Biometry, Growth, and Recruitment Pattern of a Commercially Important Nereid polychaete, Namalycastis fauveli, from the East Coast of Bangladesh
Abstract
1. Introduction
2. Materials and Methods
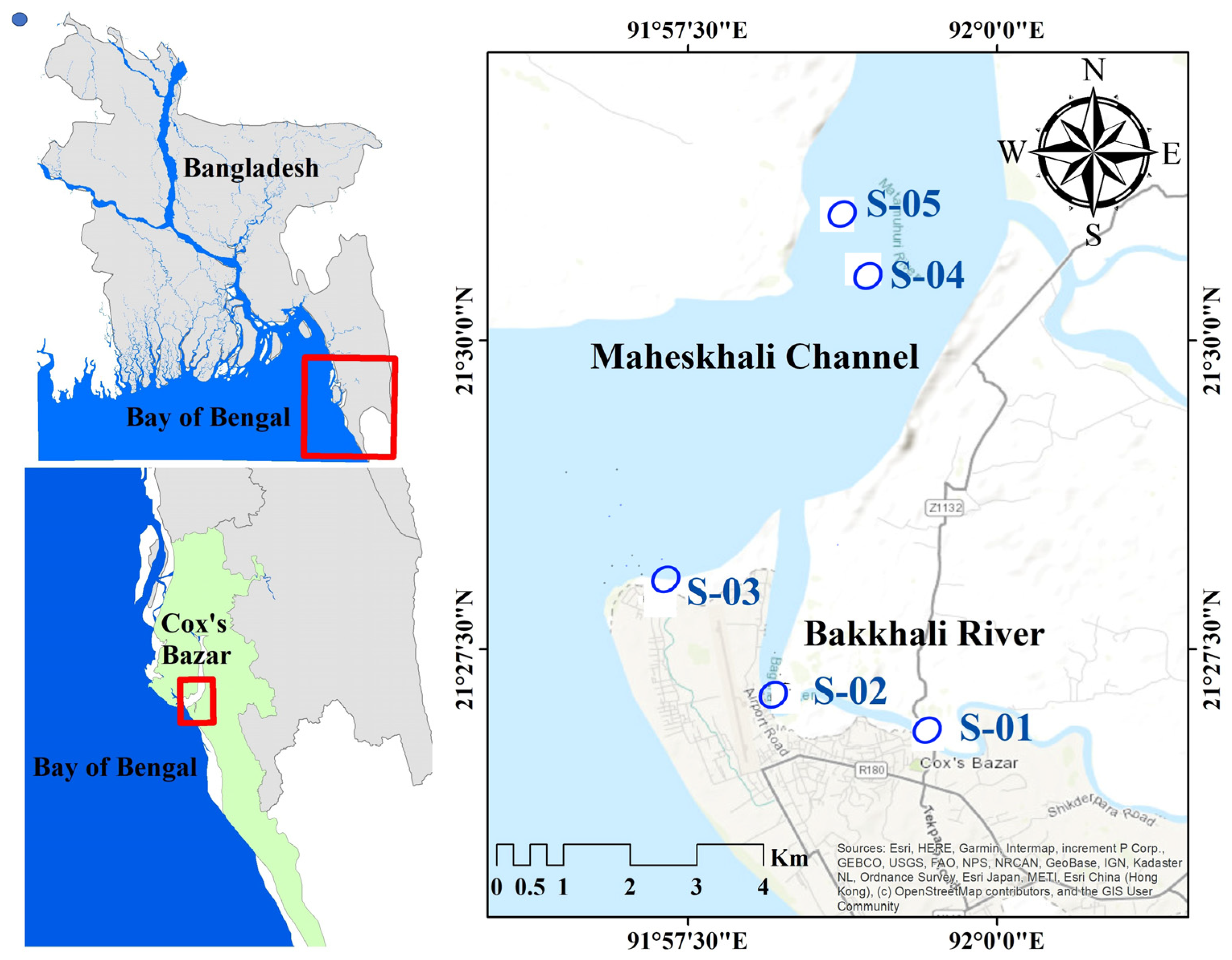
2.1. Study Area
2.2. Polychaetes Sample Collection and Identification
2.3. Estimation of Population Parameters
2.4. Statistical Analysis
3. Results
3.1. Length–Weight (L–W) Relationship of N. fauveli
3.2. Population Dynamics of N. fauveli
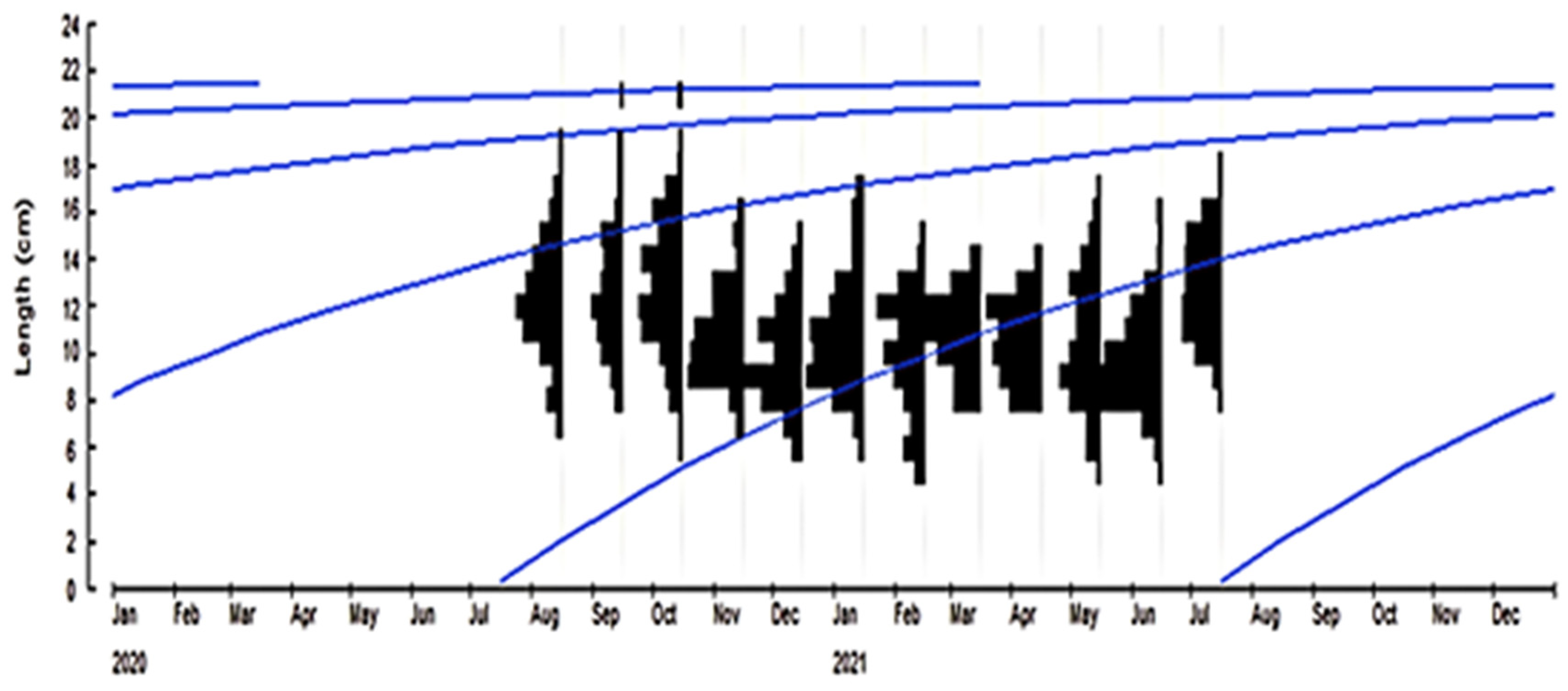
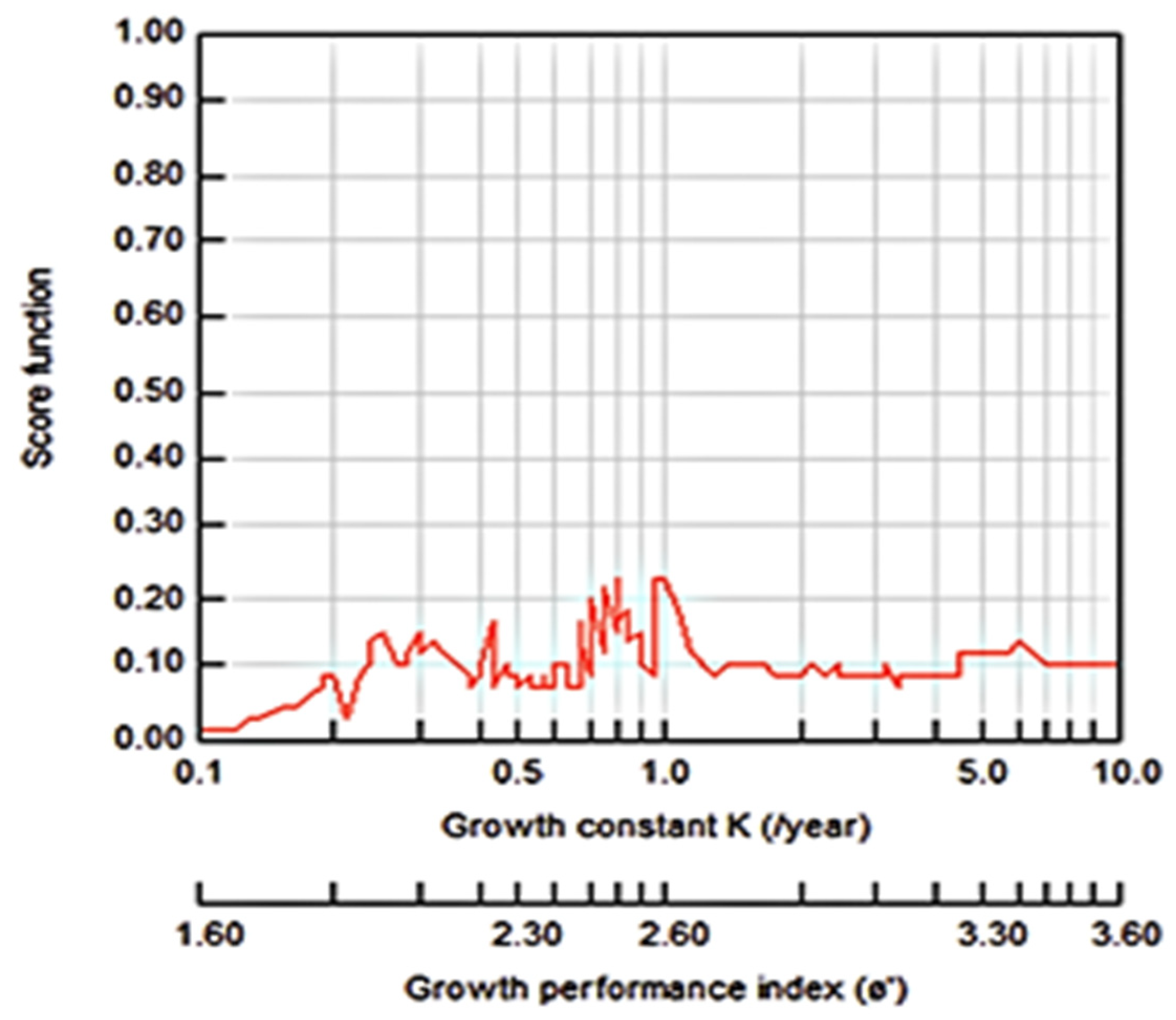
3.2.1. Growth Parameters
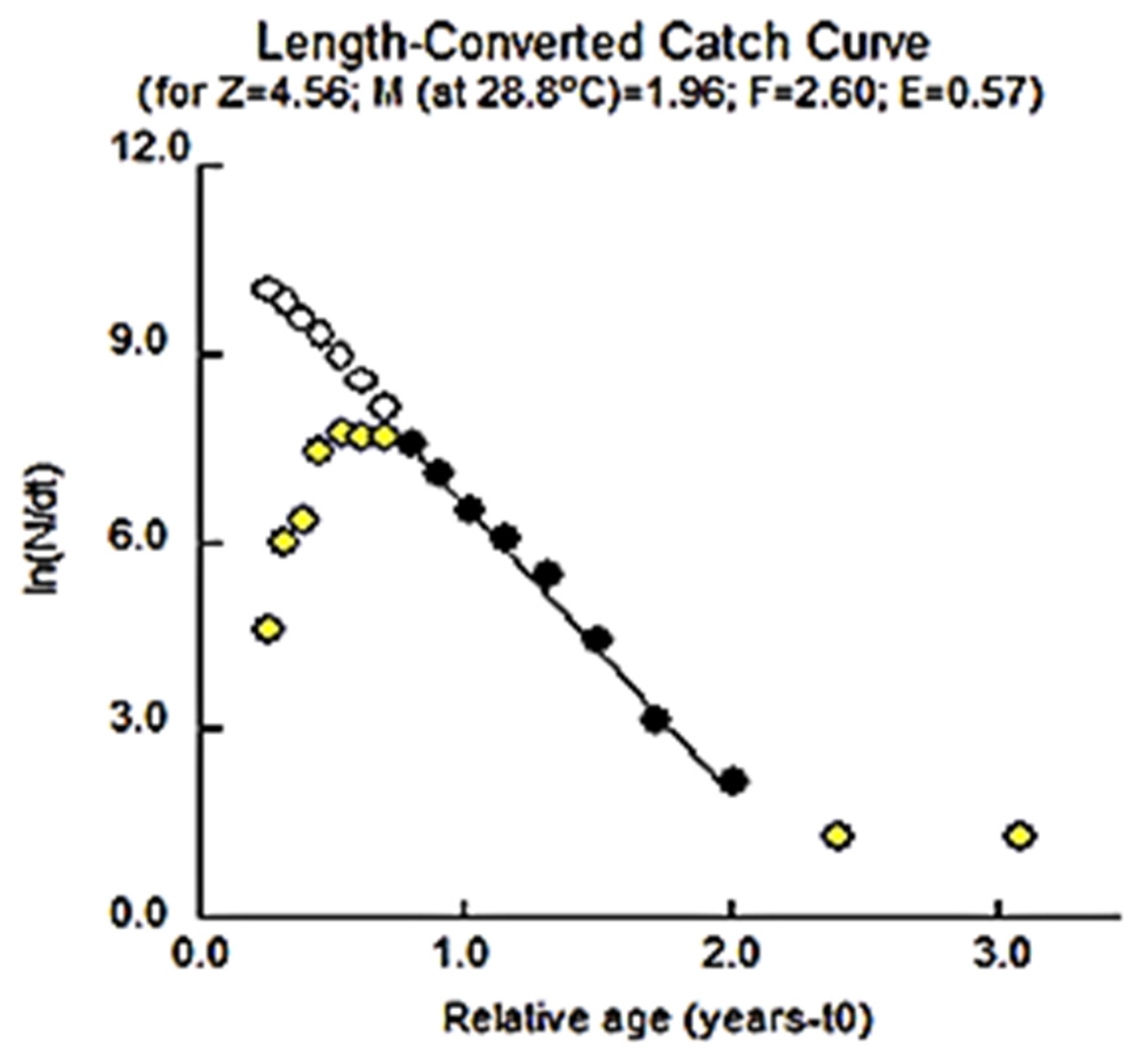
3.2.2. Mortality and Exploitation of the Species
3.2.3. Recruitment Pattern
3.2.4. Virtual Population Analysis (VPA)
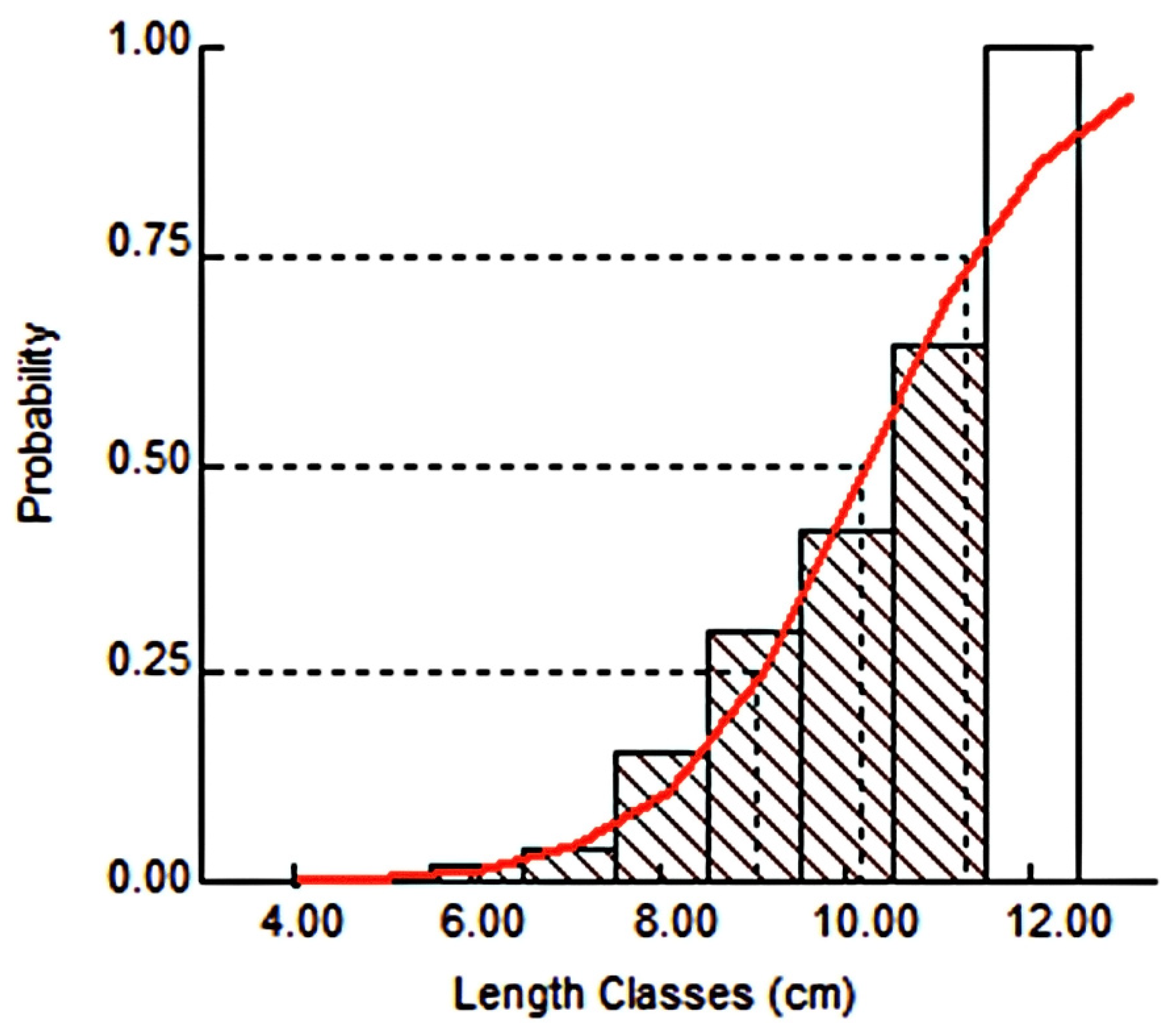
