seabass
Abstract
1. Introduction
2. Materials and Methods
2.1. Codium sp. and Osmundea sp.
2.2. Experimental Diets
2.3. Ethics Statements
2.4. Growth Trial
2.5. Digestibility Trial
2.6. Sampling
2.7. Proximate Analysis
2.8. Gene Expression Analysis
2.9. Enzymatic Activity
2.10. Histological Processing and Morphological Evaluation
2.11. Statistical Analysis
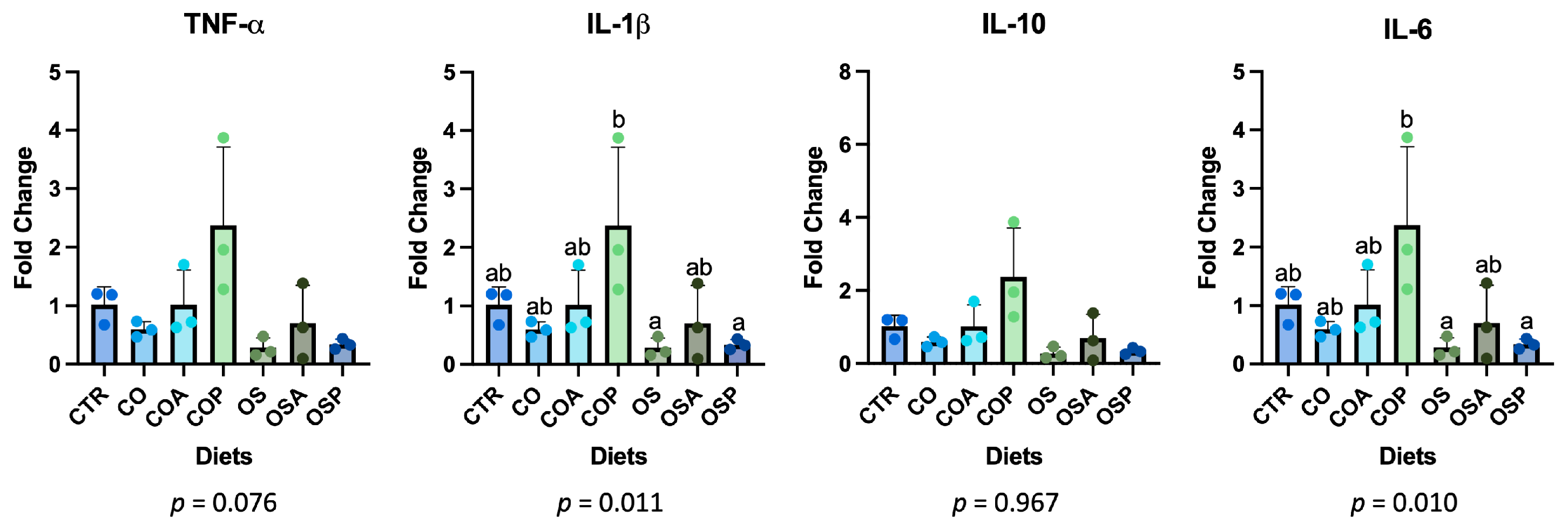
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent digestibility coefficient |
| CAT | Catalase |
| CO | Codium |
| COA | Codium autoclaved |
| COP | Codium polysaccharides |
| CTR | Control |
| GR | Glutathione reductase |
| il-10 | Interleukin-10 |
| il-1β | Interleukin-1-beta |
| il-6 | Interleukin-6 |
| OS | Osmundea |
| OSA | Osmundea autoclaved |
| OSP | Osmundea polysaccharides |
| SOD | Superoxide dismutase |
| tnf-α | Tumor necrosis factor-alpha |
References
- FAO. The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024. [CrossRef]
- Eurostat. EU Fisheries Remained Ahead of Aquaculture in 2022. 2024. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20240731-1 (accessed on 3 September 2024).
