Added Value of Water Column Spatiotemporal Monitoring to Assess Variability of Trace Metal Elements and Organotin Compounds in Mediterranean Harbours
Abstract
1. Introduction
2. Materials and Methods
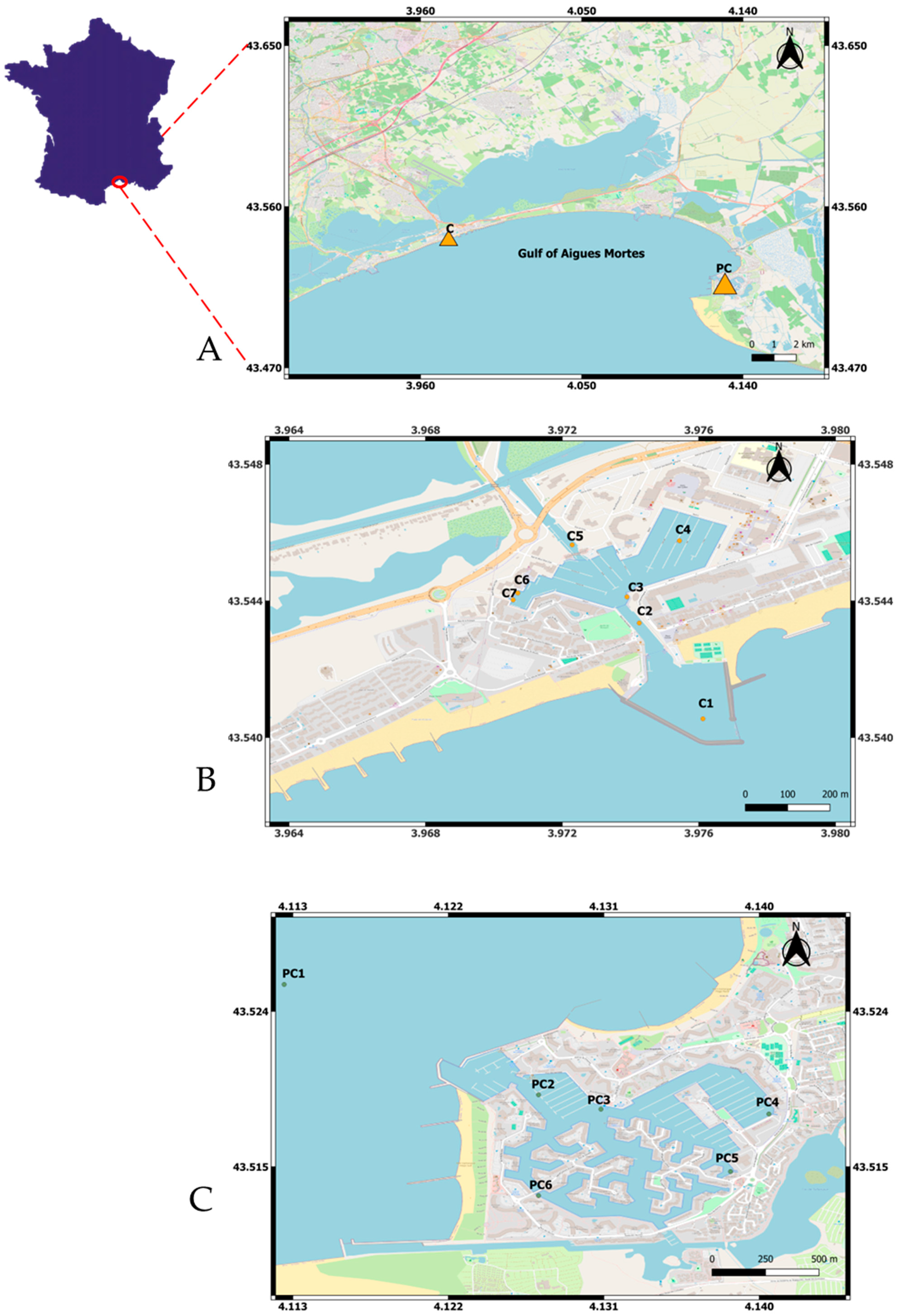
2.1. Study Areas
2.1.1. Port Carnon
2.1.2. Port Camargue
2.2. Sediment and Seawater Sampling Campaigns and Sample Pre-Treatment
2.2.1. Description of Sampling Campaigns
2.2.2. Surface Sediment Sampling and Characterisation
2.2.3. Water Sampling
2.3. Chemical Analyses
2.3.1. TME Analysis
2.3.2. Butyltin Measurements
2.4. Statistical Analysis
3. Results and Discussion
3.1. Sediments
3.1.1. General Characteristics of Sediments
3.1.2. Trace Metals and Organotins in the Sediments
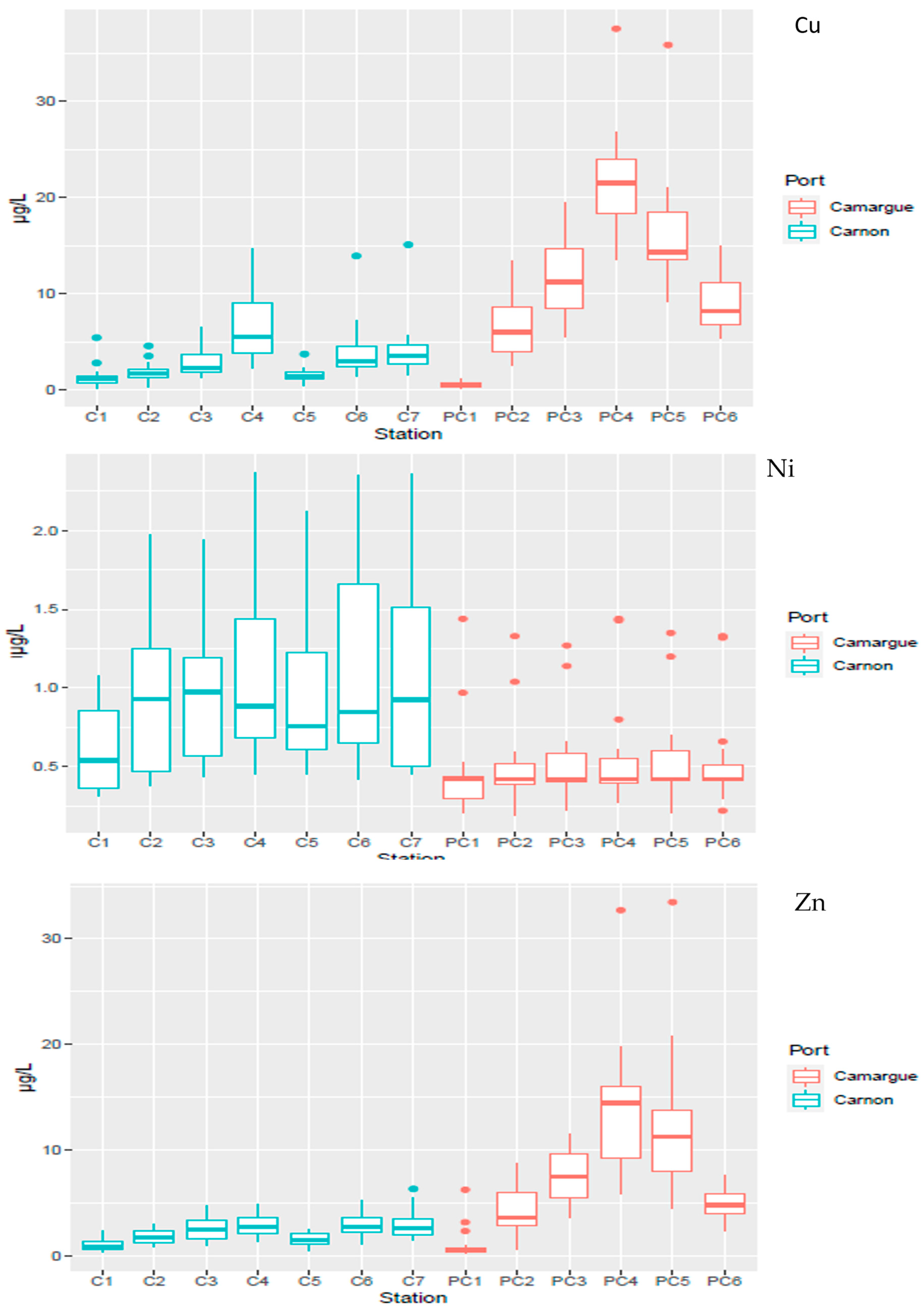
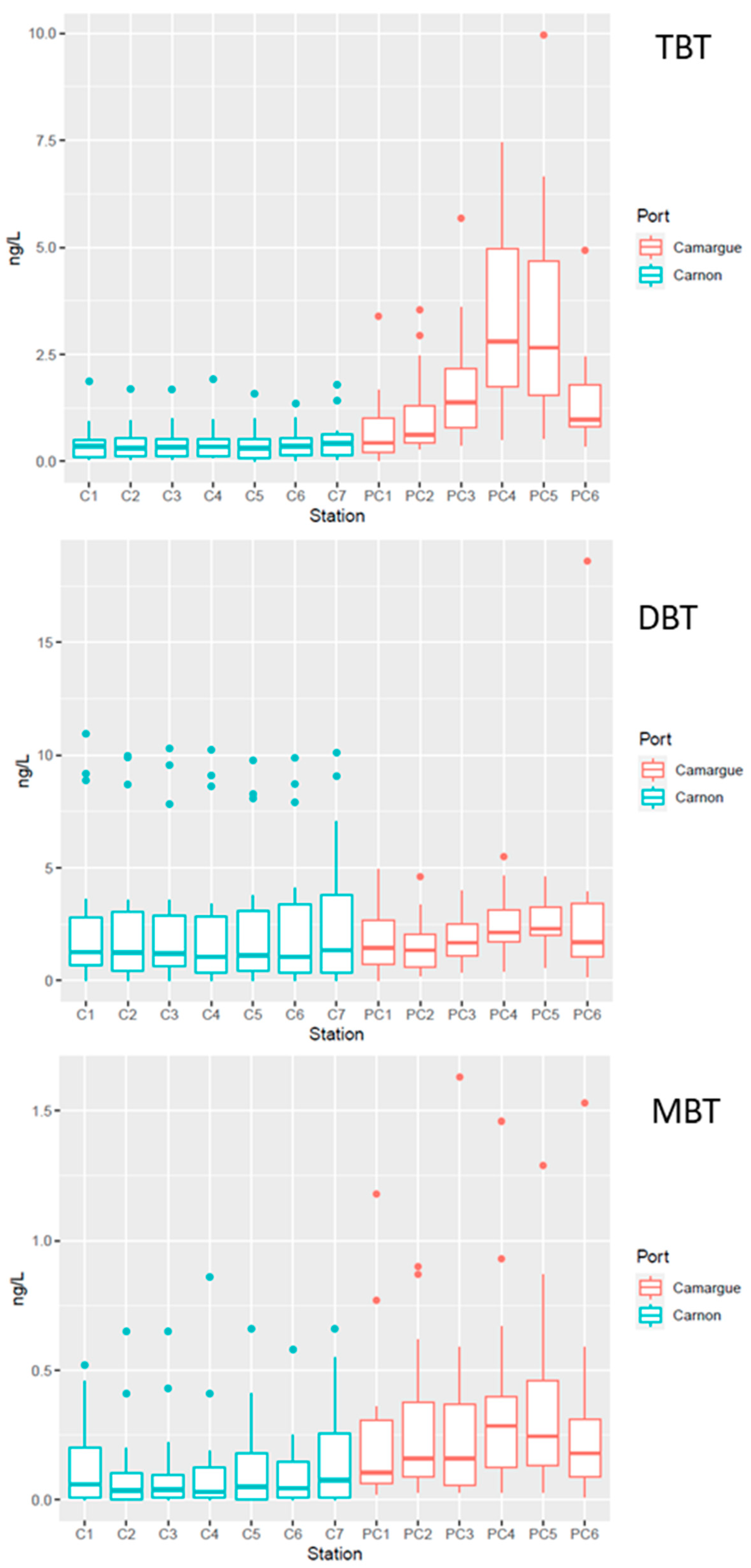
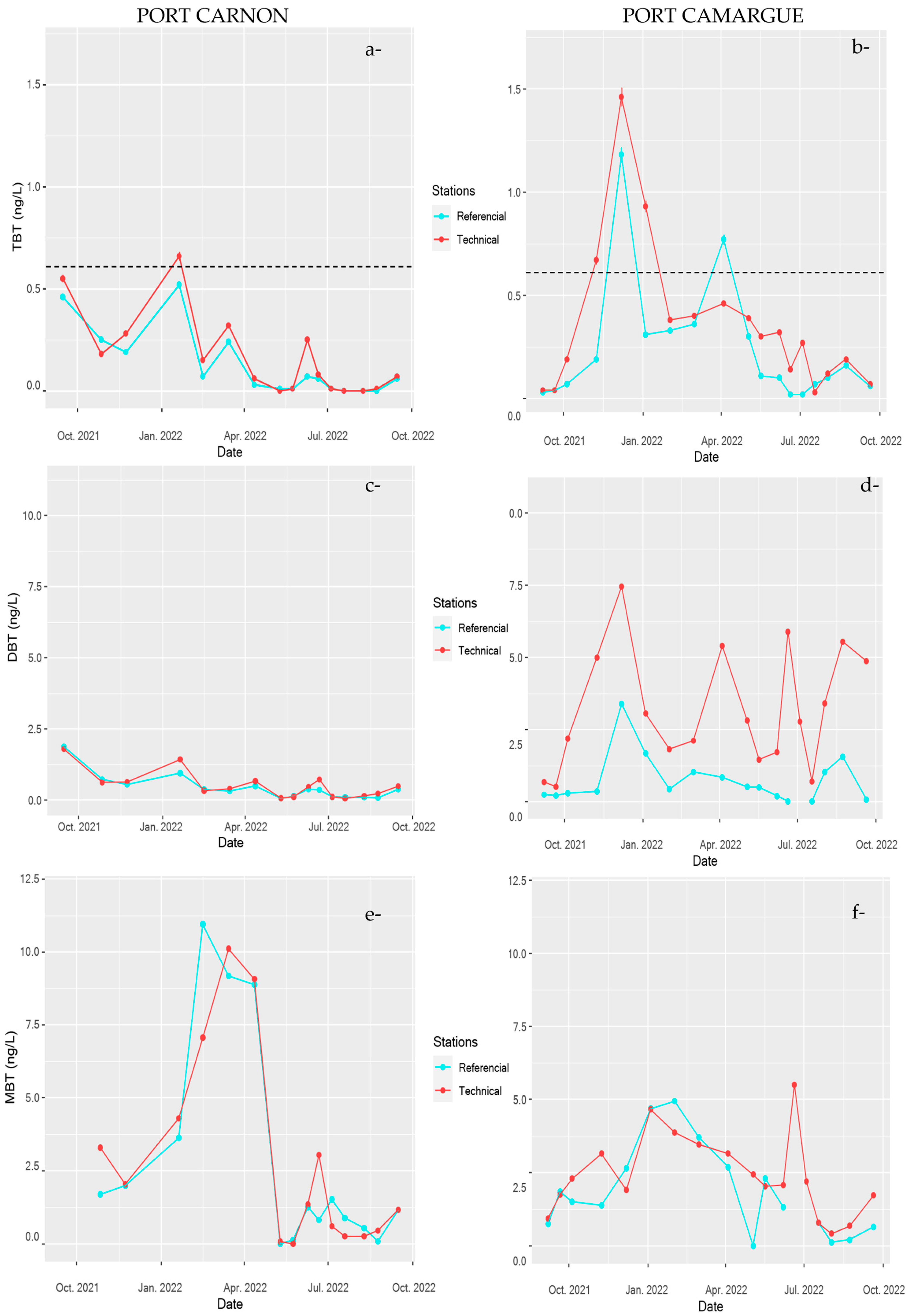
3.2. General Quality Features of the Water Column
3.2.1. Physico-Chemical Parameters
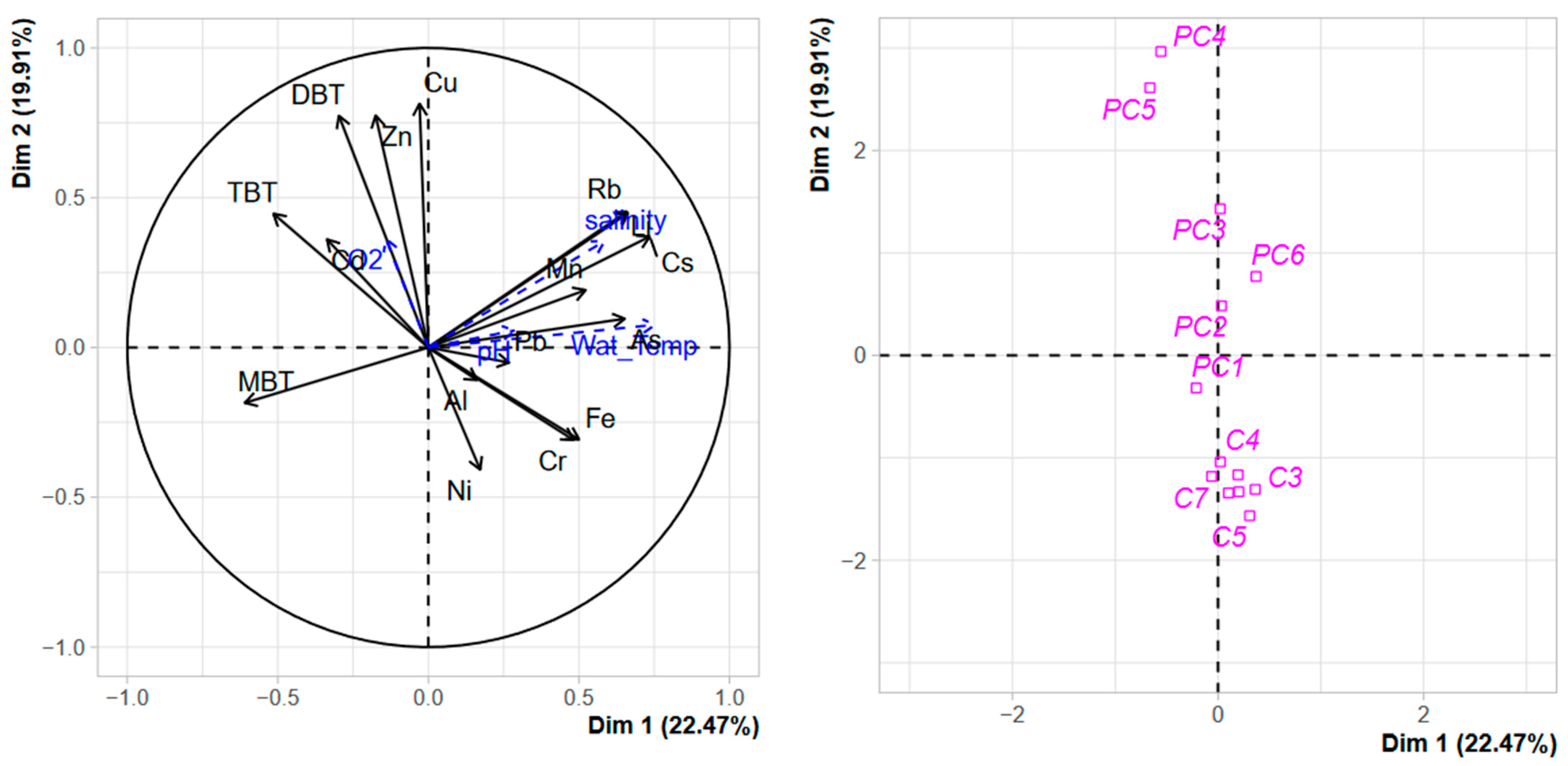
3.2.2. Temporal and Spatial Distribution of Contaminants in the Water Column
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Labonne, M.; Ben Othman, D.; Luck, J.-M. Pb Isotopes in Mussels as Tracers of Metal Sources and Water Movements in a Lagoon (Thau Basin, S. France). Chem. Geol. 2001, 181, 181–191. [Google Scholar] [CrossRef]
- Choueri, R.B.; Cesar, A.; Abessa, D.M.S.; Torres, R.J.; Riba, I.; Pereira, C.D.S.; Nascimento, M.R.L.; Morais, R.D.; Mozeto, A.A.; DelValls, T.A. Harmonised Framework for Ecological Risk Assessment of Sediments from Ports and Estuarine Zones of North and South Atlantic. Ecotoxicol. Lond. Engl. 2010, 19, 678–696. [Google Scholar] [CrossRef]
