Long-Term Field Monitoring of Biofouling Characteristics in the Yellow Sea off Jeju Island, South Korea
Abstract
1. Introduction
2. Materials and Methods
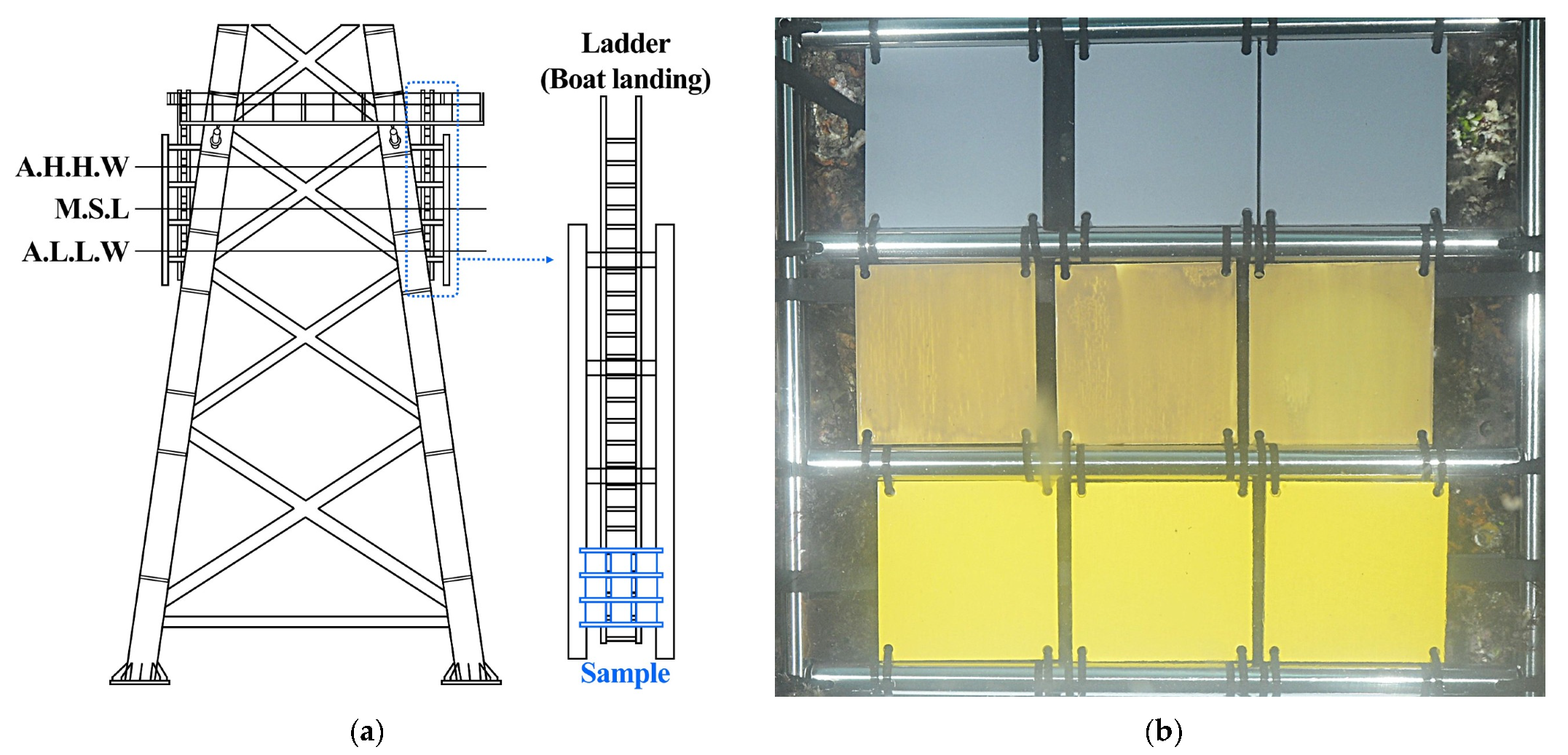