- Glencross, B.; Fracalossi, D.M.; Hua, K.; Izquierdo, M.; Mai, K.; Øverland, M.; Robb, D.; Roubach, R.; Schrama, J.; Small, B.; et al. Harvesting the benefits of nutritional research to address global challenges in the 21st century. J. World Aquac. Soc. 2023, 54, 343–363. [Google Scholar] [CrossRef]
- Campos, A.M.; Matos, J.; Afonso, C.; Gomes, R.; Bandarra, N.M.; Cardoso, C. Azorean macroalgae (Petalonia binghamiae, Halopteris scoparia and Osmundea pinnatifida) bioprospection: A study of fatty acid profiles and bioactivity. Int. J. Food Sci. Technol. 2019, 54, 880–890. [Google Scholar] [CrossRef]
- Silva, P.; Pereira, L. Concise review of Osmundea pinnatifida (Hudson) stackhouse. J. Appl. Phycol. 2020, 32, 2761–2771. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Choi, J.S. A concise review of the bioactivity and pharmacological properties of the genus Codium (Bryopsidales, Chlorophyta). J. Appl. Phycol. 2022, 34, 2827–2845. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The underexplored potential of green macroalgae in aquaculture. Rev. Aquac. 2022, 14, 5–26. [Google Scholar] [CrossRef]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar] [CrossRef]
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional value of selected macroalgae. J. Appl. Phycol. 2011, 23, 205–208. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Arguelles, E.D. Evaluation of nutritional composition and in vitro antioxidant and antibacterial activities of Codium intricatum Okamura from Ilocos Norte (Philippines). Jordan J. Biol. Sci. 2020, 13, 375–382. [Google Scholar]
- Echave, J.; Lourenço-Lopes, C.; Carreira-Casais, A.; Chamorro, F.; Fraga-Corral, M.; Otero, P.; Garcia-Perez, P.; Baamonde, S.; Fernández-Saa, F.; Cao, H.; et al. Nutritional composition of the Atlantic seaweeds Ulva rigida, Codium tomentosum, Palmaria palmata and Porphyra purpurea. Chem. Proc. 2021, 5, 67. [Google Scholar] [CrossRef]
- Freitas, M.V.; Inácio, L.G.; Martins, M.; Afonso, C.; Pereira, L.; Mouga, T. Primary composition and pigments of 11 red seaweed species from the center of Portugal. J. Mar. Sci. Eng. 2022, 10, 1168. [Google Scholar] [CrossRef]
- Negm, M.S.; Elrayess, R.A.; El-Shoubaky, G.A.; Osman, N.A. Chemical composition and bioactivity of Codium dwarkense collected from the earthquake crack region of Ras Muhammad National Park, Red Sea, Egypt. S. Afr. J. Bot. 2024, 173, 245–254. [Google Scholar] [CrossRef]
- Sousa, C.; Sousa-Pinto, I.; Oliveira, I.; Marinho, G.S. Seasonal variation in the composition and antioxidant potential of Codium tomentosum and Ulva lacinulata produced in a land-based integrated multi-trophic aquaculture system. J. Appl. Phycol. 2025, 37, 1557–1572. [Google Scholar] [CrossRef]
- Wan, A.H.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Biancacci, C.; Abell, R.; McDougall, G.J.; Day, J.G.; Stanley, M.S. Annual compositional variation in wild Osmundea pinnatifida (Hudson) Stackhouse from the west coast of Scotland. J. Appl. Phycol. 2022, 34, 1661–1675. [Google Scholar] [CrossRef]
- Ibañez, E.; Cifuentes, A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Boukid, F.; Castellari, M. Algae as nutritional and functional food sources. Foods 2022, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Kamble, M.T.; Wongprasert, K.; Chavan, B.R.; Daunde, V.V.Y.; Palekar, G.K.R.; Tayade, S.H.; Thompson, K.D.; Gabriel, N.N.; Medhe, S.V.; Pirarat, N. Red seaweeds in aquaculture: Impacts on growth, immunity, antioxidant status, gene expression, and gut health. Ann. Anim. Sci. 2025; ahead of print. [Google Scholar] [CrossRef]