- Briant, N.; Elbaz-Poulichet, F.; Freydier, R.; Bancon-Montigny, C.; Delpoux, S. Deciphering As and Cu Cycling in Sediment Pore Waters in a Large Marina (Port Camargue, Southern France) Using a Multi-Tracer (Fe, Mn, U, Mo) Approach. Appl. Geochem. 2016, 66, 242–249. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Navarro-Barranco, C.; Martínez-Laiz, G.; Moreira, J.; Giráldez, I.; Morales, E.; Fernández-Romero, A.; Florido, M.; Ros, M. Assessing Environmental Pollution Levels in Marinas. Sci. Total Environ. 2021, 762, 144169. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.G.; Valdor, P.F.; Ondiviela, B.; Díaz, J.L.; Juanes, J.A. Mapping the Environmental Risk Assessment of Marinas on Water Quality: The Atlas of the Spanish Coast. Mar. Pollut. Bull. 2019, 139, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Naser, H.A. Assessment and Management of Heavy Metal Pollution in the Marine Environment of the Arabian Gulf: A Review. Mar. Pollut. Bull. 2013, 72, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Couet, D.; Pringault, O.; Bancon-Montigny, C.; Briant, N.; Elbaz Poulichet, F.; Delpoux, S.; Kefi-Daly Yahia, O.; Hela, B.; Charaf, M.; Hervé, F.; et al. Effects of Copper and Butyltin Compounds on the Growth, Photosynthetic Activity and Toxin Production of Two HAB Dinoflagellates: The Planktonic Alexandrium Catenella and the Benthic Ostreopsis Cf. Ovata. Aquat. Toxicol. 2018, 196, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Bandara, P.C.; Novo, L.A.B.; Kumar, A.; Ambade, B.; Naveendrakumar, G.; Ranagalage, M.; Magana-Arachchi, D.N. Deposition of Trace Metals Associated with Atmospheric Particulate Matter: Environmental Fate and Health Risk Assessment. Chemosphere 2022, 303, 135051. [Google Scholar] [CrossRef]