3.2.5. Probability of Capture (P-Cap)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchings, P. Biodiversity and functioning of polychaetes in benthic sediments. Biodivers. Conserv. 1998, 7, 1133–1145. [Google Scholar] [CrossRef]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Annu. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef]
- Martins, A.D.; Barros, F. Ecological Functions of Polychaetes Along Estuarine Gradients. Front. Mar. Sci. 2022, 9, 780318. [Google Scholar] [CrossRef]
- Pauls, L.D. Optimization of Rearing Techniques for Cultured Marine Polychaetes (Nereis virens) Using Sustainably Sourced Ingredients. Ph.D. Thesis, University of Wales, Cardiff, UK, 2009. Available online: http://cronfa.swan.ac.uk/Record/cronfa42755 (accessed on 4 January 2020).
- Wang, H.; Seekamp, I.; Malzahn, A.; Hagemann, A.; Carvajal, A.K.; Slizyte, R.; Standal, I.B.; Handå, A.; Reitan, K.I. Growth and nutritional composition of the polychaete Hediste diversicolor (OF Müller, 1776) cultivated on waste from land-based salmon smolt aquaculture. Aquaculture 2019, 502, 232–241. [Google Scholar] [CrossRef]
- Yuwono, E.; Haryadi, B.; Susilo, U.; Siregar, A.; Sugiharto, S. Fertilization and Maintenance of Larvae and Juveniles as Efforts to Develop Lur Worm Cultivation Techniques. Majalah Ilmiah Biologi BIOSFERA. 2002, 9, 1–8. [Google Scholar]
- Chung, M.Y.; Liu, C.H.; Chen, Y.N.; Cheng, W. Enhancing the reproductive performance of tiger shrimp, Penaeus monodon, by incorporating sodium alginate in the broodstock and larval diets. Aquaculture 2011, 312, 180–184. [Google Scholar] [CrossRef]
- Yuwono, E. Kebutuhan Nutrisi Crustacea dan Potensi Cacing Lur (Nereis sp., Polychaeta) Untuk Pakan Udang. J. Pemb. Pedesaan 2005, 5, 42–49. [Google Scholar]
- Cole, V.J.; Chick, R.C.; Hutchings, P.A. A review of global fisheries for Polychaeta worms as a resource for recreational fishers: Diversity, sustainability and research needs. Rev. Fish Bio. Fish. 2018, 28, 543–565. [Google Scholar] [CrossRef]
- Górska, B.; Gromisz, S.; Włodarska-Kowalczuk, M. Size assessment in polychaetes worms—Application of morphometric correlations for common North Atlantic taxa Barbara. Limnol. Oceanogr. Methods 2019, 17, 254–265. [Google Scholar] [CrossRef]
- Glasby, C.J. The Namanereidinae (Polychaeta: Nereididae). Part 1, Taxonomy and Phylogeny Records of the Australian Museum. In Records of the Australian Museum; Supplement 25; 1999; Available online: https://journals.australian.museum/glasby-1999-rec-aust-mus-suppl-25-1129/ (accessed on 4 January 2020).
