Abstract
The alien Red Sea goatfish, Parupeneus forsskali (Fourmanoir & Guézé, 1976), is a Lessepsian migrant, entering the Mediterranean through the Suez Canal. This study explores its seasonal diet and biological traits in Cyprus, its non-native habitat. From August 2019 to July 2021, a total of 249 specimens were collected, ranging in total length (TL) from 5.8 to 27.7 cm, with a mean TL of 15.1 ± 4.54 cm (SD). These specimens were examined with respect to sex and season. Through various methods (F%, N%, W%, and IRI), the feeding habits of the species were analysed, revealing a diet dominated by Crustacea (mainly Decapoda) and Polychaeta, with the secondary consumption of Mollusca, Echinodermata, and others. Significant differences were found between males and juveniles and females and juveniles, with adults preferring Decapoda and juveniles Copepoda, while seasonal variations were mainly influenced by prey availability. The Red Sea goatfish exhibited ontogenetic niche shifts in its depth and habitat distribution, influenced by size and age. The trophic level (TROPH) of P. forsskali ranged from 3.22 to 3.46, corresponding to an omnivorous diet with a preference for animals. These findings suggest that P. forsskali is an opportunistic predator with a diverse diet, thriving in its new habitat and potentially impacting the local marine food webs by competing with economically important native species for resources.
1. Introduction
The intense human use of available resources has severely impacted biodiversity, increasing the extinction rate enough to characterise the Anthropocene as “the sixth mass extinction” [1]. The Mediterranean basin, in particular, faces negative consequences from a plethora of drivers such as climate change, land and sea use, overfishing, and the introduction of alien species [2]. These alien species, often transported via human-mediated pathways such as shipping, aquaculture, and corridors pose significant threats to native ecosystems by altering community dynamics, modifying habitat structures, and spreading diseases. Once established, controlling their spread becomes challenging, and their impacts are typically irreversible [3], especially in the marine environment. A pertinent example is the Suez Canal, which serves as a major pathway for introducing alien species into the Mediterranean, contributing to a decline in the native marine biodiversity. This influx has led to 183 fish species entering the Levantine, making it one of the most invaded basins in the world [4].
Despite functional redundancy, which may allow ecosystems to maintain some level of stability, important native species are still faced with the risk of extinction [5]. This risk is particularly evident in cases when native species appear to be outcompeted or displaced by alien counterparts. Such displacements not only threaten individual species, but also signify a broader, human-induced transformation of marine environments [3]. For instance, Mullus barbatus and M. surmuletus, two indigenous goatfish species in the Mediterranean, are potentially threatened by the presence of three analogous alien species: Upeneus moluccensis, U. pori, and Parupeneus forsskali [6]. These alien species may compete with native goatfishes for resources, among others, exacerbating the risk for the indigenous populations.
One such species of concern is the Red Sea goatfish, P. forsskali (Fourmanoir & Guézé, 1976). Native to the Indo-Pacific Ocean and endemic in the Red Sea and the Gulf of Aden, it represents an example of a Lessepsian migrant [7,8]. Despite being a recent addition to the Mediterranean fauna, first documented in 2004 in the Gulf of Mersin, Turkey [9], the species has rapidly spread and established in the eastern Mediterranean [7,10,11,12], underscoring its ecological significance and potential impact on native ecosystems. In Cyprus, P. forsskali was first recorded in 2014 [6]. Since then, it has become economically important, emerging as one of the most abundant species of the Mullidae family in the area [13]. Currently, there is limited research on the biology of this species, both in its native and non-native habitats.
Here, we attempt to record and evaluate the seasonal feeding habits of P. forsskali from populations from Cyprus through stomach content analysis. We further investigate their trophic level and possible diet changes due to different ontogenetic and sexual characteristics. By addressing these objectives, we aim to enhance our understanding of the ecological implications of P. forsskali as an alien species and its potential interactions with native fauna. Moreover, investigating the feeding habits of alien species contributes to broader knowledge of alien species dynamics in the Mediterranean region and can serve as a crucial indicator of ecosystem disturbances, providing pivotal insights for the development of effective management strategies to mitigate potential impacts.
2. Materials and Methods
2.1. Sample Collection
A total of 249 specimens of Parupeneus forsskali were randomly collected each season between August 2019 and July 2021 by local fishers using fishing nets (36–40 mm mesh size) at depths from 1 to 30 m (Figure 1). The sampling sites exhibited a mixed bottom composition of soft and hard substrates. To address the lack of smaller individuals and ensure that fish of all sizes were examined, additional sampling was conducted in July 2021 with hand nets of a smaller mesh size.
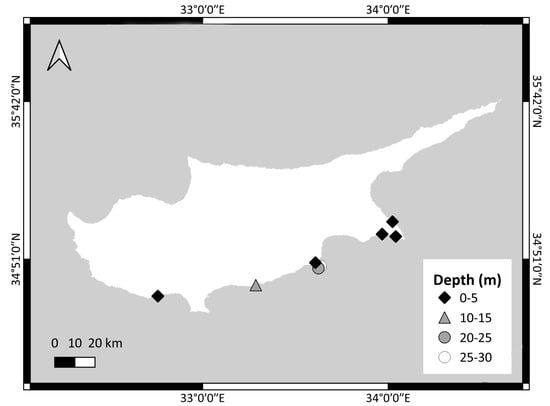
Figure 1.
Sampling stations of collected specimens of P. forsskali using fishing nets (36–40 mm mesh size) between August 2019 and July 2021.
Upon the completion of each capture, the specimens were retained and frozen. In the laboratory, the fish were defrosted overnight, blotted dry with absorbent paper towels, measured to the nearest 0.01 cm (total length, TL), and weighted to the nearest 0.001 g (total weight, TW). Next, each specimen was dissected, and the stomach and gonads were excised and weighed (±0.001 g). The stomach contents were examined, and all the prey items were identified to the lowest possible taxonomic level, counted, and weighed (±0.001 g). The gonads were examined macroscopically, and data on the sex and maturity status of each individual were collected based on the developmental stages described by Nikoslky [14]. Sex was further confirmed macroscopically based on the morphological characteristics of the gonads, as outlined by Follesa and Carbonara [15].