- Bastami, K.D.; Afkhami, M.; Mohammadizadeh, M.; Ehsanpour, M.; Chambari, S.; Aghaei, S.; Esmaeilzadeh, M.; Neyestani, M.R.; Lagzaee, F.; Baniamam, M. Bioaccumulation and Ecological Risk Assessment of Heavy Metals in the Sediments and Mullet Liza Klunzingeri in the Northern Part of the Persian Gulf. Mar. Pollut. Bull. 2015, 94, 329–334. [Google Scholar] [CrossRef]
- LeMonte, J.J.; Stuckey, J.W.; Sanchez, J.Z.; Tappero, R.; Rinklebe, J.; Sparks, D.L. Sea Level Rise Induced Arsenic Release from Historically Contaminated Coastal Soils. Environ. Sci. Technol. 2017, 51, 5913–5922. [Google Scholar] [CrossRef]
- de Oliveira, C.R.; dos Santos, D.; dos Santos Madureira, L.A.; Rodrigues de Marchi, M.R. Speciation of Butyltin Derivatives in Surface Sediments of Three Southern Brazilian Harbors. J. Hazard. Mater. 2010, 181, 851–856. [Google Scholar] [CrossRef]
- Martinez-Llado, X.; Gibert, O.; Marti, V.; Diez, S.; Romo, J.; Bayona, J.M.; de Pablo, J. Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) and Tributyltin (TBT) in Barcelona Harbour Sediments and Their Impact on Benthic Communities. Environ. Pollut. 2007, 149, 104–113. [Google Scholar] [CrossRef]
- Alzieu, C.; Michel, P.; Tolosa, I.; Bacci, E.; Mee, L.D.; Readman, J.W. Organotin Compounds in the Mediterranean: A Continuing Cause for Concern. Mar. Environ. Res. 1991, 32, 261–270. [Google Scholar] [CrossRef]
- Wake, H. Oil Refineries: A Review of Their Ecological Impacts on the Aquatic Environment. Estuar. Coast. Shelf Sci. 2005, 62, 131–140. [Google Scholar] [CrossRef]
- Omae, I. Chemistry and Fate of Organotin Antifouling Biocides in the Environment. In Antifouling Paint Biocides; Konstantinou, I.K., Ed.; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2006; pp. 17–50. ISBN 978-3-540-32844-5. [Google Scholar]