- Glasby, C.J.; Timm, T. Global diversity of polychaetes (Polychaeta; Annelida) in freshwater. Hydrobiologia 2008, 595, 107–115. [Google Scholar] [CrossRef]
- Conde-vela, V.M. The troglomorphic adaptations of Namanereidinae (Annelida, Nereididae) revisited, including a redescription of Namanereis cavernicola (Solís-Weiss & Espinasa, 1991), and a new Caribbean species of Namanereis Chamberlin, 1919. Subterr. Biol. 2017, 23, 19–46. [Google Scholar] [CrossRef][Green Version]
- Raknuzzaman, M.; Ahmed, M.K.; Islam, M.S.; Habibullah-Al-Mamun, M.; Tokumura, M.; Sekine, M.; Masunaga, S. Assessment of Trace Metals in Surface Water and Sediment Collected from Polluted Coastal Areas of Bangladesh. J. Wat. Environ. Technol. 2016, 14, 247–259. [Google Scholar] [CrossRef]
- Hena, M.K.A.; Kohinoor, S.M.S.; Siddique, M.A.M.; Ismail, J.; Idris, M.H.; Amin, S.M.N. Composition of Macrobenthos in the Bakkhali Channel System, Cox’s Bazar with Notes on the Soil Parameter. Pak. J. Biol. Sci. 2012, 15, 641–646. [Google Scholar] [CrossRef]
- Islam, M.S.; Sikder, M.N.A.; Al-Imran, M.; Hossain, M.B.; Mallick, D.; Morshed, M.M. Intertidal Macrobenthic Fauna of the Karnafuli Estuary: Relations with Environmental Variables. Wor. Appl. Scis. J. 2013, 21, 1366–1373. [Google Scholar]
- Aura, C.M.; Raburu, P.O.; Herrmann, J. Macro invertebrate’s community structure in Rivers Kipkaren and Sosiani, River Nzoia Basin, Kenya. J. Ecol. Nat. Environ. 2011, 3, 39–46. Available online: http://www.academicjournals.org/jene (accessed on 4 January 2020).
- Omena, E.P.; Amaral, A.C.Z. Population dynamics and production of Laeonereis acuta. Bull. Mar. Sci. 2000, 67, 421–431. Available online: https://www.researchgate.net/publication/233675884_Population_dynamics_and_secondary_production_of_Laeonereis_acuta_Treadwell_1923_Nereididae_Polychaeta (accessed on 4 January 2020).
- Pagliosa, P.R.; Lana, P.C. Population dynamics and secondary production of Nereis oligohalina (Nereididae: Polychaeta) from a subtropical marsh in southeast Brazil. Bull. Mar. Sci. 2000, 67, 259–268. Available online: https://www.researchgate.net/publication/298293084_Population_dynamics_and_secondary_production_of_Nereis_oligohalina_Nereididae_Polychaeta_from_a_subtropical_marsh_in_Southeast_Brazil (accessed on 14 January 2020).