2.1. Study Site
2.2. Sample Preparation and Field Exposure
2.3. Surface Properties
3. Results
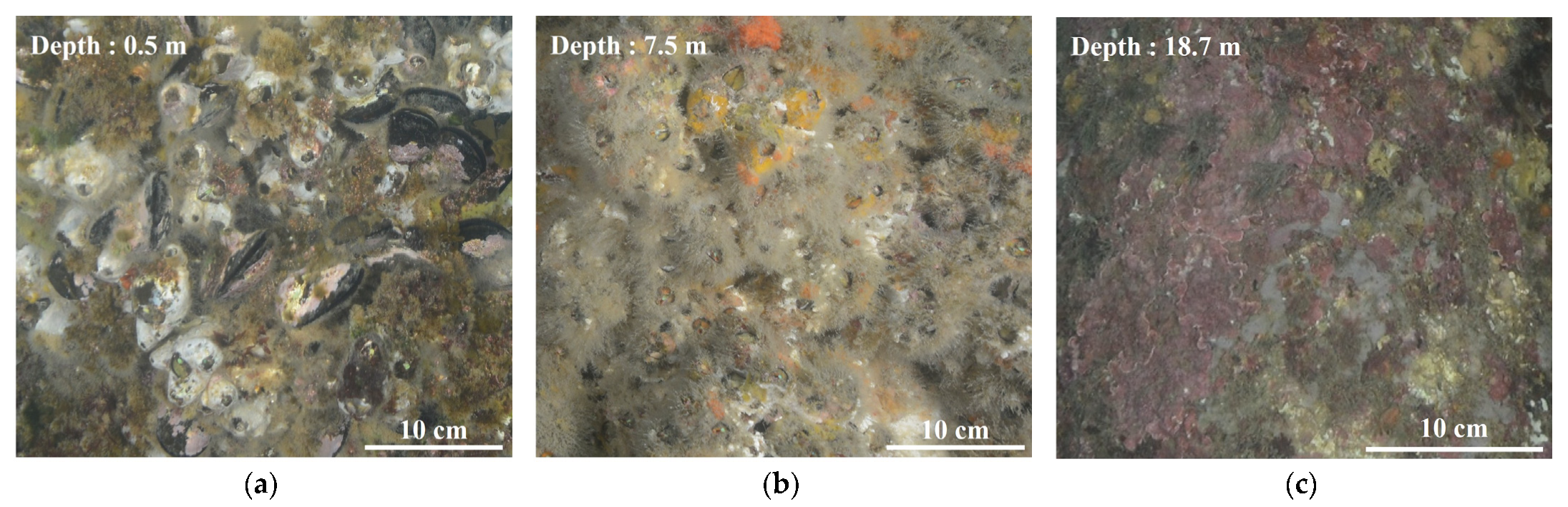
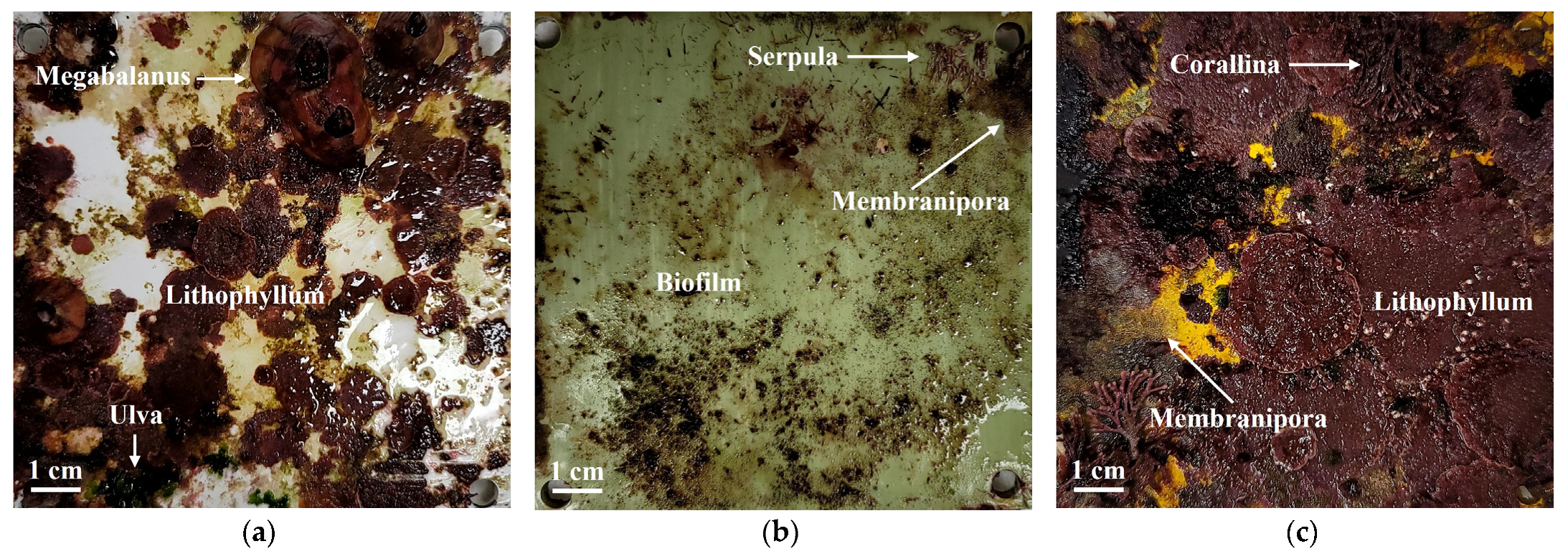