- Yang, Y.; Park, J.; You, S.G.; Hong, S. Immuno-stimulatory effects of sulfated polysaccharides isolated from Codium fragile in olive flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2019, 87, 609–614. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Je, J.G.; Jayawardena, T.U.; Kim, Y.S.; Ko, J.Y.; Fu, X.; Jeon, Y.J. Protective effects of sulfated polysaccharides isolated from the enzymatic digest of Codium fragile against hydrogen peroxide-induced oxidative stress in in vitro and in vivo models. Algal Res. 2020, 48, 101891. [Google Scholar] [CrossRef]
- Yang, Y.; Lim, J.; Li, C.; Lee, S.; Hong, S. Effects of sulfated polysaccharides isolated from Codium fragile on inflammatory cytokine gene expression and Edwardsiella tarda infection in rockfish, Sebastes schlegelii. Fish Shellfish Immunol. 2021, 112, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.J.; Coves, D.; Dutto, G.; Blanc, D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 2004, 230, 391–404. [Google Scholar] [CrossRef]
- Dias, J.; Conceição, L.E.; Ribeiro, A.R.; Borges, P.; Valente, L.M.P.; Dinis, M.T. Practical diet with low fish-derived protein is able to sustain growth performance in gilthead seabream (Sparus aurata) during the grow-out phase. Aquaculture 2009, 293, 255–262. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Sæther, B.S.; Albrektsen, S.; Noble, C. Review on European sea bass (Dicentrarchus labrax, Linnaeus, 1758) nutrition and feed management: A practical guide for optimizing feed formulation and farming protocols. Aquac. Nutr. 2015, 21, 129–151. [Google Scholar] [CrossRef]
- Torrecillas, S.; Robaina, L.; Caballero, M.J.; Montero, D.; Calandra, G.; Mompel, D.; Karalazos, V.; Kaushik, S.; Izquierdo, M.S. Combined replacement of fishmeal and fish oil in European sea bass (Dicentrarchus labrax): Production performance, tissue composition and liver morphology. Aquaculture 2017, 474, 101–112. [Google Scholar] [CrossRef]
- Batista, S.; Pereira, R.; Oliveira, B.; Baião, L.F.; Jessen, F.; Tulli, F.; Messina, M.; Silva, J.L.; Abreu, H.; Valente, L.M.P. Exploring the potential of seaweed Gracilaria gracilis and microalga Nannochloropsis oceanica, single or blended, as natural dietary ingredients for European seabass Dicentrarchus labrax. J. Appl. Phycol. 2020, 32, 2041–2059. [Google Scholar] [CrossRef]
- Passos, R.; Correia, A.P.; Pires, D.; Pires, P.; Ferreira, I.; Simões, M.; do Carmo, B.; Santos, P.; Pombo, A.; Afonso, C.; et al. Potential use of macroalgae Gracilaria gracilis in diets for European seabass (Dicentrarchus labrax): Health benefits from a sustainable source. Fish Shellfish Immunol. 2021, 119, 105–113. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Batista, S.; Ribeiro, C.; Pereira, R.; Oliveira, B.; Garrido, I.; Baião, L.F.; Tulli, F.; Messina, M.; Pierre, R.; et al. Physical processing or supplementation of feeds with phytogenic compounds, alginate oligosaccharide or nucleotides as methods to improve the utilization of Gracilaria gracilis by juvenile European seabass (Dicentrarchus labrax). Aquaculture 2021, 530, 735914. [Google Scholar] [CrossRef]