- Daas, T.; Younsi, M.; Daas-Maamcha, Q.; Gillet, P.; Scaps, P. Reproduction, population dynamics and production of Nereis falsa (Nereididae: Polychaeta) on the rocky coast of El Kala National Park, Algeria. Helgol. Mar. Res. 2011, 65, 165–173. [Google Scholar] [CrossRef]
- Otegui, M.B.P.; Blankensteyn, A.; Pagliosa, P.R. Population structure, growth and production of Thoracophelia furcifera (Polychaeta: Opheliidae) on a sandy beach in Southern Brazil. Helgol. Mar. Res. 2012, 66, 479–488. [Google Scholar] [CrossRef][Green Version]
- Peixoto, A.J.M.; Costa, T.D.M.M.; Santos, C.S.G. Population structure and dynamics of Perinereis anderssoni (Polychaeta: Nereididae) in a subtropical Atlantic Beach. Pan-Am. J. Aquat. Sci. 2018, 13, 188–199. [Google Scholar]
- Robinson, L.A.; Greenstreet, S.P.R.; Reiss, H.; Callaway, R.; Craeymeersch, J.; De Boois, I.; Degraer, S.; Ehrich, S.; Fraser, H.M.; Goffin, A.; et al. Length–weight relationships of 216 North Sea benthic Invertebrates and fish. J. Mar. Biol. Assoc. 2010, 90, 95–104. [Google Scholar] [CrossRef]
- Hua, E.; Zhang, Z.; Warwick, R.M.; Deng, K.; Lin, K.; Wang, R.; Yu, Z. Pattern of benthic biomass size spectra from shallow waters in the East China seas. Mar. Biol. 2013, 160, 1723–1736. [Google Scholar] [CrossRef]
- Górska, B.; Włodarska-Kowalczuk, M. Food and disturbance effects on Arctic benthic biomass and production size spectra. Prog. Oceanogr. 2017, 152, 50–61. [Google Scholar] [CrossRef]
- Costa-Paiva, E.M.; Paiva, P.C. A morphometric analysis of Eunice Cuvier (Annelida, Polychaeta) species. Rev. Bras. Zool. 2007, 24, 353–358. [Google Scholar] [CrossRef]
- Mistri, M.; Fano, E.A.; Rossi, R. Macrofaunal secondary production in a lagoon of the Po River Delta: An evaluation of estimation methods. Ital. J. Zool. 2001, 68, 147–151. [Google Scholar] [CrossRef]
- Benke, A.C.; Huryn, A.D.; Smock, L.A.; Wallace, J.B. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the south-eastern United States. J. N. Am. Benthol. Soc. 1999, 18, 308–343. [Google Scholar] [CrossRef]
- Rosati, I.; Barbone, E.; Basset, A. Length-mass relationships for transitional water benthic macroinvertebrates in Mediterranean and Black Sea ecosystems. Estuar. Coas. Shelf Sci. 2012, 113, 231–239. [Google Scholar] [CrossRef]
- Gayanilo, F.C., Jr.; Sparre, P.; Pauly, D. FAO-ICLARM Stock Assessment Tools II (FiSAT II). Revised version. In User’s Guide, FAO Computerized Information Series (Fisheries). No. 8, Revised version; FAO: Rome, Italy, 2005; 168p, Available online: https://www.fao.org/3/y5997e/y5997e.pdf (accessed on 14 January 2020).
- Hossain, M.B.; Banik, P.; Nur, A.U.; Rahman, T. Abundance and characteristics of microplastics in sediments from the world’s longest natural beach, Cox’s Bazar, Bangladesh. Mar. Poll. Bull. 2021, 163, 111956. [Google Scholar] [CrossRef] [PubMed]
- Paavo, B.; Ziegelmeyer, A.; Lavric, E.; Probert, P.K. Morphometric correlations and body mass regressions for Armandiam aculata, Aglaophamus macroura (Polychaeta), and Zethalia zelandica (Gastropoda), New Zealand. J. Mar. Freshwat. Res. 2008, 42, 85–91. [Google Scholar] [CrossRef]
- Pramanik, M.N.; Chowdhury, S.H.; Kabir, S.M.H. Annelida. In Encyclopedia of Flora and Fauna of Bangladesh; Ahmad, M., Ahmed, A.T.A., Rahman, A.K.A., Ahmed, Z.U., Begum, Z.N.T., Hassan, M.A., Khondker, M., Eds.; Annelida, Echinodermata, Acanthocephala and Minor Phyla; Asiat. Society of Bangladesh: Dhaka, Bengal, 2009; Volume 16, pp. 1–91. [Google Scholar]
- Muir, A.I.; Hossain, M.M.M. The intertidal polychaete (Annelida) fauna of the Sitakunda coast (Chittagong, Bangladesh), with notes on the Capitellidae, Glyceridae, Lumbrineridae, Nephtyidae, Nereididae and Phyllodocidae of the “Northern Bay of Bengal Ecoregion”. ZooKeys 2014, 419, 1–27. [Google Scholar] [CrossRef]
- Pauly, D.; Munro, J.L. Once more on the comparison of growth in fish and invertebrates. Fishbyte 1984, 2, 1–21. Available online: http://pubs.iclarm.net/Naga/na_1951.pdf (accessed on 24 January 2020).
- Beverton, R.J.H. A review of methods for estimating mortality rates in fish populations, with special reference to sources of bias in catch sampling. Rapp. Procès-Verbaux Reun. 1956, 140, 67–83. Available online: https://www.scienceopen.com/document?vid=5ce3b272-dd06-4925-94ab-41995bbd5d47 (accessed on 14 January 2020).
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Gulland, J.A. The fish resources of the Ocean West poly fleet, survey fishing news (Books) Ltd. FAO Tech. Pap. 1971, 97, 428. Available online: https://www.fao.org/3/al937e/al937e.pdf (accessed on 4 January 2020).
- Gulland, J.A. Estimation of mortality rates. In Annex to Arctic Fisheries Working Group Report (Meeting in Hamburg, January 1965); ICES, C.M.: Copenhagen, Denmark, 1965; Doc. No. 3 (mimeographed); Available online: https://www.ices.dk/sites/pub/CM%20Doccuments/1965/1965_3A%20-%20Annex%20-%20Estimation%20of%20Mortality%20Rates.pdf (accessed on 14 January 2020).
- Sakib, M.H.; Ahmmed, S.; Washim, M.R.; Islam, M.L.; Chowdhury, P. Population dynamics of mud crab, Scylla olivacea (Herbst, 1796) from the Sundarbans of Bangladesh. Aquac. Fish Fish. 2022, 2, 224–232. [Google Scholar] [CrossRef]
- Fischer, A. Reproductive and developmental phenomena in annelids: A source of exemplary research problems. Hydrobiologia 1999, 402, 1–20. [Google Scholar] [CrossRef]
- Hossain, M.B.; Hutchings, P. Nephtys bangladeshi n. sp., a new species of Nephtyidae (Annelida: Phyllodocida) from Bangladesh coastal waters. Zootaxa 2016, 4079, 41–52. [Google Scholar] [CrossRef]
- Komendantov, A.Y.; Aladin, N.V.; Ezhova, E.E. Environmental dependence of osmoregulation in Lycastopsis augeneri polychaeta nereidae. Zool. Žurnal 1989, 68, 137–140. Available online: https://eurekamag.com/research/007/305/007305572.php (accessed on 4 January 2020).