3.1. Depth-Related Biofouling Characteristics
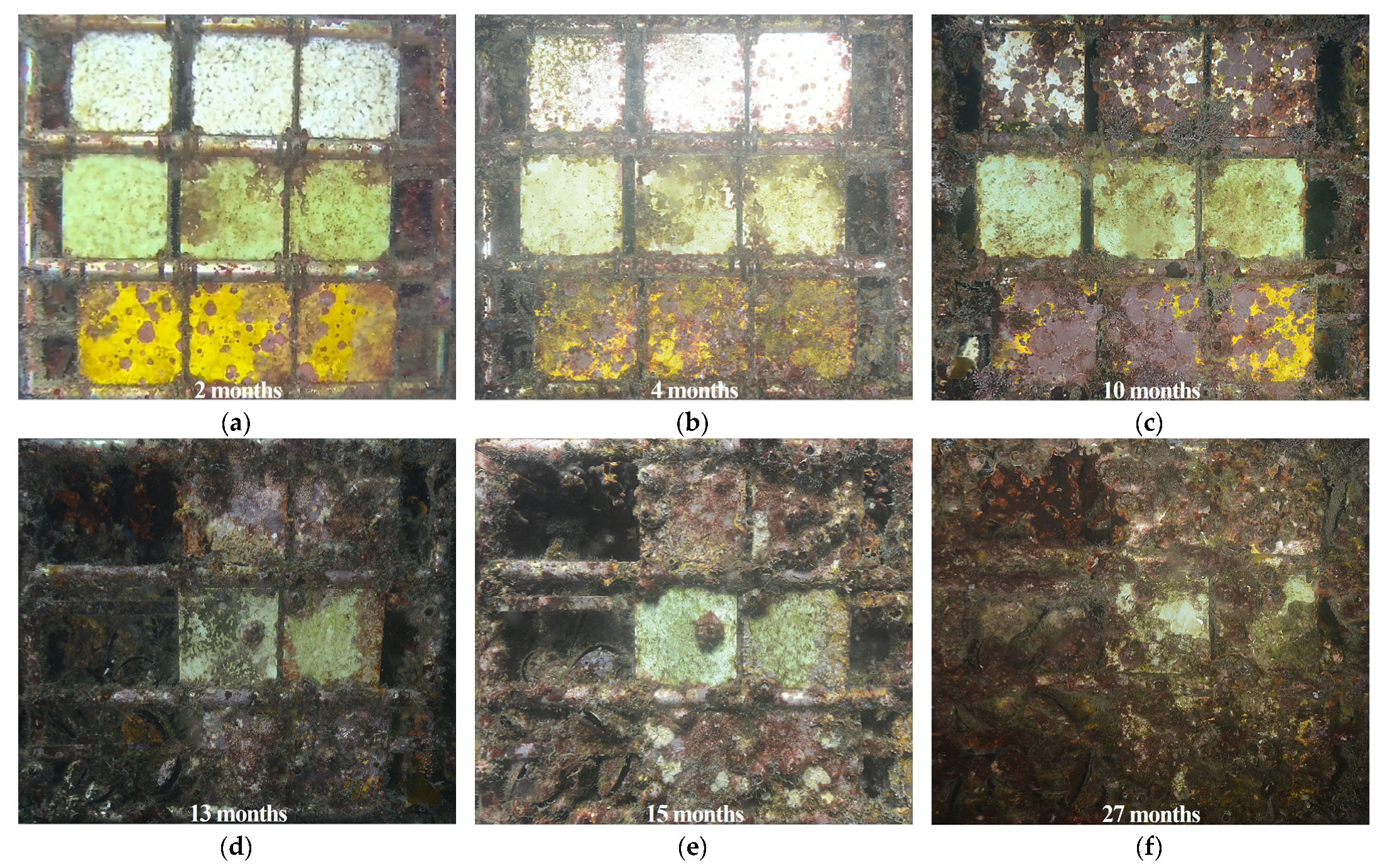
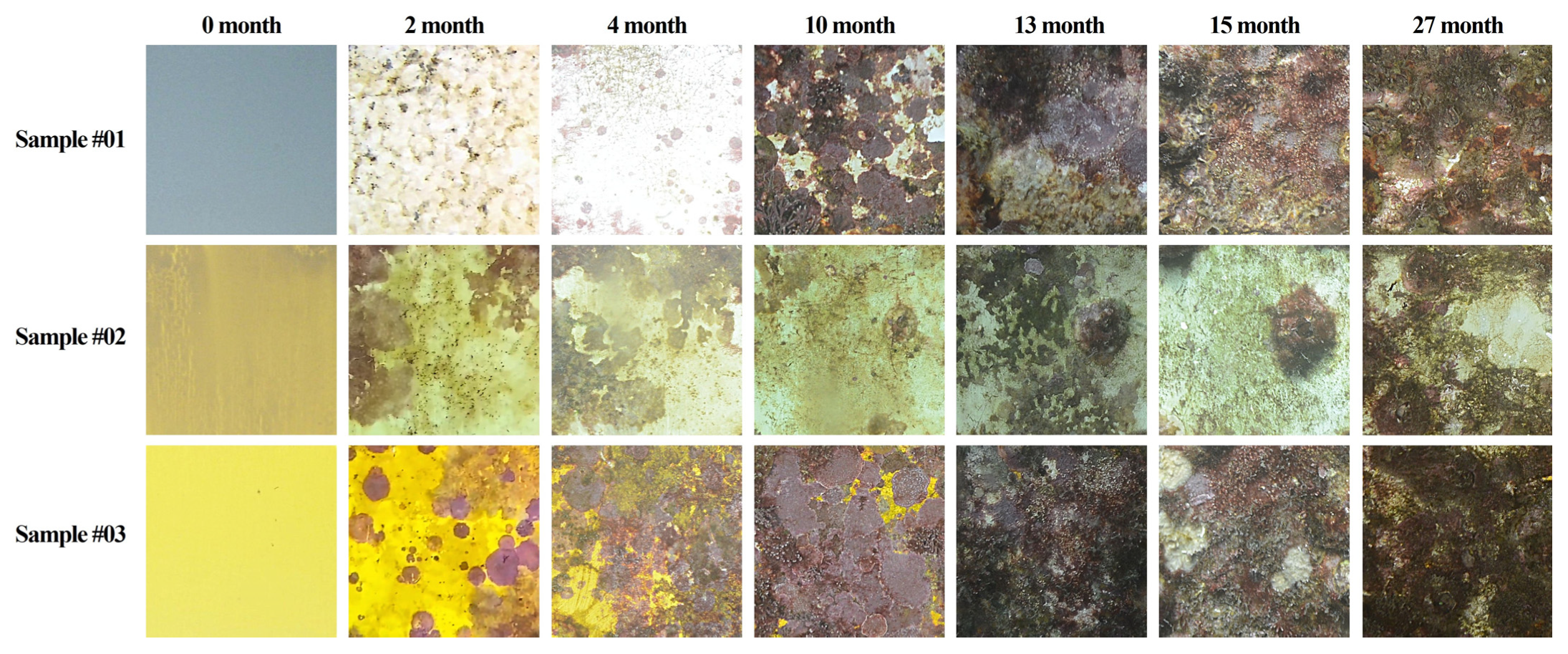
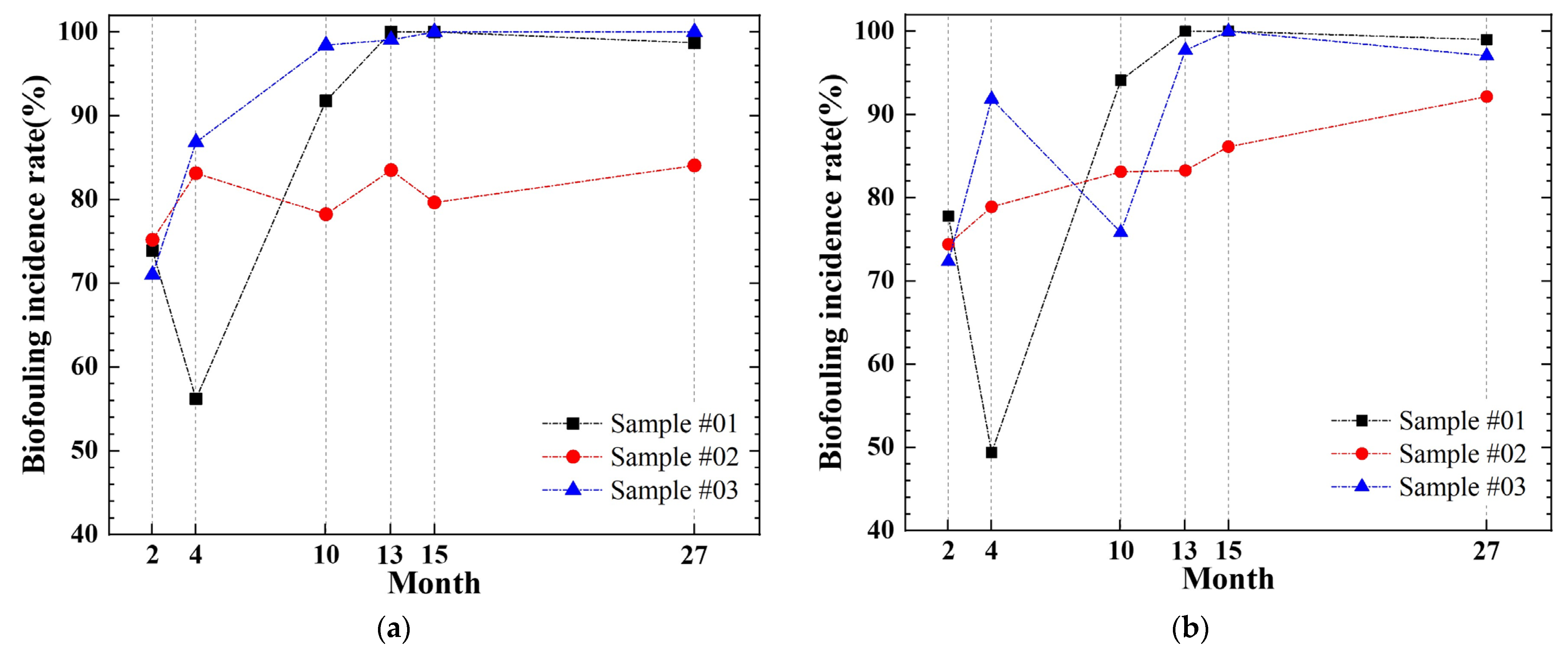