- Fonseca, F.; Fuentes, J.; Vizcaíno, A.J.; Alarcón, F.J.; Mancera, J.M.; Martínez-Rodríguez, G.; Martos-Sitcha, J.A. From invasion to fish fodder: Inclusion of the brown algae Rugulopteryx okamurae in aquafeeds for European sea bass Dicentrarchus labrax (L., 1758). Aquaculture 2023, 568, 739318. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Sáez, M.I.; Galafat, A.; Galindo-Melero, R.; Perera, E.; Casal-Porras, I.; Zubía, E.; Vega, J.; Figueroa, F.L.; Martínez, T.F.; et al. Effects of feeding European seabass (Dicentrarchus labrax) juveniles with crude, hydrolysed and fermented biomass of the invasive macroalga Rugulopteryx okamurae (Ochrophyta). Aquac. Rep. 2024, 34, 101877. [Google Scholar] [CrossRef]
- Fernandes, H.; Martins, N.; Vieira, L.; Salgado, J.M.; Castro, C.; Oliva-Teles, A.; Belo, I.; Peres, H. Pre-treatment of Ulva rigida improves its nutritional value for European seabass (Dicentrarchus labrax) juveniles. Algal Res. 2022, 66, 102803. [Google Scholar] [CrossRef]
- González-Meza, G.M.; Elizondo-Luevano, J.H.; Cuellar-Bermudez, S.P.; Sosa-Hernández, J.E.; Iqbal, H.M.; Melchor-Martínez, E.M.; Parra-Saldívar, R. New perspective for macroalgae-based animal feeding in the context of challenging sustainable food production. Plants 2023, 12, 3609. [Google Scholar] [CrossRef] [PubMed]
- Batista, S.; Pintado, M.; Marques, A.; Abreu, H.; Silva, J.L.; Jessen, F.; Tulli, F.; Valente, L.M.P. Use of technological processing of seaweed and microalgae as strategy to improve their apparent digestibility coefficients in European seabass (Dicentrarchus labrax) juveniles. J. Appl. Phycol. 2020, 32, 3429–3446. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Galafat, A.; Sáez, M.I.; Martínez, T.F.; Alarcón, F.J. Partial characterization of protease inhibitors of Ulva ohnoi and their effect on digestive proteases of marine fish. Mar. Drugs 2020, 18, 319. [Google Scholar] [CrossRef]
- Güroy, B.; Ergün, S.; Merrifield, D.L.; Güroy, D. Effect of autoclaved Ulva meal on growth performance, nutrient utilization and fatty acid profile of rainbow trout, Oncorhynchus mykiss. Aquac. Int. 2013, 21, 605–615. [Google Scholar] [CrossRef]
- Gonçalves, A.T.; Simões, M.; Costa, C.; Passos, R.; Baptista, T. Modulatory effect of Gracilaria gracilis on European seabass gut microbiota community and its functionality. Sci. Rep. 2022, 12, 14836. [Google Scholar] [CrossRef] [PubMed]
- Pappou, S.; Bakopoulos, V.; Valsamidis, M.A.; Krokida, M.; Batjakas, I. Supplementation of a commercial diet of European seabass by an algal ethanolic extract of Ulva lactuca. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2024, 80, 157–162. [Google Scholar] [CrossRef]
- Khanzadeh, M.; Beikzadeh, B.; Hoseinifar, S.H. The effects of Laurencia caspica algae extract on hemato-immunological parameters, antioxidant defense, and resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2023, 2023, 8882736. [Google Scholar] [CrossRef]
- Kiadaliri, M.; Firouzbakhsh, F.; Deldar, H. Effects of feeding with red algae (Laurencia caspica) hydroalcoholic extract on antioxidant defense, immune responses, and immune gene expression of kidney in rainbow trout (Oncorhynchus mykiss) infected with Aeromonas hydrophila. Aquaculture 2020, 526, 735361. [Google Scholar] [CrossRef]
- Cho, C.Y.; Slinger, S.J.; Bayley, H.S. Bioenergetics of salmonid fishes: Energy intake, expenditure and productivity. Comp. Biochem. Physiol. B 1982, 73, 25–41. [Google Scholar] [CrossRef]
- Besednova, N.N.; Zaporozhets, T.S.; Kuznetsova, T.A.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Ermakova, S.P. Extracts and marine algae polysaccharides in therapy and prevention of inflammatory diseases of the intestine. Mar. Drugs 2020, 18, 289. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Furukawa, A.; Tsukahara, H. On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility of fish feed. Bull. Japan. Soc. Sci. Fish 1966, 32, 502–506. [Google Scholar] [CrossRef]