- Ezhova, E.E. Spawning and Early Ontogenesis of the Littoral Polychaete Namanereis littoralis (Grube, 1876) (Nereididae, Namanereidinae). Russi. J. Develop. Biol. 2011, 42, 159–166. [Google Scholar] [CrossRef]
- Wilson, R.S. Nerididae. In Fauna of Australia Volume 4A Polychaetes & Allies Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula Subtitle (1–30); Beesley, P.L., Ed.; Australian Biological Resources Study/CSIRO Publishing: Sydney, Australia, 2000. [Google Scholar]
- Fischer, A.; Dorresteijn, A. The polychaete Platynereis dumerilii (Annelida): A laboratory animal with spiralian cleavage, lifelong segment proliferation and a mixed benthic/pelagic life cycle. BioEssays 2004, 26, 314–325. [Google Scholar] [CrossRef]
- Johnston, T.A.; Cunjak, R.A. Dry mass-length relationships for benthic insects: A review with new data from Catamaran Brook, New Brunswick, Canada. Freshwat. Biol. 1999, 41, 653–674. [Google Scholar] [CrossRef]
- Wenzel, E.; Meyer, E.; Schwoerbel, J. Morphology and biomass determination of dominan mayfly larvae (Ephemeroptera) in running waters. Arch. Hydrobiol. 1990, 118, 31–46. [Google Scholar] [CrossRef]
- Towers, D.J.; Henderson, I.M.; Veltman, C.J. Predicting dry weight of New Zealand aquatic macroinvertebrates from linear dimensions. N. Z. J. Mar. Freshwat. Res. 1994, 28, 159–166. [Google Scholar] [CrossRef]
- Sette, C.S.C.; Shinozaki-Mendes, R.A.; Barros, T.L.; Souza, J.R.B. Age and growth of Alitta succinea (Polychaeta; Nereididae) in a tropical estuary of Brazil. J. Mar. Biol. Assoc. 2013, 93, 2123–2128. [Google Scholar] [CrossRef]
- Olive, P.J.W.; Lewis, C.; Beardall, V. Fitness components of seasonal reproduction: An analysis using Nereis virens as a life history model. Oceano. Acta 2000, 23, 377–389. [Google Scholar] [CrossRef]
- Scaps, P.; Rouabah, A.; Leprêtre, A. Morphological and biochemical evidence that Perinereis cultrifera (Polychaeta: Annelida) is a complex of species. J. Mar. Biol. Assoc. 2000, 80, 735–736. [Google Scholar] [CrossRef]
- Basset, A.; Glazier, D.S. Resource limitation and intraspecific patterns of weight length variation among spring detritivores. Hydrobiologia 1995, 316, 127–137. Available online: https://link.springer.com/article/10.1007/BF00016894 (accessed on 4 January 2020). [CrossRef]
- Basset, A.; Rossi, L. Competitive trophic niche modifications in three populations of detritivores. Functional Ecol. 1990, 4, 685–694. [Google Scholar] [CrossRef]
- Basset, A. Resource-mediated effects of stream pollution on food absorption of Asellus aquaticus (L.) populations. Oecologia 1993, 93, 315–321. Available online: https://link.springer.com/article/10.1007/BF00317872 (accessed on 4 January 2020). [CrossRef]
- Scaps, P. The exploitation and aquaculture of marine polychaetes. Bull. Soc. Zool. Fr. 2003, 128, 21–33. Available online: https://www.researchgate.net/publication/286049351_The_exploitation_and_aquaculture_of_marine_polychaetes (accessed on 4 January 2020).