- Alzieu, C.L.; Sanjuan, J.; Deltreil, J.P.; Borel, M. Tin Contamination in Arcachon Bay: Effects on Oyster Shell Anomalies. Mar. Pollut. Bull. 1986, 17, 494–498. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Bryan, G.W. Reproductive Failure in Populations of the Dog-Whelk, Nucella Lapillus, Caused by Imposex Induced by Tributyltin from Antifouling Paints. J. Mar. Biol. Assoc. U. K. 1986, 66, 767–777. [Google Scholar] [CrossRef]
- Bettin, C.; Oehlmann, J.; Stroben, E. TBT-Induced Imposex in Marine Neogastropods Is Mediated by an Increasing Androgen Level. Helgoländer Meeresunters. 1996, 50, 299–317. [Google Scholar] [CrossRef]
- Díez, S.; Ábalos, M.; Bayona, J.M. Organotin Contamination in Sediments from the Western Mediterranean Enclosures Following 10 Years of TBT Regulation. Water Res. 2002, 36, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Sabah, A.; Bancon-Montigny, C.; Rodier, C.; Marchand, P.; Delpoux, S.; Ijjaali, M.; Tournoud, M.-G. Occurrence and Removal of Butyltin Compounds in a Waste Stabilisation Pond of a Domestic Waste Water Treatment Plant of a Rural French Town. Chemosphere 2016, 144, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Briant, N.; Bancon-Montigny, C.; Freydier, R.; Delpoux, S.; Elbaz-Poulichet, F. Behaviour of Butyltin Compounds in the Sediment Pore Waters of a Contaminated Marina (Port Camargue, South of France). Chemosphere 2016, 150, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, S.I.; Apeti, D.A.; Mason, A.L.; Pait, A.S. An Assessment of Butyltins and Metals in Sediment Cores from the St. Thomas East End Reserves, USVI. Environ. Monit. Assess. 2016, 188, 642. [Google Scholar] [CrossRef]
- Mikac, N.; Furdek Turk, M.; Petrović, D.; Bigović, M.; Krivokapić, S. First Assessment of Butyltins (BuTs) Contamination of the Montenegrin Coast (Southeast Adriatic): Tributyltin (TBT) Poses a Threat to the Marine Ecosystem. Mar. Pollut. Bull. 2022, 185, 114270. [Google Scholar] [CrossRef]