- Monteiro, M.; Lavrador, A.S.; Santos, R.; Rangel, F.; Iglesias, P.; Tárraga, M.; Couto, A.; Serra, C.R.; Tafalla, C.; Da Costa, E.; et al. Evaluation of the potential of marine algae extracts as a source of functional ingredients using zebrafish as animal model for aquaculture. Mar. Biotechnol. 2021, 23, 529–545. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Carvalhais, A.; Pereira, B.; Sabato, M.; Seixas, R.; Dolbeth, M.; Marques, A.; Guilherme, S.; Pereira, P.; Pacheco, M.; Mieiro, C. Mild effects of sunscreen agents on a marine flatfish: Oxidative stress, energetic profiles, neurotoxicity and behaviour in response to titanium dioxide nanoparticles and oxybenzone. Int. J. Mol. Sci. 2021, 22, 1567. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Baeverfjord, G.; Krogdahl, A. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. J. Fish Dis. 1996, 19, 375–387. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Bakke-McKellep, A.M.; Baeverfjord, G. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2003, 9, 361–371. [Google Scholar] [CrossRef]
- Penn, M.H.; Bendiksen, E.Å.; Campbell, P.; Krogdahl, Å. High level of dietary pea protein concentrate induces enteropathy in Atlantic salmon (Salmo salar L.). Aquaculture 2011, 310, 267–273. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Gouveia, A.; Rema, P.; Matos, J.; Gomes, E.F.; Pinto, I.S. Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2006, 252, 85–91. [Google Scholar] [CrossRef]
- Azaza, M.S.; Mensi, F.; Ksouri, J.; Dhraief, M.N.; Brini, B.; Abdelmouleh, A.; Kraïem, M.M. Growth of Nile tilapia (Oreochromis niloticus L.) fed with diets containing graded levels of green algae ulva meal (Ulva rigida) reared in geothermal waters of southern Tunisia. J. Appl. Ichthyol. 2008, 24, 202–207. [Google Scholar] [CrossRef]
- Marinho, G.; Nunes, C.; Sousa-Pinto, I.; Pereira, R.; Rema, P.; Valente, L.M.P. The IMTA-cultivated Chlorophyta Ulva spp. as a sustainable ingredient in Nile tilapia (Oreochromis niloticus) diets. J. Appl. Phycol. 2013, 25, 1359–1367. [Google Scholar] [CrossRef]
- Silva, D.M.; Valente, L.M.P.; Sousa-Pinto, I.; Pereira, R.; Pires, M.A.; Seixas, F.; Rema, P. Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. J. Appl. Phycol. 2015, 27, 1671–1680. [Google Scholar] [CrossRef]
- Zhu, D.; Wen, X.; Xuan, X.; Li, S.; Li, Y. The green alga Ulva lactuca as a potential ingredient in diets for juvenile white spotted snapper Lutjanus stellatus Akazaki. J. Appl. Phycol. 2016, 28, 703–711. [Google Scholar] [CrossRef]
- Sotoudeh, E.; Mardani, F. Antioxidant-related parameters, digestive enzyme activity and intestinal morphology in rainbow trout (Oncorhynchus mykiss) fry fed graded levels of red seaweed, Gracilaria pygmaea. Aquac. Nutr. 2018, 24, 777–785. [Google Scholar] [CrossRef]
- Dallaire, V.; Lessard, P.; Vandenberg, G.; De La Noüe, J. Effect of algal incorporation on growth, survival and carcass composition of rainbow trout (Oncorhynchus mykiss) fry. Bioresour. Technol. 2007, 98, 1433–1439. [Google Scholar] [CrossRef]
- Pereira, R.; Valente, L.M.P.; Sousa-Pinto, I.; Rema, P. Apparent nutrient digestibility of seaweeds by rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus). Algal Res. 2012, 1, 77–82. [Google Scholar] [CrossRef]
- ALGALUP Green Future. Composición de Macroalgas en Galicia y Portugal. Available online: https://algalup.eu/wp-content/uploads/2022/07/Composicion.pdf (accessed on 18 September 2025).