3.2. Temporal Development of Biofouling Coverage
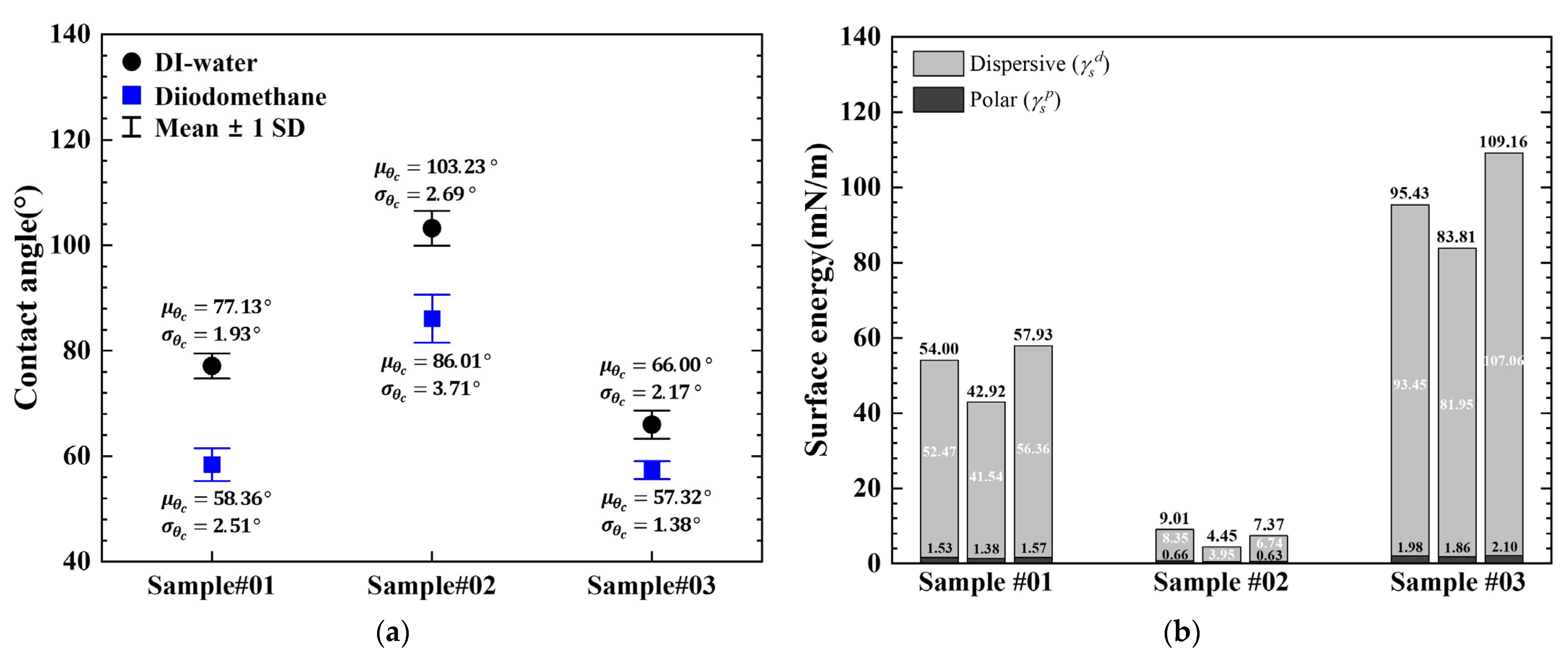
3.3. Contact Angle and Surface Energy Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- REN21. Renewables 2025 Global Status Report—Global Overview; REN21 Secretariat: Paris, France, 2025; Available online: https://www.ren21.net/reports/global-status-report/ (accessed on 28 September 2025).