2.2. Reproduction
To assess the reproductive condition of P. forsskali, the gonadosomatic index (GSI) of the male and female individuals was calculated as the ratio of the gonad weight (GW, g) to the whole-body weight (BW, g) [16] following the equation:
2.3. Dietary and Trophic Level Analysis
Data obtained from the stomach content analysis were used to calculate the vacuity index (VI) based on the percentage ratio of the number of empty stomachs to the total number of stomachs [17]. The stomach fullness degree (SFD) was also estimated as the sum of the weights of all the prey in a stomach (g) divided by the total length (TL) of the fish (cm) [18].
To determine the feeding habits of P. forsskali, three indices were assessed, as described by Hyslop [19]: (1) the frequency of occurrence (%F), (2) the numerical abundance (%N), and (3) the gravimetric composition (%W). The frequency of occurrence (%F) of each prey item was calculated as the proportion of all non-empty stomachs containing each prey item. Numerical abundance (%N) was expressed as the percentage of the number of each prey item in all non-empty stomachs divided by the total number of food items in all stomachs. The gravimetric composition (%W) was obtained from the ratio of the total weight of each prey item to the sum weight of all prey items. To estimate the importance of each prey item in the stomachs of P. forsskali, these three metrics were used to calculate the index of relative importance (IRI) [19]:
The trophic levels (TROPHs) of males, females, adults (males and females combined), juveniles, and the total sample and its standard error (SE) were estimated from the quantitative diet composition data, using the gravimetric composition (%W) and the trophic level of each prey item. The TROPH was calculated with the TrophLab software [20] (it can be downloaded at https://www.fishbase.org.au/Download/index.htm, accessed on 30 April 2024). The calculation follows the equation:
where TROPHi is the species (i) trophic level, TROPHj is the fractional trophic level of prey j, DCij is the contribution of prey j to the diet of species (i), and G is the total number of prey items. TROPH values classify species as (a) pure herbivores and detritivores (2.0–2.1), (b) omnivores with a preference for plants (2.1–2.9), (c) omnivores with a preference for animals (2.9–3.7), (d) carnivores with a preference for decapods, cephalopods, and fish (3.7–4.0), (e) carnivores with a preference for fish and cephalopods (4.0–4.5), and (f) top carnivores/piscivorous organisms and specialised predators (4.5–5.0) [21,22].
2.4. Statistical Analysis
Univariate statistical analysis was performed using the open-source RStudio programme (version 4.0.2) [23]. Statistical significance was set at 0.05. The normality of residuals and homogeneity of variance were examined using a Shapiro–Wilk test [24] and a Levene’s test [25], respectively. A chi-square test was applied to estimate the sex ratio between males and females. Spearman’s rank correlation was used to test the association between SFD and TL. Differences in the SFD among males, females, and juveniles were analysed using the non-parametric Kruskal–Wallis test by post hoc pairwise comparisons with Bonferroni correction.
Multivariate statistical analysis was performed using the PRIMERv7 statistical software [26]. A similarity matrix was constructed employing the Bray–Curtis coefficient, using the presence/absence transformation on non-empty stomachs. A one-way analysis of similarities (ANOSIM) was performed on the similarity matrix to test whether the dietary samples of different sexes (males, females, and juveniles) differed significantly. The same analyses were repeated to test for statistical differences in the diets of species between seasons (summer, autumn, winter, and spring). Additionally, a similarity of percentages (SIMPER) test was used on the transformed data to identify the specific prey items contributing to any observed variations between sexes and seasons, respectively. Prey items were categorised into 23 groups including Tanaidacea, Ostracoda, Isopoda, Amphipoda, Euphausiacea, Mysida, Copepoda, Cumacea, Pleocyemata, Dendrobranchiata, Anomura, Brachyura, Gastropoda, Bivalvia, Polyplacophora, Echinoidea, Ophiuroidea, Polychaeta, Oligochaeta, Sipuncula, Actinopterygii, Plantae, and eggs.
3. Results
3.1. Morphometrics
A total of 249 individuals were collected, 121 males (48.6%) and 56 females (22.5%) with a sex ratio of 2.16:1 male to female (χ2 = 23.87, p < 0.01), and 72 juveniles (28.9%), with a mean TL of 15.1 ± 4.54 cm (SD). The juveniles were assessed as unsexed individuals with non-visible gonads following Saemundsson et al. [27]. The TL, wet weight, and gonad weights of the male, female, and juvenile P. forsskali are shown in Table 1.

Table 1.
Mean, standard deviation (SD), minimum, max values of total length (TL), wet weight and gonad weight (GW), and stomach fullness degree (SFD) of male, female, and juvenile Parupeneus forsskali, collected in the coastal water of Cyprus between August 2019 and July 2021.
3.2. Reproduction
The GSI values of Parupeneus forsskali ranged from 0.01 to 3.93 in May 2020 and August 2019, respectively. The minimum and maximum GSI values of the male P. forsskali were found in May 2020 at 0.01 and 1.04, respectively, whilst for females, the lowest values were recorded in December 2019 at 0.02 and the highest in August 2019 at 3.92. The highest average GSI values for both sexes appeared in August 2019, followed by a decline during the autumn and winter months, whilst in May 2020, the GSI increased again (Figure 2).
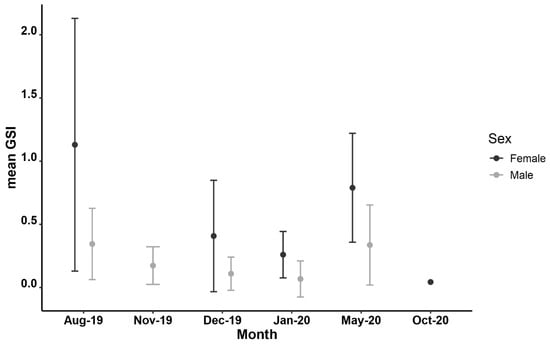
Figure 2.