- Juul, L.; Stødkilde, L.; Ingerslev, A.K.; Bruhn, A.; Jensen, S.K.; Dalsgaard, T.K. Digestibility of seaweed protein from Ulva sp. and Saccharina latissima in rats. Algal Res. 2022, 63, 102644. [Google Scholar] [CrossRef]
- Prashant, N.; Sangwan, M.; Singh, P.; Das, P.; Srivastava, U.; Bast, F. Anti-nutritional factors and heavy metals in edible seaweeds: Challenges, health implications, and strategies for safer consumption. J. Food Compos. Anal. 2025, 140, 107283. [Google Scholar] [CrossRef]
- Peres, H.; Lim, C.; Klesius, P.H. Nutritional value of heat-treated soybean meal for channel catfish (Ictalurus punctatus). Aquaculture 2003, 225, 67–82. [Google Scholar] [CrossRef]
- Guerreiro, I.; Magalhães, R.; Coutinho, F.; Couto, A.; Sousa, S.; Delerue-Matos, C.; Domingues, V.F.; Oliva-Teles, A.; Peres, H. Evaluation of the seaweeds Chondrus crispus and Ulva lactuca as functional ingredients in gilthead seabream (Sparus aurata). J. Appl. Phycol. 2019, 31, 2115–2124. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef]
- Khongthong, S.; Theapparat, Y.; Roekngam, N.; Tantisuwanno, C.; Otto, M.; Piewngam, P. Characterization and immunomodulatory activity of sulfated galactan from the red seaweed Gracilaria fisheri. Int. J. Biol. Macromol. 2021, 189, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Barrows, F.T.; Stone, D.A.; Hardy, R.W. The effects of extrusion conditions on the nutritional value of soybean meal for rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 265, 244–252. [Google Scholar] [CrossRef]
- Ferreira, M.; Sousa, V.; Oliveira, B.; Canadas-Sousa, A.; Abreu, H.; Dias, J.; Kiron, V.; Valente, L.M.P. An in-depth characterisation of European seabass intestinal segments for assessing the impact of an algae-based functional diet on intestinal health. Sci. Rep. 2023, 13, 11686. [Google Scholar] [CrossRef]
- Ferreira, M.; Machado, M.; Mota, C.S.C.; Abreu, H.; Silva, J.; Maia, M.R.G.; Kiron, V.; Costas, B.; Valente, L.M.P. Micro- and macroalgae blend modulates the mucosal and systemic immune responses of European seabass (Dicentrarchus labrax) upon infection with Tenacibaculum maritimum. Aquaculture 2023, 566, 739222. [Google Scholar] [CrossRef]

| Diets | |||||||
|---|---|---|---|---|---|---|---|
| CTR | CO | COA | COP | OS | OSA | OSP | |
| Ingredients (% dry weight basis) | |||||||
| Fishmeal 1 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Osmundea 2 | - | - | - | - | 5.0 | - | - |
| Osmundea autoclaved 2 | - | - | - | - | - | 5.0 | - |
| Codium 3 | - | 5.0 | - | - | - | - | - |
| Codium autoclaved 3 | - | - | 5.0 | - | - | - | - |
| Osmundea polysaccharide extract 4 | - | - | - | - | - | - | 0.5 |
| Codium polysaccharide extract 4 | - | - | - | 0.5 | - | - | - |
| Wheat gluten 5 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Corn gluten 6 | 9.1 | 9.2 | 9.2 | 9.1 | 8.4 | 8.4 | 9.1 |