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Abed, R.M.M.; Teplitski, M. Mini-review: Inhibition of biofouling by marine microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Aboobucker, S.I.; Satheesh, S. Characterization and assessment of barnacle larval settlement-inducing activity of extracellular polymeric substances isolated from marine biofilm bacteria. Sci. Rep. 2019, 9, 17849. [Google Scholar] [CrossRef] [PubMed]
- Rajitha, K.; Selvakumar, P.; Subramanian, G.; Satheesh, S. Insight into bacterial biofilm–barnacle larvae interactions for environmentally benign antifouling strategies. Int. Biodeterior. Biodegrad. 2020, 149, 104937. [Google Scholar] [CrossRef]
- Zupan, M.; Rumes, B.; Vanaverbeke, J.; Degraer, S.; Kerckhof, F. Long-term succession on offshore wind farms and the role of species interactions. Diversity 2023, 15, 288. [Google Scholar] [CrossRef]
- Degraer, S.; Carey, D.A.; Coolen, J.W.P.; Hutchison, Z.; Kerckhof, F.; Rumes, B.; Vanaverbeke, J. Offshore wind farm artificial reefs affect ecosystem structure and functioning. Oceanography 2020, 33, 48–57. [Google Scholar] [CrossRef]
- Schultz, M.P. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 2007, 23, 331–341. [Google Scholar] [CrossRef]
- Yang, S.-H.; Ringsberg, J.W.; Johnson, E.; Hu, Z. Biofouling on mooring lines and power cables used in wave energy converter systems—Analysis of fatigue life and energy performance. Appl. Ocean Res. 2017, 65, 166–177. [Google Scholar] [CrossRef]
- Shi, W.; Park, H.C.; Baek, J.H.; Kim, H.M.; Lee, S.H. Study on the marine growth effect on the dynamic response of offshore wind turbines. Int. J. Precis. Eng. Manuf. 2012, 13, 1167–1176. [Google Scholar] [CrossRef]
- Mallat, C.; Corbett, A.; Harris, G.; Lefranc, M. Marine growth on North Sea fixed steel platforms: Insights from the decommissioning industry. In Proceedings of the ASME 2014 33rd International Conference on Ocean, Offshore and Arctic Engineering (OMAE 2014), San Francisco, CA, USA, 8–13 June 2014; Volume 1A. Offshore Technology, V01AT01A021. [Google Scholar] [CrossRef]
- Titah-Benbouzid, H.; Benbouzid, M. Marine renewable energy converters and biofouling: A review on impacts and prevention. In Proceedings of the 11th European Wave and Tidal Energy Conference (EWTEC 2015), Nantes, France, 6–11 September 2015; pp. 1–8. [Google Scholar]
- Liniger, J.; Jensen, A.L.; Pedersen, S.; Sørensen, H.; Mai, C. On the autonomous inspection and classification of marine growth on subsea structures. In Proceedings of the OCEANS 2022—Chennai, Chennai, India, 21–24 February 2022; pp. 1–7. [Google Scholar] [CrossRef]
- Pedersen, S.; Liniger, J.; Sørensen, F.F.; von Benzon, M. On marine growth removal on offshore structures. In Proceedings of the OCEANS 2022—Chennai, Chennai, India, 21–24 February 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Poozesh, P.; Nieto, F.; Fernández, P.M.; Ríos, R.; Díaz-Casás, V. Biofouling on Offshore Wind Energy Structures: Characterization, Impacts, Mitigation Strategies, and Future Trends. J. Mar. Sci. Eng. 2025, 13, 1363. [Google Scholar] [CrossRef]