Mean gonadosomatic index (GSI) of female (dark grey, n = 56) and male (light grey, n = 121) P. forsskali, collected in the coastal waters of Cyprus between August 2019 and October 2020. Error bars represent standard deviation (SD).
3.3. Diet Analysis
Out of the 249 stomachs examined, 49 were empty (19.68% of the total stomachs), including those of 31 males (25.62% of males), 16 females (28.57% of females), and 2 juveniles (2.78% of juveniles). The percentage of the VI of P. forsskali varied according to sex and season. The highest values of the vacuity index (VI) were recorded during winter across all sexes (males: 37.31%, females: 39.29%, juveniles: 100%). Following winter, summer recorded the next highest values for the males and females with 37.50% and 23.81%, respectively. The lowest values were detected during spring and autumn. During spring, the VI was at 0% across all sexes, while in autumn, it remained at 0% for males and females and 1.49% for juveniles (Figure 3).
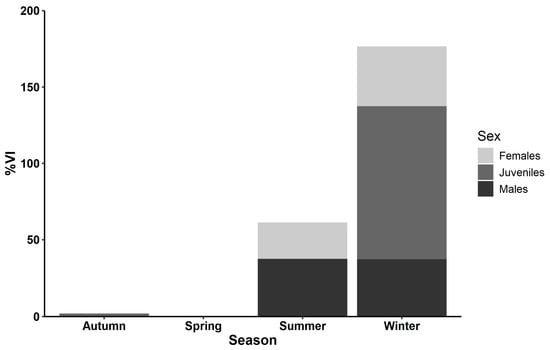
Figure 3.
Seasonal variation in the vacuity index percentage (VI%) of male (black), female (light grey), and juvenile (dark grey) P. forsskali collected between August 2019 and July 2021.
The stomach fullness degree (SFD) ranged between 0 and 0.075 (mean = 0.006, SD = 0.009). Table 1 displays the minimum, maximum, and average values of the SFD index for males, females, and juveniles of P. forsskali. Spearman’s rank correlation coefficient indicated a significant negative correlation between the SFD and TL (rho = −0.16, p = 0.013). The SFD values varied across sexes (Figure 4; Kruskal–Wallis H (2) = 9.46, p = 0.009). Significant differences in the SFD among sexes were observed, with pairwise comparisons indicating significant differences between females and juveniles (p = 0.003) and between males and juveniles (p = 0.021), while no significant difference was found between females and males (p = 0.063).
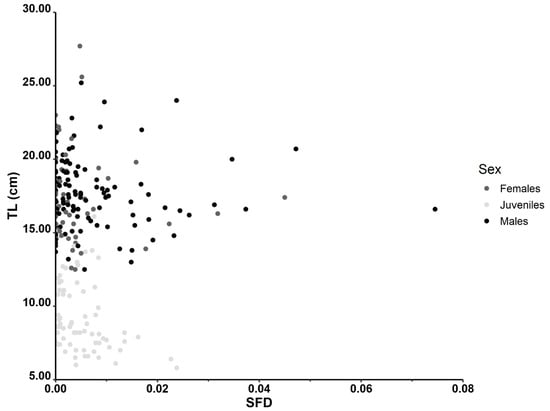
Figure 4.
Stomach fullness degree (SFD) of males, females, and juvenile individuals of P. forsskali.
The stomach content analysis revealed 5323 prey items belonging to 47 food categories from 6 Phyla (Arthropoda, Mollusca, Echinodermata, Annelida, Sipuncula, and Chordata), with an overall weight of 21.23 g (Supplementary Materials S1). Foraminifera, sand, sediment, and plastic particles were also present in the stomachs of P. forsskali and ranked as accidental prey. Nematodes were also present in the stomachs, considered as parasites.
Crustacea were the most important prey items for the total examined stomachs (IRI = 12,698.75) of males (IRI = 14,219.76), females (IRI = 12,237.12), and juveniles (IRI = 10,349.73), followed by Annelida (IRItotal = 3624.81, IRImales = 2006.45, IRIfemales = 1874.15, IRIjuveniles = 6879.15) (Figure 5, Supplementary Materials S1). In terms of abundance (%N), weight (%W), and frequency (%F), Crustacea dominated the composition of the total food items in all the examined stomachs, accounting for 61.83%, 69.77%, and 96.50%, respectively. Crustacea were also the predominant prey items for all sex categories, constituting 94.44% of male diets, 95% of female dietsm and 100% of juvenile diets, as indicated by their respective percentage frequencies (%F). In terms of abundance (%N) and weight (%W), Crustacea accounted for 74.75% and 75.81% of male stomach contents, 65.33% and 63.48% of female stomach contents, and 54.26% and 49.24% of juvenile stomach contents, respectively (Figure 5).
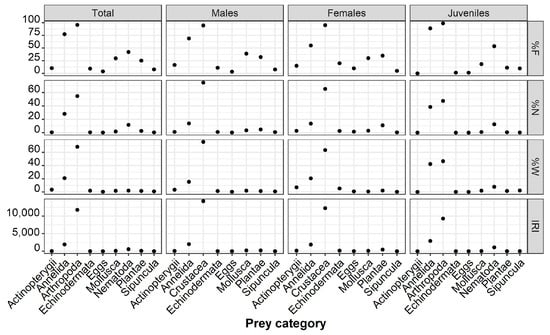
Figure 5.
Frequency of occurrence (F%), numerical abundance (N%), gravimetric composition (W%), and index of relative importance (IRI) of the major prey categories of total individuals, male, female and juvenile P. forsskali.