- Cossa, D.; Durrieu de Madron, X.; Schäfer, J.; Guédron, S.; Marusczak, N.; Castelle, S.; Naudin, J.-J. Sources and Exchanges of Mercury in the Waters of the Northwestern Mediterranean Margin. Prog. Oceanogr. 2018, 163, 172–183. [Google Scholar] [CrossRef]
- Zohra, B.S.; Habib, A. Assessment of Heavy Metal Contamination Levels and Toxicity in Sediments and Fishes from the Mediterranean Sea (Southern Coast of Sfax, Tunisia). Environ. Sci. Pollut. Res. 2016, 23, 13954–13963. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Chemical Contaminants Entering the Marine Environment from Sea-Based Sources: A Review with a Focus on European Seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef]
- Flouty, R.; Estephane, G. Bioaccumulation and Biosorption of Copper and Lead by a Unicellular Algae Chlamydomonas Reinhardtii in Single and Binary Metal Systems: A Comparative Study. J. Environ. Manage. 2012, 111, 106–114. [Google Scholar] [CrossRef]
- Fortibuoni, T.; Noventa, S.; Rampazzo, F.; Gion, C.; Formalewicz, M.; Berto, D.; Raicevich, S. Evidence of Butyltin Biomagnification along the Northern Adriatic Food-Web (Mediterranean Sea) Elucidated by Stable Isotope Ratios. Environ. Sci. Technol. 2013, 47, 3370–3377. [Google Scholar] [CrossRef]
- Pachés, M.; Martínez-Guijarro, R.; Romero, I.; Aguado, D. Assessment of Metal Pollution and Its Environmental Impact on Spanish Mediterranean Coastal Ecosystems. J. Mar. Sci. Eng. 2023, 11, 89. [Google Scholar] [CrossRef]
- Briant, N.; Bancon-Montigny, C.; Elbaz-Poulichet, F.; Freydier, R.; Delpoux, S.; Cossa, D. Trace Elements in the Sediments of a Large Mediterranean Marina (Port Camargue, France): Levels and Contamination History. Mar. Pollut. Bull. 2013, 73, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, P.; Viehberg, F.; Kirsten, K.; Newman, B.; Frenzel, P.; Gildeeva, O.; Green, A.; Hahn, A.; Haberzettl, T. Spatial Distribution and Consequences of Contaminants in Harbour Sediments—A Case Study from Richards Bay Harbour, South Africa. Mar. Pollut. Bull. 2021, 172, 112764. [Google Scholar] [CrossRef] [PubMed]
- Alprol, A.E.; Ashour, M.; Mansour, A.T.; Alzahrani, O.M.; Mahmoud, S.F.; Gharib, S.M. Assessment of Water Quality and Phytoplankton Structure of Eight Alexandria Beaches, Southeastern Mediterranean Sea, Egypt. J. Mar. Sci. Eng. 2021, 9, 1328. [Google Scholar] [CrossRef]
- Layglon, N.; Abdou, M.; Massa, F.; Castellano, M.; Bakker, E.; Povero, P.; Tercier-Waeber, M.-L. Speciation of Cu, Cd, Pb and Zn in a Contaminated Harbor and Comparison to Environmental Quality Standards. J. Environ. Manage. 2022, 317, 115375. [Google Scholar] [CrossRef]
- Leredde, Y.; Denamiel, C.; Brambilla, E.; Lauer-Leredde, C.; Bouchette, F.; Marsaleix, P. Hydrodynamics in the Gulf of Aigues-Mortes, NW Mediterranean Sea: In Situ and Modelling Data. Cont. Shelf Res. 2007, 27, 2389–2406. [Google Scholar] [CrossRef]
- Burton, G., Jr. Allen Sediment Quality Criteria in Use around the World. Limnology 2002, 3, 65–76. [Google Scholar] [CrossRef]
- Long, E.; Macdonald, D.; Smith, S.; Calder, F. Incidence of Adverse Biological Effects Within Ranges of Chemical Concentration in Marine and Estuarine Sediments. Environ. Manage. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Thompson, R.; Wasserman, H. Sediment Quality Guidelines (SQGs): A Review and Their Use in Practice. Available online: https://www.geoengineer.org/education/web-class-projects/cee-549-geoenvironmental-engineering-fall-2015/assignments/sediment-quality-guidelines-sqgs-a-review-and-their-use-in-practice#references (accessed on 23 January 2024).