| Rapeseed meal 7 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean meal 8 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Sunflower meal 9 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Wheat meal 10 | 8.4 | 3.2 | 3.2 | 8.4 | 3.9 | 4.0 | 8.4 |
| α-cellulose | 0.5 | 0.5 | 0.5 | - | 0.5 | 0.5 | - |
| Fish oil | 15.0 | 15.0 | 15.0 | 15.0 | 15.1 | 15.1 | 15.0 |
| Vitamin premix 11 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral premix 12 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Binder 13 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline chloride (50%) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Dibasic calcium phosphate | 0.05 | 0.1 | 0.1 | 0.05 | 0.1 | 0.1 | 0.05 |
| Chromium oxide | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Methionine 14 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 |
| Taurine 15 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Proximate analyses (% dry weight basis) | |||||||
| Dry matter | 97.8 | 97.0 | 97.0 | 97.6 | 97.8 | 97.2 | 98.1 |
| Crude protein | 41.7 | 41.7 | 42.0 | 42.3 | 41.7 | 42.3 | 42.5 |
| Crude lipid | 17.1 | 17.6 | 17.8 | 17.5 | 17.2 | 17.6 | 16.9 |
| Ash | 7.6 | 9.6 | 9.7 | 7.2 | 8.8 | 8.6 | 7.7 |
| Energy (kJ g−1) | 23.2 | 22.9 | 23.1 | 23.5 | 22.8 | 22.8 | 23.7 |
| Gene | Sequence | Efficiency | Accession Number |
|---|---|---|---|
| 18S | F: AGGGTGTTGGCAGACGTTAC | 2.0 | AM490061 |
| R: CTTCTGCCTGTTGAGGAACC | |||
| IL-1β | F: ATCTGGAGGTGGTGGACAAA | 1.9 | AJ311925 |
| R: AGGGTGCTGATGTTCAAACC | |||
| IL-6 | F: ACTCCTCGGTCTCTCCTCGTATCCGC | 1.9 | AM490062 |
| R:CTGTGTCGAGATCATCGTTGGCTTCATAAAAGTC | |||
| TNF-α | F: CACAAGAGCGGCCATTCATTTACAAGGAG | 2.0 | DQ200910 |
| R: GGAAAGACGCTTGGCTGTAGATGG | |||
| IL-10 | F: ATCACAGTTCCGGCGTATTT | 2.0 | DQ821114 |
| R: ATGGACACGTCAAAGGTGCC |
| Diets | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CTR | CO | COA | COP | OS | OSA | OSP | SEM | p Value | |
| Final body weight | 70.5 b | 59.7 a | 62.6 ab | 63.3 ab | 68.9 b | 63.9 ab | 63.6 ab | 0.95 | 0.01 |
| Daily Growth Index 1 | 2.48 b | 2.08 a | 2.19 ab | 2.22 ab | 2.42 b | 2.24 ab | 2.23 ab | 0.04 | 0.01 |
| Feed Intake (g Kg ABW−1 day−1) 2 | 20.7 | 19.5 | 19.5 | 19.5 | 20.8 | 19.5 | 20.0 | 0.16 | 0.05 |
| Feed Efficiency 3 | 0.95 | 0.89 | 0.92 | 0.93 | 0.93 | 0.93 | 0.91 | 0.01 | 0.27 |
| Protein Efficiency Ratio 4 | 2.28 | 2.14 | 2.19 | 2.20 | 2.22 | 2.19 | 2.14 | 0.01 | 0.14 |
| Survival (%) | 97.3 | 97.3 | 98.7 | 100.0 | 98.7 | 98.7 | 100.0 | 0.41 | 0.50 |
| Diets | |||||||
|---|---|---|---|---|---|---|---|
| CTR | CO | COA | OS | OSA | SEM | p Value | |
| Dry matter | 73.8 c | 64.6 a | 68.5 abc | 73.3 bc | 67.4 ab | 0.99 | 0.002 |
| Protein | 94.4 c | 91.9 a | 92.9 ab | 93.9 bc | 93.1 abc | 0.23 | 0.000 |
| Energy | 99.84 b | 99.83 b | 99.79 a | 99.80 ab | 99.82 ab | 0.01 | 0.014 |