Crustacea were represented by the orders Tanaidacea, Ostracoda, Amphipoda, Euphausiacea, Mysida, Copepoda, Cumacea, and Decapoda, along with the suborders Pleocyemata and Dendrobranchiata (shrimps) and the infraorders Anomura and Brachyura. Among Crustacea, the dominant category in terms of importance was Decapoda for males and females (IRImales = 4614.58, IRIfemales = 4394.89) and Copepoda for juveniles (IRI = 3733.40, Supplementary Materials S1). In the diets of both males and females, Tanaidacea, Cumacea, and Oligochaeta were absent, whilst in the diet of juveniles, the latter prey item was especially pronounced (IRIOligochaeta = 1098.27). Euphausiacea were not found in the stomachs of the examined males and juveniles and Polyplacophora were absent from the diets of females and juveniles, whereas Echinoidea and Actinopterygii were absent from the stomachs of juveniles.
Similar results were revealed when the diets of the species were compared regarding different seasons, showing a predominant consumption of Crustacea and Annelida (mainly Polychaeta) (Figure 6, Supplementary Materials S2). Crustacea showed a consistent prevalence throughout the year, ranging from an 89.83% to 100% occurrence (%F). However, their contribution to stomach contents (%W and %N) varied, peaking in spring (71.68% and 53.00%, respectively) and autumn (62.95% and 63.84%, respectively). Other taxa such as Isopoda and Amphipoda showed fluctuations in their prevalence, with varying percentages across seasons (Figure 6, Supplementary Materials S2).
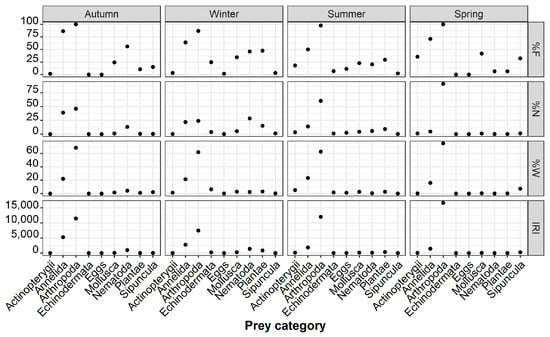
Figure 6.
Seasonal changes in the frequency of occurrence (F%), numerical abundance (N%), gravimetric composition (W%), and index of relative importance (IRI) of the major prey categories of P. forsskali.
Additionally, some taxa exhibited sporadic occurrences across seasons. Specifically, Tanaidacea, Cumacea, and Oligochaeta appeared only in autumn, while Euphausiacea were exclusively observed in winter. Polyplacophora were exclusively recorded during summer. In spring, Mysida and Cumacea were absent from the diet of P. forsskali. Echinoidea were present in both winter and summer, whereas Ophiuroidea and eggs were absent during autumn (Supplementary Materials S2).
Regarding the diets of sexes, ANOSIM revealed significant differences in the diet composition between sexes. Pairwise comparisons revealed a significant dissimilarity between the diets of females and juveniles (R = 0.229, p = 0.0001) and between juveniles and males (R = 0.051, p = 0.018). However, there were no significant differences observed between the female and male dietary profiles (R = 0.027, p = 0.155).
When examining the differences between sexes, the SIMPER analysis revealed that females exhibited an average similarity of 13.71%, juveniles showed an average similarity of 35.80%, and males exhibited an average similarity of 18.87%. Comparing dissimilarities between pairs of sexes, females and juveniles displayed an average dissimilarity of 82.45%, with notable contributors such as Copepoda (14.61%), Polychaeta (12.29%), and Isopoda (11.72%). Females and males displayed an average dissimilarity of 83.40%, mainly driven by Polychaeta (13.76%), Brachyura (11.96%), and Isopoda (10.23%). Juveniles and males showed an average dissimilarity of 79.11%, featuring substantial contributions from Copepoda (14.07%), Polychaeta (11.90%), and Isopoda (11.50%).
ANOSIM also showed that the diet of P. forsskali differed across seasons (R = 0.083, p = 0.001). Pairwise comparisons highlighted significant dissimilarities between spring and autumn (R = 0.194, p = 0.0001), winter and autumn (R = 0.134, p = 0.0001), and autumn and summer (R = 0.222, p = 0.0001). No significant differences in the diets of the species were recorded between spring and winter (R = −0.039, p = 0.811), spring and summer (R = −0.007, p = 0.509), and winter and summer (R = 0.002, p = 0. 399).
The SIMPER analysis showed that spring exhibited an average similarity of 66.26%, winter showed an average similarity of 12.29%, autumn had an average similarity of 37.78%, and summer exhibited an average similarity of 17.48%. SIMPER also revealed an average dissimilarity of 82.27% between spring and winter, while spring and autumn exhibited a 65.63% dissimilarity. Comparatively, winter and autumn displayed an average dissimilarity of 84.99% and spring and summer showed 71.02%. The dissimilarity between winter and summer was 86.82%, and between autumn and summer was 80.24%.
The key contributors to dissimilarities varied among pairs of seasons. The dissimilarity between spring and winter was influenced by Amphipoda (18.49%), Brachyura (17.40%), and Isopoda (12.24%). Spring and autumn’s dissimilarity was driven by Brachyura (12.53%), Amphipoda (12.50%), and Copepoda (10.86%). Similarly, winter and autumn’s dissimilarity was attributed to Copepoda (17.27%), Polychaeta (12.58%), and Isopoda (8.02%). The dissimilarity between spring and summer was primarily influenced by Brachyura (16.17%), Amphipoda (15.81%), and Polychaeta (11.420%), whilst winter and summer’s dissimilarity was driven by Polychaeta (14.57%), Isopoda (14.02%), and Plantae (8.96%). Lastly, the dissimilarity between autumn and summer was influenced by Copepoda (15.77%), Polychaeta (11.68%), and Isopoda (9.25%).
3.4. Trophic Level
The trophic level of P. forsskali was estimated at 3.22 (SE = 0.37) for juveniles, 3.44 (SE = 0.48) for males, and 3.42 (SE = 0.47) for females. When considering adult individuals (males and females combined), the average trophic level (TROPH) was 3.46 (SE = 0.48), while the overall trophic level for the combined individuals was 3.41 (SE = 0.37).