- Telloli, C.; Tagliavini, S.; Passarini, F.; Salvi, S.; Rizzo, A. ICP-MS Triple Quadrupole as Analytical Technique to Define Trace and Ultra-Trace Fingerprint of Extra Virgin Olive Oil. Food Chem. 2023, 402, 134247. [Google Scholar] [CrossRef]
- Renzi, M.; Perra, G.; Guerranti, C.; Mariottini, M.; Baroni, D.; Volterrani, M.; Graziosi, M.; Specchiulli, A.; Focardi, S. Assessment of Environmental Pollutants in Ten Southern Italy Harbor Sediments. Toxicol. Ind. Health 2009, 25, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, M.H.; Ibrahim, M.I.A.; Abo Elkhair, E.M.; Mohamed, L.A.; Halim, A.A.; Shaban, K.S.; Fahmy, M. The Modified Canadian Water Index with Other Sediment Models for Assessment of Sediments from Two Harbours on the Egyptian Mediterranean Coast. J. Hazard. Mater. Adv. 2022, 8, 100180. [Google Scholar] [CrossRef]
- Briant, N.; Freydier, R.; Araújo, D.F.; Delpoux, S.; Elbaz-Poulichet, F. Cu Isotope Records of Cu-Based Antifouling Paints in Sediment Core Profiles from the Largest European Marina, The Port Camargue. Sci. Total Environ. 2022, 849, 157885. [Google Scholar] [CrossRef] [PubMed]
- Rebai, N.; Mosbahi, N.; Dauvin, J.-C.; Neifar, L. Ecological Risk Assessment of Heavy Metals and Environmental Quality of Tunisian Harbours. J. Mar. Sci. Eng. 2022, 10, 1625. [Google Scholar] [CrossRef]
- Pougnet, F.; Schäfer, J.; Dutruch, L.; Garnier, C.; Tessier, E.; Dang, D.H.; Lanceleur, L.; Mullot, J.-U.; Lenoble, V.; Blanc, G. Sources and Historical Record of Tin and Butyl-Tin Species in a Mediterranean Bay (Toulon Bay, France). Environ. Sci. Pollut. Res. 2014, 21, 6640–6651. [Google Scholar] [CrossRef]
- Bibi, M.H.; Ahmed, F.; Ishiga, H. Assessment of Metal Concentrations in Lake Sediments of Southwest Japan Based on Sediment Quality Guidelines. Environ. Geol. 2007, 52, 625–639. [Google Scholar] [CrossRef]
- Concha-Graña, E.; Moscoso-Pérez, C.; Fernández-González, V.; López-Mahía, P.; Gago, J.; León, V.M.; Muniategui-Lorenzo, S. Phthalates, Organotin Compounds and per-Polyfluoroalkyl Substances in Semiconfined Areas of the Spanish Coast: Occurrence, Sources and Risk Assessment. Sci. Total Environ. 2021, 780, 146450. [Google Scholar] [CrossRef]
- Directive 2008/105/EC of the European Parliament and of the Council (16 December 2008) on Environmental Quality Standards in the Field of Water Policy. Off. J. Eur. Union 2008, L 348, 84–97. Available online: https://Eur-Lex.Europa.Eu/Eli/Dir/2008/105/Oj (accessed on 25 January 2024).
- Légifrance—Publications Officielles—Journal Officiel—JORF N° 0198 Du 28/08/2015. Available online: https://www.legifrance.gouv.fr/download/pdf?id=CzMk9C9TuiNiDv8adNATce6yplGEb0Xgie4-T-nS53g= (accessed on 25 January 2024).
- Seligman, P.F.; Valkirs, A.O.; Stang, P.M.; Lee, R.F. Evidence for Rapid Degradation of Tributyltin in a Marina. Mar. Pollut. Bull. 1988, 19, 531–534. [Google Scholar] [CrossRef]
- Brosillon, S.; Bancon-Montigny, C.; Mendret, J. Study of Photocatalytic Degradation of Tributyltin, Dibutylin and Monobutyltin in Water and Marine Sediments. Chemosphere 2014, 109, 173–179. [Google Scholar] [CrossRef]
- Tueros, I.; Rodriguez, J.G.; Borja, A.; Solaun, O.; Valencia, V.; Millan, E. Dissolved Metal Background Levels in Marine Waters, for the Assessment of the Physico-Chemical Status, within the European Water Framework Directive. Sci. Total Environ. 2008, 407, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Morsy, A.; Ebeid, M.; Soliman, A.; Halim, A.A.; Ali, A.E.; Fahmy, M. Evaluation of the Water Quality and the Eutrophication Risk in Mediterranean Sea Area: A Case Study of the Port Said Harbour, Egypt. Environ. Chall. 2022, 7, 100484. [Google Scholar] [CrossRef]
- Layglon, N.; Misson, B.; Durieu, G.; Coclet, C.; D’Onofrio, S.; Dang, D.H.; François, D.; Mullot, J.-U.; Mounier, S.; Lenoble, V.; et al. Long-Term Monitoring Emphasizes Impacts of the Dredging on Dissolved Cu and Pb Contamination along with Ultraplankton Distribution and Structure in Toulon Bay (NW Mediterranean Sea, France). Mar. Pollut. Bull. 2020, 156, 111196. [Google Scholar] [CrossRef] [PubMed]
- Furdek, M.; Vahčič, M.; Ščančar, J.; Milačič, R.; Kniewald, G.; Mikac, N. Organotin Compounds in Seawater and Mytilus Galloprovincialis Mussels along the Croatian Adriatic Coast. Mar. Pollut. Bull. 2012, 64, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Centineo, G.; Blanco González, E.; Sanz-Medel, A. Multielemental Speciation Analysis of Organometallic Compounds of Mercury, Lead and Tin in Natural Water Samples by Headspace-Solid Phase Microextraction Followed by Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2004, 1034, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, A.M.A. Occurrence of Organotin Compounds in Water and Biota from Alexandria Harbours. Chemosphere 1995, 30, 707–715. [Google Scholar] [CrossRef]