- Dubois, A.; Schoefs, F.; Cognie, B.; Reynaud, M.; Soulard, T.; Dumay, J. Spatio-temporal evolution and engineering implications of biofouling communities on floating wind turbines mooring lines. Estuar. Coast. Shelf Sci. 2025, 320, 109302. [Google Scholar] [CrossRef]
- De Mesel, I.; Kerckhof, F.; Norro, A.; Rumes, B.; Degraer, S. Succession and seasonal dynamics of the epifauna community on offshore wind farm foundations and their role as stepping stones for non-indigenous species. Hydrobiologia 2015, 756, 37–50. [Google Scholar] [CrossRef]
- Dannheim, J.; Bergström, L.; Birchenough, S.N.R.; Brzana, R.; Boon, A.R.; Coolen, J.W.P.; Gill, A.B.; Hutchison, Z.L.; Jackson, A.C.; Janas, U.; et al. Benthic effects of offshore renewables: Identification of knowledge gaps and urgently needed research. ICES J. Mar. Sci. 2020, 77, 1092–1108. [Google Scholar] [CrossRef]
- Krone, R.; Gutow, L.; Joschko, T.J.; Schröder, A. Epifauna dynamics at an offshore foundation—Implications of future wind power farming in the North Sea. Mar. Environ. Res. 2013, 85, 1–12. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Wu, S. Research Progress of Marine Anti-Fouling Coatings. Coatings 2024, 14, 1227. [Google Scholar] [CrossRef]
- Callau, M.; Fajolles, C.; Leroy, J.; Verneuil, E.; Guenoun, P. A silicone-based slippery polymer coating with humidity-dependent nanoscale topography. J. Colloid Interface Sci. 2023, 641, 725–737. [Google Scholar] [CrossRef]
- Finlay, J.A.; Callow, M.E.; Schultz, M.P.; Swain, G.W.; Callow, J.A. Adhesion strength of settled spores of the green alga Enteromorpha. Biofouling 2002, 18, 251–256. [Google Scholar] [CrossRef]
- Callow, M.E.; Callow, J.A.; Ista, L.K.; Lopez, G.P. Effect of substratum surface chemistry and surface energy on attachment of marine bacteria and algal spores. Appl. Environ. Microbiol. 2004, 70, 4151–4157. [Google Scholar] [CrossRef]
- Ministry of Oceans and Fisheries. Annual Report on Marine Environment Monitoring in Korea 2022; Ministry of Oceans and Fisheries: Sejong, Republic of Korea, 2022. [Google Scholar]
- Ministry of Oceans and Fisheries. Annual Report on Marine Environment Monitoring in Korea 2023; Ministry of Oceans and Fisheries: Sejong, Republic of Korea, 2023. [Google Scholar]
- Ministry of Oceans and Fisheries. Annual Report on Marine Environment Monitoring in Korea 2024; Ministry of Oceans and Fisheries: Sejong, Republic of Korea, 2024. [Google Scholar]
- Ma, C.; Yang, H.; Li, X.; Zhang, G. Functional polymer materials for modern marine biofouling control. Prog. Polym. Sci. 2022, 125, 101473. [Google Scholar] [CrossRef]
- Li, L.; Hong, H.; Cao, J.; Yang, Y. Progress in Marine Antifouling Coatings: Current Status and Prospects. Coatings 2023, 13, 1893. [Google Scholar] [CrossRef]