4. Discussion
The present study analysed the feeding habits of Parupeneus forsskali with respect to sex and season from populations in Cyprus. The gonadosomatic index of P. forsskali, showed seasonal variability; an increase in the GSI was observed in May, indicative of gonad maturation. The peak of the index was observed in summer, suggesting spawning, whilst the lowest values of the index were recorded in autumn and winter. Additionally, the presence of juveniles (unsexed individuals) during autumn, coupled with the low GSI values of the male and female specimens during the same period, support our observations that reproduction occurred in the previous months. Our findings are consistent with the results of Farrag et al. [28] and Heneish et al. [29], who examined specimens of P. forsskali in the Egyptian Red Sea and reported that spawning takes place in summer. Similar results have also been documented through histological analyses of P. forsskali collected in Cyprus, revealing that the most advanced maturation stages are found in summer and spawning occurs from May until August, with a peak in July, which possibly extends until September [27].
The stomach fullness degree (SFD) showed that the feeding intensity of P. forsskali was affected by size and decreased with an increase in length. This can be attributed to the fact that juveniles, in the first stages of their development, have greater energetic demands than adults, thus, they need more intense feeding activity to rapidly increase their size in order to reduce predation and increase their survival ability [30,31]. According to our results and those of other studies [27], this species matures in relatively small sizes, supporting the hypothesis of a rapid growth rate, which can promote the maturation of P. forsskali at smaller sizes [30]. As is mentioned in Saemundsson et al. [27], the size at maturity for this species was estimated at 14.2 cm for males and 11.8 cm for females, which shows that they are able to mature in their first year of life. Early maturation is one of the key characteristics of alien species, benefiting their successful introduction to and establishment in non-native regions [32,33].
The highest percentages of VI were recorded during winter and summer (37.11% and 20% of the total stomachs, respectively). These proportions comprised almost entirely adult individuals, mainly males, whilst the majority of juveniles contained prey in their stomachs, indicating again that intensive feeding is essential for growth during these early life stages. This decrease in feeding intensity and the subsequent increase in VI observed in adults may have been the result of variations in fish abundance, temperature, spawning time, or food availability [18,34,35]. Conversely, the increased energy requirements associated with gonad development prior to spawning can explain the low VI values observed in autumn and spring [36].
In this study, the diet of P. forsskali revealed a total of 47 food categories, consisting mainly of Crustacea and Annelida. Secondary prey taxa included Mollusca, Echinodermata, Sipuncula, Actinopterygii, Plantae, and eggs. A preliminary examination of the main prey items consumed by P. forsskali in Cyprus was conducted by Evagelopoulos et al. [13], suggesting that the species mainly consumes Crustacea, followed by other invertebrates, Actinopterygii, and Foraminifera. Our results differed, to some extent, from those of Evagelopoulos et al. [13], who studied 73 individuals of P. forsskali during summer. While they classified Foraminifera as prey items, in our analysis, Foraminifera were classified as accidental prey, given their abundance in sediment and the species’ primary predatory behaviour of substrate borrowing and prey capture [37]. Notably, upon examination of the Foraminifera within the stomach of P. forsskali, no signs of predatory activities such as mechanical or chemical damage were observed [38]. Moreover, our study revealed abundant nematode parasites in the stomachs of P. forsskali.
Furthermore, a recent study examining the diet of P. forsskali in Greece [39] found a preference for Decapoda and Amphipoda, followed by Ostracoda, Anomura, and Isopoda, while fish remnants, Polychaeta, and Mollusca considered occasional or accidental prey types. Our study suggests some differentiation in diet compared to the findings from Greece, with Polychaeta playing a major role in the diet of the species alongside various Crustacea. Similar feeding habits with P. forsskali were also reported for other Mullidae, including Mullus barbatus, M. surmuletus, Upeneus asymmetricus, and U. moluccensis, with Crustacea and/or Annelida (Polychaeta) being the most common prey items [40,41,42,43,44].
Our study indicated that there were significant differences in the dietary patterns between males and juveniles, as well as between females and juveniles. However, the low R-statistic values of ANOSIM suggested that the actual dissimilarity in the diet composition between these groups was relatively minor. This observation is likely explained by the broad diet of P. forsskali, resulting in some overlap in dietary preferences.
However, the IRI showed that adults (males and females) seemed to prefer Decapoda, whilst juveniles mainly consumed Copepoda. Also, Polyplacophora, Echinoidea, and Actinopterygii were absent from the diet of juveniles. These differences can be explained by dissimilarities in the mouth characteristics between smaller and larger individuals. According to Karachle and Stergiou [45], mouth gape is one of the most important and limiting factors affecting food consumption, determining the size and range of prey, influencing the efficiency of prey capture and consumption. As proposed by Uiblein [46], our findings further support that P. forsskali exhibits ontogenetic changes in substrate preference, with juveniles mainly occupying sandy substrates and shifting to rocky substrates when they reach adulthood. This ontogenetic habitat shift is further validated by sandy and muddy substrates being the preferred habitat by all prey items. Additionally, juveniles seemed to be mainly found in the first 15 m of the water column, whilst adult individuals could be found at an even greater depth (authors’ personal observations). Therefore, in addition to the changes in the habitat use of P. forsskali, there is also a potential shift in depth. These ontogenetic changes in habitat and depth can also be related to changes in the feeding behaviour of adult and juvenile individuals, reflecting the resource availability in each habitat. These changes in dietary preferences during different ontogenetic stages may further support the greater ability of this species to expand its distribution.
A seasonal change in dietary composition was also found for P. forsskali. An increased consumption of Crustacea and Annelida was recorded at all seasons, however, there was a difference in the order of Crustacea that was preferred more at each season. In summer, there was an increase in the consumption of Isopoda and Amphipoda in terms of importance, Copepoda and Decapoda in autumn, and Isopoda and Decapoda in spring and winter. These findings suggest that the observed seasonal patterns in the diet of P. forsskali were influenced by factors such as prey availability during different seasons.