- Garcia-Alonso, J.; Muller, C.T.; Hardege, J.D. Influence of food regimes and seasonality on fatty acid composition in the ragworm. Aqua. Biol. 2008, 4, 7–13. [Google Scholar] [CrossRef]


| References | Groups | Months | Equation | n | Intercept (a) | Slope (b) | SE | p-Value | log r2 | 1 r2 | pw r2 | e r2 | p r2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present Study | N. fauveli, Nereididae | August, 2020 | W = aL1.50 | 87 | 0.741 | 1.50 | 0.047 | <0.001 | 0.82 | 0.89 | 0.90 | 0.92 | 0.92 |
| September, 2020 | W = aL1.53 | 77 | 0.732 | 1.53 | 0.068 | <0.001 | 0.79 | 0.80 | 0.82 | 0.80 | 0.80 | ||
| October, 2020 | W = aL1.36 | 122 | 0.820 | 1.36 | 0.148 | <0.001 | 0.43 | 0.44 | 0.48 | 0.47 | 0.45 | ||
| November, 2020 | W = aL1.80 | 109 | 0.599 | 1.80 | 0.086 | <0.001 | 0.70 | 0.75 | 0.76 | 0.77 | 0.77 | ||
| December, 2020 | W = aL1.72 | 111 | 0.779 | 1.72 | 0.254 | <0.001 | 0.69 | 0.64 | 0.69 | 0.70 | 0.66 | ||
| January, 2021 | W = aL1.52 | 130 | 0.742 | 1.52 | 0.062 | <0.001 | 0.70 | 0.78 | 0.85 | 0.88 | 0.87 | ||
| February, 2021 | W = aL1.63 | 105 | 0.824 | 1.63 | 0.058 | <0.001 | 0.74 | 0.83 | 0.86 | 0.89 | 0.89 | ||
| March, 2021 | W = aL2.12 | 104 | 0.720 | 2.12 | 0.039 | <0.001 | 0.88 | 0.87 | 0.90 | 0.87 | 0.87 | ||
| April, 2021 | W = aL2.03 | 104 | 0.698 | 2.03 | 0.030 | <0.001 | 0.93 | 0.94 | 0.94 | 0.92 | 0.94 | ||
| May, 2021 | W = aL1.53 | 106 | 0.715 | 1.53 | 0.890 | <0.001 | 0.80 | 0.87 | 0.90 | 0.90 | 0.91 | ||
| June, 2021 | W = aL1.18 | 129 | 0.372 | 1.18 | 0.740 | <0.001 | 0.12 | 0.19 | 0.20 | 0.37 | 0.59 | ||
| July, 2021 | W = aL1.72 | 101 | 0.657 | 1.72 | 0.103 | <0.001 | 0.80 | 0.81 | 0.82 | 0.81 | 0.82 | ||
| Overall | W = aL1.65 | 1300 | 0.72 | 1.65 | 0.138 | <0.001 | 0.63 | 0.70 | 0.72 | 0.71 | 0.73 | ||
| [32] | Armandia maculata Opheliidae | March, 2003 | eFW = L3.52 | 69 | 0.0014 | 3.52 | − | <0.05 | 0.69 | 0.77 | 0.88 | 0.80 | 0.79 |
| March, 2003 | DW = L3.24 | 69 | 0.0006 | 3.24 | − | <0.05 | 0.69 | 0.77 | 0.88 | 0.80 | 0.79 | ||
| March, 2003 | AFW = W2.39 | 58 | 0.3102 | 2.39 | − | <0.05 | 0.55 | 0.60 | 0.76 | 0.73 | 0.61 | ||
| [32] | Aglaophamus macroura, Nephtyidae | March, 2003 | W7 = e0.04L | 56 | 0.2867 | 0.04 | − | <0.05 | 0.62 | 0.78 | 0.70 | 0.77 | 0.82 |
| March, 2003 | eFW = e0.16L | 56 | 0.1871 | 0.16 | − | <0.05 | 0.50 | 0.73 | 0.89 | 0.90 | 0.92 | ||
| March, 2003 | DW= e0.15L | 56 | 0.0359 | 0.15 | − | <0.05 | 0.50 | 0.72 | 0.90 | 0.91 | 0.90 | ||
| March, 2003 | AFW = e0.14L | 0.0312 | 0.14 | − | <0.05 | 0.48 | 0.69 | 0.87 | 0.91 | 0.86 | |||
| March, 2003 | eFW = TA1.5 | 0.1847 | 1.5 | − | <0.05 | 0.60 | 0.93 | 0.97 | 0.75 | 0.97 | |||
| [29] | Phyllodocida, Nereididae | November, 2004 and May 2005 | W = aL1.11 | 5 | 0.0841 | 1.11 | − | − | − | 0.54 | − | − | − |
| [32] | Hediste diversicolor, Nereididae | November, 2004 and May, 2005 | W = aL2.20 | 207 | 0.0023 | 2.20 | − | − | − | 0.90 | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, M.J.; Sarker, P.K.; Islam, M.A.; Rima, N.N.; Joydas, T.V.; Sultana, N.; Islam, M.M.; Hossain, M.Y.; Hossain, M.B. Biometry, Growth, and Recruitment Pattern of a Commercially Important Nereid polychaete, Namalycastis fauveli, from the East Coast of Bangladesh. J. Mar. Sci. Eng. 2024, 12, 312. https://doi.org/10.3390/jmse12020312
Sarker MJ, Sarker PK, Islam MA, Rima NN, Joydas TV, Sultana N, Islam MM, Hossain MY, Hossain MB. Biometry, Growth, and Recruitment Pattern of a Commercially Important Nereid polychaete, Namalycastis fauveli, from the East Coast of Bangladesh. Journal of Marine Science and Engineering. 2024; 12(2):312. https://doi.org/10.3390/jmse12020312
Chicago/Turabian StyleSarker, Md. Jahangir, Pallab Kumer Sarker, Md. Ariful Islam, Nazmun Naher Rima, Thadickal Viswanathan Joydas, Nahid Sultana, Md. Monirul Islam, Md. Yeamin Hossain, and Mohammad Belal Hossain. 2024. "Biometry, Growth, and Recruitment Pattern of a Commercially Important Nereid polychaete, Namalycastis fauveli, from the East Coast of Bangladesh" Journal of Marine Science and Engineering 12, no. 2: 312. https://doi.org/10.3390/jmse12020312
APA StyleSarker, M. J., Sarker, P. K., Islam, M. A., Rima, N. N., Joydas, T. V., Sultana, N., Islam, M. M., Hossain, M. Y., & Hossain, M. B. (2024). Biometry, Growth, and Recruitment Pattern of a Commercially Important Nereid polychaete, Namalycastis fauveli, from the East Coast of Bangladesh. Journal of Marine Science and Engineering, 12(2), 312. https://doi.org/10.3390/jmse12020312