- Weber, F.; Esmaeili, N. Marine biofouling and the role of biocidal coatings in balancing environmental impacts. Biofouling 2023, 39, 661–681. [Google Scholar] [CrossRef]
- Shi, X.; Liang, H.; Li, Y. Review of progress in marine anti-fouling coatings: Manufacturing techniques and copper- and silver-doped antifouling coatings. Coatings 2024, 14, 1454. [Google Scholar] [CrossRef]
- Soma Raju, K.R.C.; Subasri, R.; Srinivasa Rao, K. Ceramic–polymer hybrid coatings for diverse applications. Front. Coatings Dyes Interface Eng. 2024, 2, 1386920. [Google Scholar] [CrossRef]

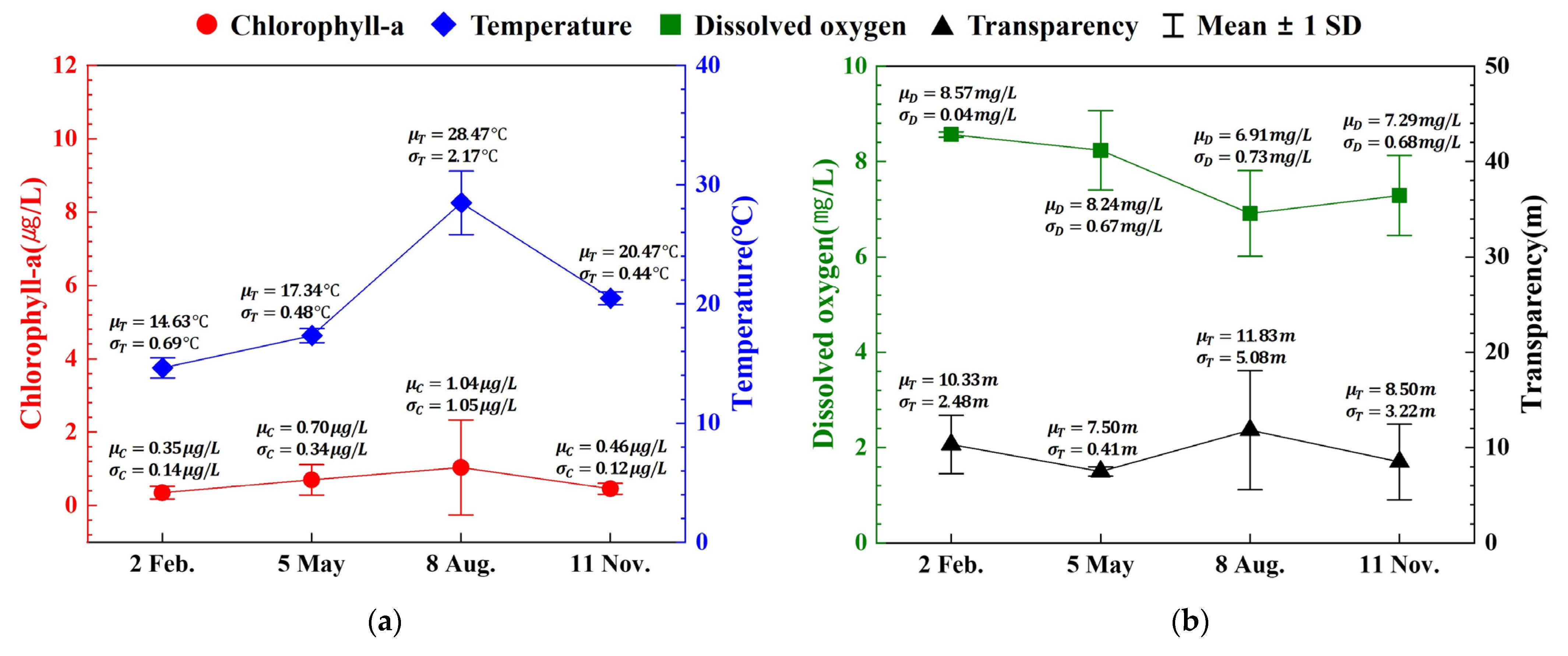
| Month | Depth (m) | Temperature (°C) | Salinity (‰) | Dissolved Oxygen (mg/L) | Total Nitrogen (μg/L) | Total Phosphorus (μg/L) | Chlorophyll a (μg/L) | Transparency (m) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sur. * | Bot. ** | Sur. | Bot. | Sur. | Bot. | Sur. | Bot. | Sur. | Bot. | Sur. | Bot. | |||
| 2 February | 27.33 | 14.63 | 14.57 | 34.38 | 34.40 | 8.57 | 8.58 | 261.30 | 164.13 | 20.10 | 18.30 | 0.35 | 0.32 | 10.33 |
| 5 May | 21.00 | 17.34 | 17.05 | 34.24 | 34.27 | 8.24 | 8.14 | 123.93 | 100.80 | 13.70 | 14.47 | 0.70 | 0.61 | 7.50 |
| 8 August | 26.33 | 28.47 | 24.29 | 29.67 | 32.44 | 6.91 | 6.64 | 167.17 | 129.57 | 11.43 | 9.63 | 1.04 | 0.35 | 11.83 |
| 11 November | 21.10 | 20.47 | 20.39 | 34.18 | 34.19 | 7.29 | 7.30 | 113.03 | 141.53 | 16.97 | 16.87 | 0.46 | 0.55 | 8.50 |
| Sample | Category | Functionality | Substrate | Thickness (μm) |
|---|---|---|---|---|
| #01 | Self-polishing copolymer (SPC)—A | Antifouling (self-polishing) | PVC | 100 |
| #02 | Self-polishing copolymer (SPC)—B | |||
| #03 | Non-antifouling coating | Baseline (no antifouling) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Jung, H.; Chae, Y.S.; Kim, H.M.; Lim, J.-S.; Kim, H.-J.; Lee, S.H. Long-Term Field Monitoring of Biofouling Characteristics in the Yellow Sea off Jeju Island, South Korea. J. Mar. Sci. Eng. 2025, 13, 1877. https://doi.org/10.3390/jmse13101877
Kim JH, Jung H, Chae YS, Kim HM, Lim J-S, Kim H-J, Lee SH. Long-Term Field Monitoring of Biofouling Characteristics in the Yellow Sea off Jeju Island, South Korea. Journal of Marine Science and Engineering. 2025; 13(10):1877. https://doi.org/10.3390/jmse13101877
Chicago/Turabian StyleKim, Ji Hyung, Hoon Jung, Yoon Seok Chae, Ho Min Kim, Jin-Seok Lim, Hae-Jong Kim, and Sung Hoon Lee. 2025. "Long-Term Field Monitoring of Biofouling Characteristics in the Yellow Sea off Jeju Island, South Korea" Journal of Marine Science and Engineering 13, no. 10: 1877. https://doi.org/10.3390/jmse13101877
APA StyleKim, J. H., Jung, H., Chae, Y. S., Kim, H. M., Lim, J.-S., Kim, H.-J., & Lee, S. H. (2025). Long-Term Field Monitoring of Biofouling Characteristics in the Yellow Sea off Jeju Island, South Korea. Journal of Marine Science and Engineering, 13(10), 1877. https://doi.org/10.3390/jmse13101877