The trophic level values of P. forsskali showed that this species is omnivorous with a preference for animals (3.22 < TROPH < 3.46, total = 3.41), consuming a wide variety of prey. In Greece, in the Aegean Sea, a study by Vagenas et al. [39] revealed slightly higher trophic values (TROPH = 3.58 ± 0.53 SD). However, their study was based on a relatively low number of individuals. The relative Mullidae species were consistent with our results, yielding similar trophic level values in the Mediterranean (Mullus barbatus: 2.79 < TROPH < 3.57, M. surmulettus: 3.16 < TROPH < 3.58, Upeneus moluccensis: 3.40 < TROPH < 3.89, U. pori: TROPH = 3.53; [22]), suggesting potential competition among species sharing the same habitat. Although it is known that stomach content analyses rely on samples that represent snapshots in time [47], in the current study, this was subdued by collecting seasonal specimens.
In conclusion, the generalist feeding behaviour of P. forsskali is an important trait of this alien species, allowing them to thrive in non-native habitats and exploit the available resources in their newly introduced areas [48,49]. It is also noteworthy that our study showed that the species consumes both native species (e.g., Echinodermata: Echinocyamus pusillus, Amphiura chiajei, Decapoda: Galathea squamifera, G. intermedia, Mysida: Gastrosaccus spinifer) and alien species (e.g., Decapoda: Thalamita poissonii). Therefore, the species seems to be able to adjust its foraging behaviour to maximize its energy intake, based on changes in prey availability in its non-native habitat, appearing as an opportunistic predator [50]. This broad dietary range and opportunistic ability of P. forsskali could possibly be some of the most important factors that favour the introduction and expansion of the species in the Mediterranean. This is also supported by Tsadok et al. [50], who used stable isotopes to compare the dietary compositions of Lessepsian fish migrants in the Eastern Mediterranean Sea, including P. forsskali, with their original Red Sea populations. The authors found significant differences in the carbon isotopes in the food consumed by the same species across various environments and suggested that Lessepsian fishes are capable of adjusting their nutritional habits, even if they differ from their original diet, and can adapt more effectively to their new environment [50].
Until now, there have been no records to prove that P. forsskali threatens the biodiversity of Cyprus. However, further spreading and increases in its population could negatively impact the local biodiversity, reducing indigenous populations, mainly Crustacea and other common prey, disrupting the local food ecosystems and competing with economically important native species for resources. A similar opinion was adopted by Evagelopoulos et al. [13], who reported that P. forsskali compete for resources and space with the native Mullus barbatus, with the former displacing the latter, as shown by recent artisanal fisheries’ catches in Cyprus, already indicating the negative effects of the introduction of P. forsskali on the local economy and biodiversity, since M. barbatus is a species of high economic importance. However additional research is needed to prove this statement.
Future studies are required to further examine the biology and ecology of the species, given the challenges in predicting its long-term effects on non-native ecosystems. Questions regarding the species’ population dynamics and their effect on local food webs remain crucial. Furthermore, coordination at the local, national, and international levels is essential in order to address the conservation challenged posed by this and other alien species [51]. The present study could set the baseline for future studies on the potential impact of P. forsskali in Cyprus. Such information is not only a basic requirement for understanding the community ecology and structure of food webs, but also serves as a valuable tool to inform and facilitate sustainable fisheries’ management, thereby mitigating the socioeconomic and ecological impacts of established alien species populations [52].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse12071122/s1. The supplementary material regards detailed prey categories found in each individual per sex (Supplementary Material S1) and season (Supplementary Material S2).
Author Contributions
Conceptualization, C.M. and N.C.; methodology, C.M. and N.C.; formal analysis, C.M.; writing—original draft, C.M.; writing—review and editing, all authors; supervision, N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the findings of this study are available upon request. Researchers interested in accessing the data may contact Christina Michail at cmichail@merresearch.com to request access.
Acknowledgments
All authors wish to thank the anonymous reviewers for their essential comments on the manuscript. Special thanks to the fisher Panicos Eleftheriou for his invaluable assistance in providing the smaller individuals, which greatly contributed to the completion of this study and Latchi Fishmarket for the valuable help with samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the Curve of Global Freshwater Biodiversity Loss: An Emergency Recovery Plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Cherif, S.; Doblas-Miranda, E.; Lionello, P.; Borrego, C.; Giorgi, F.; Inglesias, A.; Jebari, S.; Mahmoudi, E.; Moriondo, M.; Pringault, O.; et al. Drivers of Change. In Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future. First Mediterranean Assessment Report; Cramer, W., Guiot, J., Marini, K., Eds.; Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; pp. 59–180. [Google Scholar]