- Krishna, A.K.; Satyanarayanan, M.; Govil, P.K. Assessment of Heavy Metal Pollution in Water Using Multivariate Statistical Techniques in an Industrial Area: A Case Study from Patancheru, Medak District, Andhra Pradesh, India. J. Hazard. Mater. 2009, 167, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chang-Chien, G.-P.; Chiang, P.-C.; Chen, W.-H.; Lin, Y.-C. Multivariate Analysis of Heavy Metal Contaminations in Seawater and Sediments from a Heavily Industrialized Harbor in Southern Taiwan. Mar. Pollut. Bull. 2013, 76, 266–275. [Google Scholar] [CrossRef]



| Location of Sampling Stations | Sampling Stations | Date of Water Sampling Campaigns | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sept.-21 | Oct.-21 | Nov.-21 | Dec.-21 | Jan.-22 | Feb.-22 | Mar-22 | Apr.-22 | May-22 | Jun-22 | Jul.-22 | Aug-22 | Sept.-22 | |||
| PORT CARNON | Reference: Port entrance | C1 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X |
| Channel entrance | C2 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| Harbour master’s office | C3 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| Eastern Basin | C4 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| Contact Channel (lagoon to sea) | C5 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| Western Basin | C6 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| Western Basin (Dock) | C7 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| PORT CAMARGUE | Reference: Outside the Port | PC1 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X |
| Harbour master’s office | PC2 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| Fuel station | PC3 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| Technical station (Dock) | PC4 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| Technical station (Dock) | PC5 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| Marina Area | PC6 | X | X | X | X | X | X | X | X | XX | XX | XX | XX | X | |
| PORT CARNON | PORT CAMARGUE | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | ||||
| Trace elements | ERL 1 | ERM 2 | ||||||||||||||
| As | mg kg−1 | 16 | 12.2 | 13.4 | 16 | 11.9 | 12.6 | 14.1 | 12.0 | 11.9 | 12.0 | 15.6 | 15.4 | 8.4 | 8.2 | 70 |
| Cd | 0.1 | 0.2 | 0.4 | 0.4 | 0.3 | 0.4 | 0.4 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 1.2 | 9.6 | |
| Cr | 32.2 | 51 | 77.5 | 88.6 | 67.1 | 75.1 | 84.8 | 61.7 | 53.5 | 53.9 | 66.4 | 52.2 | 39.2 | 81 | 370 | |
| Cu | 4.2 | 46.2 | 73.7 | 169 | 111.5 | 111.7 | 309 | 82.7 | 26.7 | 94.2 | 1210 | 713 | 292 | 34 | 270 | |
| Ni | 18 | 22.5 | 33.3 | 37.8 | 29 | 33.9 | 36.4 | 1.3 | 13.3 | 396 | 0.3 | 2.9 | 133 | 20.9 | 51.6 | |
| Pb | 13.2 | 28.7 | 48.5 | 57 | 43.2 | 48.3 | 55.4 | 24.8 | 20.3 | 20.2 | 81.3 | 58.6 | 27.9 | 46.7 | 218 | |
| Zn | 43.2 | 79.9 | 126 | 199 | 127.6 | 144 | 242 | 106 | 76.2 | 89.2 | 425 | 270 | 174 | 150 | 410 | |
| Organotin compounds | ||||||||||||||||
| TBT | µg (Sn) kg−1 | 0.6 | 3 | 9 | 19 | 1 | 15 | 198 | 5 | 4 | 13 | 4678 | 2803 | 29 | ||
| DBT | 0.9 | 3 | 5 | 9 | 0 | 7 | 69 | 6 | 6 | 21 | 1532 | 1519 | 38 | |||
| MBT | 5.3 | 29 | 17 | 15 | 7 | 13 | 35 | 20 | 17 | 54 | 568 | 856 | 95 | |||
| Σbutyltins | 6.8 | 35 | 31 | 43 | 8 | 35 | 302 | 31 | 27 | 88 | 6778 | 5178 | 163 | |||
| BDI | 10.3 | 12 | 2.3 | 1.2 | 5.1 | 1.4 | 0.5 | 5.7 | 5.3 | 5.6 | 0.4 | 0.8 | 4.5 | |||
| Port Carnon Harbour | Port Camargue Harbour | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
| Cu | 18.8 | 62.5 | 93.8 | 100.0 | 43.8 | 93.8 | 93.8 | 0.0 | 100 | 100 | 100 | 100 | 100 |
| Zn | 0.0 | 0.0 | 37.5 | 37.5 | 0.0 | 31.3 | 37.5 | 11.1 | 61.1 | 100.0 | 100.0 | 100.0 | 94.4 |
| TBT | 0.0 | 6.3 | 6.3 | 6.3 | 6.3 | 0.0 | 6.3 | 11.1 | 16.7 | 5.6 | 16.7 | 22.2 | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouba, C.; Pringault, O.; Domeau, A.; Delpoux, S.; Causse, L.; Marie, M.; Freydier, R.; Montigny, C. Added Value of Water Column Spatiotemporal Monitoring to Assess Variability of Trace Metal Elements and Organotin Compounds in Mediterranean Harbours. J. Mar. Sci. Eng. 2024, 12, 399. https://doi.org/10.3390/jmse12030399
Chouba C, Pringault O, Domeau A, Delpoux S, Causse L, Marie M, Freydier R, Montigny C. Added Value of Water Column Spatiotemporal Monitoring to Assess Variability of Trace Metal Elements and Organotin Compounds in Mediterranean Harbours. Journal of Marine Science and Engineering. 2024; 12(3):399. https://doi.org/10.3390/jmse12030399
Chicago/Turabian StyleChouba, Cyrine, Olivier Pringault, Aurélien Domeau, Sophie Delpoux, Léa Causse, Mylène Marie, Rémi Freydier, and Chrystelle Montigny. 2024. "Added Value of Water Column Spatiotemporal Monitoring to Assess Variability of Trace Metal Elements and Organotin Compounds in Mediterranean Harbours" Journal of Marine Science and Engineering 12, no. 3: 399. https://doi.org/10.3390/jmse12030399
APA StyleChouba, C., Pringault, O., Domeau, A., Delpoux, S., Causse, L., Marie, M., Freydier, R., & Montigny, C. (2024). Added Value of Water Column Spatiotemporal Monitoring to Assess Variability of Trace Metal Elements and Organotin Compounds in Mediterranean Harbours. Journal of Marine Science and Engineering, 12(3), 399. https://doi.org/10.3390/jmse12030399