| Lipids | 96.2 | 95 | 95.9 | 96.7 | 95.2 | 0.30 | 0.388 |
| Diets | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CTR | CO | COA | COP | OS | OSA | OSP | SEM | p Value | |
| Superoxide dismutase | 32.5 | 33.2 | 34.8 | 37.9 | 31.5 | 34.1 | 36.9 | 1.03 | 0.687 |
| Catalase | 62.6 | 59.1 | 67.4 | 58.2 | 60.8 | 65.2 | 60.2 | 1.52 | 0.710 |
| Glutathione reductase | 12.8 | 12.0 | 13.7 | 12.1 | 12.4 | 11.5 | 12.0 | 0.37 | 0.856 |
| Diets | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CTR | CO | COA | COP | OS | OSA | OSP | SEM | p Value | |
| Villi length | 1.78 | 1.78 | 1.75 | 2.00 | 1.89 | 1.33 | 1.56 | 0.09 | 0.508 |
| Lamina propria size | 1.00 | 1.00 | 1.13 | 1.33 | 1.00 | 1.11 | 1.11 | 0.03 | 0.099 |
| Submucosa widening | 1.11 | 1.56 | 1.00 | 1.33 | 1.11 | 1.22 | 1.22 | 0.06 | 0.274 |
| Supranuclear vacuole size | 2.00 | 1.78 | 1.38 | 1.78 | 1.78 | 2.33 | 1.78 | 0.09 | 0.140 |
| Goblet cells | 1.67 | 1.33 | 1.38 | 2.00 | 1.89 | 1.56 | 1.89 | 0.08 | 0.132 |
| Eosinophilic granulocytes | 1.11 | 1.33 | 1.13 | 1.33 | 1.11 | 1.00 | 1.22 | 0.05 | 0.635 |
| Intraepithelial leukocytes | 1.22 | 1.44 | 1.38 | 1.56 | 1.22 | 1.22 | 1.22 | 0.06 | 0.616 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerreiro, I.; Fontinha, F.; Monteiro, M.; Oliveira, J.; Marçal, R.; Magalhães, R.; Pacheco, M.; Soula, M.; Oliva-Teles, A.; Enes, P.; et al. Towards Sustainable Aquafeeds: Valorization of Codium sp. and Osmundea sp. as Functional Ingredients to Enhance Nutrient and Bioactive Compounds in European Seabass. J. Mar. Sci. Eng. 2025, 13, 1884. https://doi.org/10.3390/jmse13101884
Guerreiro I, Fontinha F, Monteiro M, Oliveira J, Marçal R, Magalhães R, Pacheco M, Soula M, Oliva-Teles A, Enes P, et al. Towards Sustainable Aquafeeds: Valorization of Codium sp. and Osmundea sp. as Functional Ingredients to Enhance Nutrient and Bioactive Compounds in European Seabass. Journal of Marine Science and Engineering. 2025; 13(10):1884. https://doi.org/10.3390/jmse13101884
Chicago/Turabian StyleGuerreiro, Inês, Filipa Fontinha, Marta Monteiro, Joana Oliveira, Raquel Marçal, Rui Magalhães, Mário Pacheco, Mohamed Soula, Aires Oliva-Teles, Paula Enes, and et al. 2025. "Towards Sustainable Aquafeeds: Valorization of Codium sp. and Osmundea sp. as Functional Ingredients to Enhance Nutrient and Bioactive Compounds in European Seabass" Journal of Marine Science and Engineering 13, no. 10: 1884. https://doi.org/10.3390/jmse13101884
APA StyleGuerreiro, I., Fontinha, F., Monteiro, M., Oliveira, J., Marçal, R., Magalhães, R., Pacheco, M., Soula, M., Oliva-Teles, A., Enes, P., & Couto, A. (2025). Towards Sustainable Aquafeeds: Valorization of Codium sp. and Osmundea sp. as Functional Ingredients to Enhance Nutrient and Bioactive Compounds in European Seabass. Journal of Marine Science and Engineering, 13(10), 1884. https://doi.org/10.3390/jmse13101884