- Galil, B.S. A Sea, a Canal, a Disaster: The Suez Canal and the Transformation of the Mediterranean Biota. In The Suez Canal: Past Lessons and Future Challenges; Springer: Cham, Switzerland, 2023; pp. 199–214. [Google Scholar] [CrossRef]
- Solanou, M.; Valavanis, V.D.; Karachle, P.K.; Giannoulaki, M. Looking at the Expansion of Three Demersal Lessepsian Fish Immigrants in the Greek Seas: What Can We Get from Spatial Distribution Modeling? Diversity 2023, 15, 776. [Google Scholar] [CrossRef]
- Biggs, C.R.; Yeager, L.A.; Bolser, D.G.; Bonsell, C.; Dichiera, A.M.; Hou, Z.; Keyser, S.R.; Khursigara, A.J.; Lu, K.; Muth, A.F.; et al. Does Functional Redundancy Affect Ecological Stability and Resilience? A Review and Meta-Analysis. Ecosphere 2020, 11, e03184. [Google Scholar] [CrossRef]
- Chartosia, N.; Michailidis, N. First Confirmed Presence of the Red Sea Goatfish Parupeneus forsskali (Fourmanoir & Guézé, 1976) from Cyprus. Mar. Biodivers. Rec. 2016, 9, 1–4. [Google Scholar] [CrossRef]
- Bariche, M.; Bilecenoglu, M.; Azzurro, E. Confirmed Presence of the Red Sea Goatfish Parupeneus forsskali (Fourmanoir & Guézé, 1976) in the Mediterranean Sea. Bioinvasions. Rec. 2013, 2, 173–175. [Google Scholar] [CrossRef]
- Mehanna, S.F.; Mahmoud, U.M.; Hassanien, E.M. First Occurrence of the Red Sea Goatfish, Parupeneus forsskali (Fourmanoir Guz, 1976) in the Coastal Waters of Egyptian Mediterranean Sea. Int. J. Fish. Aquac. 2016, 8, 94–97. [Google Scholar] [CrossRef][Green Version]
- Çinar, M.E.; Bilecenoglu, M.; Öztürk, B.; Can, A. New Records of Alien Species on the Levantine Coast of Turkey. Aquat. Invasions 2006, 1, 84–90. [Google Scholar] [CrossRef]
- Sonin, O.; Salameh, P.; Edelist, D.; Golani, D. First Record of the Red Sea Goatfish, Parupeneus forsskali (Perciformes: Mullidae) from the Mediterranean Coast of Israel. Mar. Biodivers. Rec. 2013, 6, e105. [Google Scholar] [CrossRef]
- Ali, M.; Diatta, Y.; Alkusairy, H.; Saad, A.; Capapé, C. First record of Red Sea Goatfish Parupeneus forsskali (Osteichthyes: Mullidae) from the Syrian coast (Eastern Mediterranean). J. Ichthyol. 2016, 56, 616–619. [Google Scholar] [CrossRef]
- Stamouli, C.; Akel, E.H.K.; Azzurro, E.; Bakiu, R.; Bas, A.A.; Bitar, G.; Boyaci, Y.; Cakalli, M.; Corsini-Foka, M.; Crocetta, F.; et al. New Mediterranean Biodiversity Records (December 2017). Mediterr. Mar. Sci. 2017, 18, 534–556. [Google Scholar] [CrossRef]
- Evagelopoulos, A.; Nikolaou, A.; Michailidis, N.; Kampouris, T.E.; Batjakas, I.E. Progress of the Dispersal of the Alien Goatfish Parupeneus forsskali (Fourmanoir & Guézé, 1976) in the Mediterranean, with Preliminary Information on Its Diet Composition in Cyprus. Bioinvasions. Rec. 2020, 9, 209–222. [Google Scholar] [CrossRef]
- Nikolsky, G.V. Ecology of Fishes; Academic Press Inc.: London, UK, 1963. [Google Scholar]
- Follesa, M.C.; Carbonara, P. Atlas of the Maturity Stages of Mediterranean Fishery Resources. In Studies and Reviews No. 99; FAO: Rome, Italy, 2019; p. 268. [Google Scholar]
- Devlaming, V.; Grossman, G.; Chapman, F. On the Use of the Gonosomatic Index. Comp. Biochem. Physiol. A Physiol. 1982, 73, 31–39. [Google Scholar] [CrossRef]
- Molinero, A.; Flos, R. Influence of Season on the Feeding Habits of the Common Sole Solea Solea. Mar. Biol. 1992, 113, 499–507. [Google Scholar] [CrossRef]
- Bachiller, E.; Skaret, G.; Nøttestad, L.; Slotte, A. Feeding Ecology of Northeast Atlantic Mackerel, Norwegian Spring-Spawning Herring and Blue Whiting in the Norwegian Sea. PLoS ONE 2016, 11, e0149238. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, E.J. Stomach Contents Analysis—A Review of Methods and Their Application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Pauly, D.; Froese, R.; Sa-a, P.S.; Palomares, M.L.; Christensen, V.; Rius, J. TrophLab Manual; ICLARM: Manila, Philippines, 2000. [Google Scholar]
- Froese, R.; Pauly, D. World Wide Web Electronic Publication. FishBase. 2009. Available online: http://www.fishbase.org (accessed on 1 April 2024).
- Stergiou, K.I.; Karpouzi, V.S. Feeding Habits and Trophic Levels of Mediterranean Fish. Rev. Fish Biol. Fish. 2002, 11, 217–254. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 18 February 2024).
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Dytham, C. Choosing and Using Statistics A Biologist’s Guide, 3rd ed.; John Wiley & Sons: Chichister, UK, 2011. [Google Scholar]
- Clarke, K.R.; Gorley, R.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; Primer-E Ltd.: Plymouth, UK, 2014. [Google Scholar]
- Saemundsson, S.; Tsikliras, A.C.; Chartosia, N. Reproduction and Growth of the Red Sea Goatfish Parupeneus forsskali in Its New Environment (Cyprus, Eastern Mediterranean Sea). Sci. Mar. 2023, 87, e064. [Google Scholar] [CrossRef]
- Farrag, M.M.S.; Osman, A.G.M.; Mehanna, S.F.; Osman, Y.A.A. Fisheries Status of the Common Species of Family Mullidae in the Southern Red Sea, Hurghada, Egypt. Egypt. J. Aquat. Biol. Fish. 2018, 22, 249–265. [Google Scholar] [CrossRef]
- Heneish, R.A.; Sabrah, M.M.; El-Ganainy, A.A. Biological Aspects of Some Goatfish (Mullidae) from the Southern Egyptian Red Sea; Hurghada to Shalateen. Egypt. J. Aquat. Biol. Fish. 2019, 23, 91–101. [Google Scholar] [CrossRef]
- Hutchings, J.A. Adaptive Life Histories Effected by Age-Specific Survival and Growth Rate. Ecology 1993, 74, 673–684. [Google Scholar] [CrossRef]
- Daly, E.A.; Brodeur, R.D.; Weitkamp, L.A. Ontogenetic Shifts in Diets of Juvenile and Subadult Coho and Chinook Salmon in Coastal Marine Waters: Important for Marine Survival? Trans. Am. Fish. Soc. 2009, 138, 1420–1438. [Google Scholar] [CrossRef]
- Bøhn, T.; Sandlund, O.T.; Amundsen, P.A.; Primicerio, R. Rapidly Changing Life History during Invasion. Oikos 2004, 106, 138–150. [Google Scholar] [CrossRef]
- Grabowski, M.; Bacela, K.; Konopacka, A. How to Be an Invasive Gammarid (Amphipoda: Gammaroidea)—Comparison of Life History Traits. Hydrobiologia 2007, 590, 75–84. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Soofiani, N.M.; Keivany, Y.; Motlagh, S.A.T. Feeding Habits of the King Soldier Bream, Argyrops spinifer (Forsskål, 1775) (Perciformes: Sparidae), in the Northern Persian Gulf. J. Appl. Ichthyol. 2014, 30, 485–489. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of Temperature on Feeding and Digestive Processes in Fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Link, J.S.; Burnett, J. The Relationship between Stomach Contents and Maturity State for Major Northwest Atlantic Fishes: New Paradigms? J. Fish. Biol. 2001, 59, 783–794. [Google Scholar] [CrossRef]
- Gosline, W.A. Structure, Function, and Ecology in the Goatfishes (Family Mullidae). Pac. Sci. 1984, 38, 312–323. [Google Scholar]
- Culver, S.J.; Lipps, J.H. Predation on and by Foraminifera. In Predator—Prey Interactions in the Fossil Record; Springer: Boston, MA, USA, 2003. [Google Scholar] [CrossRef]
- Vagenas, G.; Dogrammatzi, A.; Kondylatos, G.; Karachle, P.K. On the Biology of the Alien Red Sea Goatfish, Parupeneus forsskali (Fourmanoir & Guézé, 1976) in the Aegean Sea, Eastern Mediterranean. Mar. Biol. Res. Res. 2023, 19, 564–573. [Google Scholar] [CrossRef]
- Esposito, V.; Andaloro, F.; Bianca, D.; Natalotto, A.; Romeo, T.; Scotti, G.; Castriota, L. Diet and Prey Selectivity of the Red Mullet, Mullus barbatus (Pisces: Mullidae), from the Southern Tyrrhenian Sea: The Role of the Surf Zone as a Feeding Ground. Mar. Biol. Res. 2014, 10, 167–178. [Google Scholar] [CrossRef]
- Golani, D.; Galil, B. Trophic Relationships of Colonizing and Indigenous Goatfishes (Mullidae) in the Eastern Mediterranean with Special Emphasis on Decapod Crustaceans. Hydrobiologia 1991, 218, 27–33. [Google Scholar] [CrossRef]
- Vassilopoulou, V.; Papaconstantinou, C.; Christides, G. Food Segregation of Sympatric Mullus barbatus and Mullus surmuletus in the Aegean Sea. Isr. J. Zool. 2001, 47, 201–211. [Google Scholar] [CrossRef]
- Chérif, M.; Ben Amor, M.M.; Selmi, S.; Gharbi, H.; Missaoui, H.; Capapé, C. Food and Feeding Habits of the Red Mullet, Mullus barbatus (Actinopterygii: Perciformes: Mullidae), off the Northern Tunisian Coast (Central Mediterranean). Acta Ichthyol. Piscat. 2011, 41, 109–116. [Google Scholar] [CrossRef]
- Mahmoud, H.H.; Fahim, R.M.; Srour, T.M.; El-Bermawi, N.; Ibrahim, M.A. Feeding Ecology of Mullus barbatus and Mullus surmuletus off the Egyptian Mediterranean Coast. Int. J. Fish. Aquat. Stud. 2017, 5, 321–325. [Google Scholar]
- Karachle, P.K.; Stergiou, K.I. Mouth Allometry and Feeding Habits of Some Mediterranean Fishes. Acta Ichthyol. Piscat. 2011, 41, 265–275. [Google Scholar] [CrossRef][Green Version]
- Uiblein, F. Goatfishes (Mullidae) as Indicators in Tropical and Temperate Coastal Habitat Monitoring and Management. Mar. Biol. Res. 2007, 3, 275–288. [Google Scholar] [CrossRef]
- Baker, R.; Buckland, A.; Sheaves, M. Fish Gut Content Analysis: Robust Measures of Diet Composition. Fish Fish. 2014, 15, 170–177. [Google Scholar] [CrossRef]
- Golani, D. Impact of Red Sea Fish Migrants through the Suez Canal on the Aquatic Environment of the Eastern Mediterranean. Bull. Ser. Yale Sch. For. Environ. Stud. 1998, 103, 375–387. [Google Scholar]
- Heng, K.; Chevalier, M.; Lek, S.; Laffaille, P. Seasonal Variations in Diet Composition, Diet Breadth and Dietary Overlap between Three Commercially Important Fish Species within a Flood-Pulse System: The Tonle Sap Lake (Cambodia). PLoS ONE 2018, 13, e0198848. [Google Scholar] [CrossRef]
- Tsadok, R.; Zemah-Shamir, Z.; Shemesh, E.; Martinez, S.; Ramon, D.; Kolski, I.; Tsemel, A.; Tchernov, D. Dietary Habits Change of Lessepsian Migrants’ Fish from the Red Sea to the Eastern Mediterranean Sea. Aquat. Invasions 2023, 18, 521–531. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Olenin, S.; Puntila-Dodd, R.; Rilov, G.; Stæhr, P.A.U.; Teixeira, H.; Tsirintanis, K.; Birchenough, S.N.R.; Jakobsen, H.H.; Knudsen, S.W.; et al. Marine Invasive Alien Species in Europe: 9 Years after the IAS Regulation. Front. Mar. Sci. 2023, 10, 1271755. [Google Scholar] [CrossRef]
- Kleitou, P.; Crocetta, F.; Giakoumi, S.; Giovos, I.; Hall-Spencer, J.M.; Kalogirou, S.; Kletou, D.; Moutopoulos, D.K.; Rees, S. Fishery Reforms for the Management of Non-Indigenous Species. J. Environ. Manag. 2021, 280, 111690. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).