Soft-Shell Production of the Invasive Atlantic Blue Crab Callinectes sapidus in the Lesina Lagoon (SE Italy): A First Assessment
Abstract
1. Introduction
- (i)
- To explore for the first time in Europe the suitability of a pond system to produce SSC; to this end, the flow-through or recirculating cultivation facilities in use in native areas to rear pre-molt individuals of C. sapidus (locally known as peelers) were taken as a model [24];
- (ii)
- To investigate the molting process in blue crabs from the Lesina Lagoon and verify if the population is characterized by a predictable molting pattern similar to that observed in native areas.
2. Materials and Methods
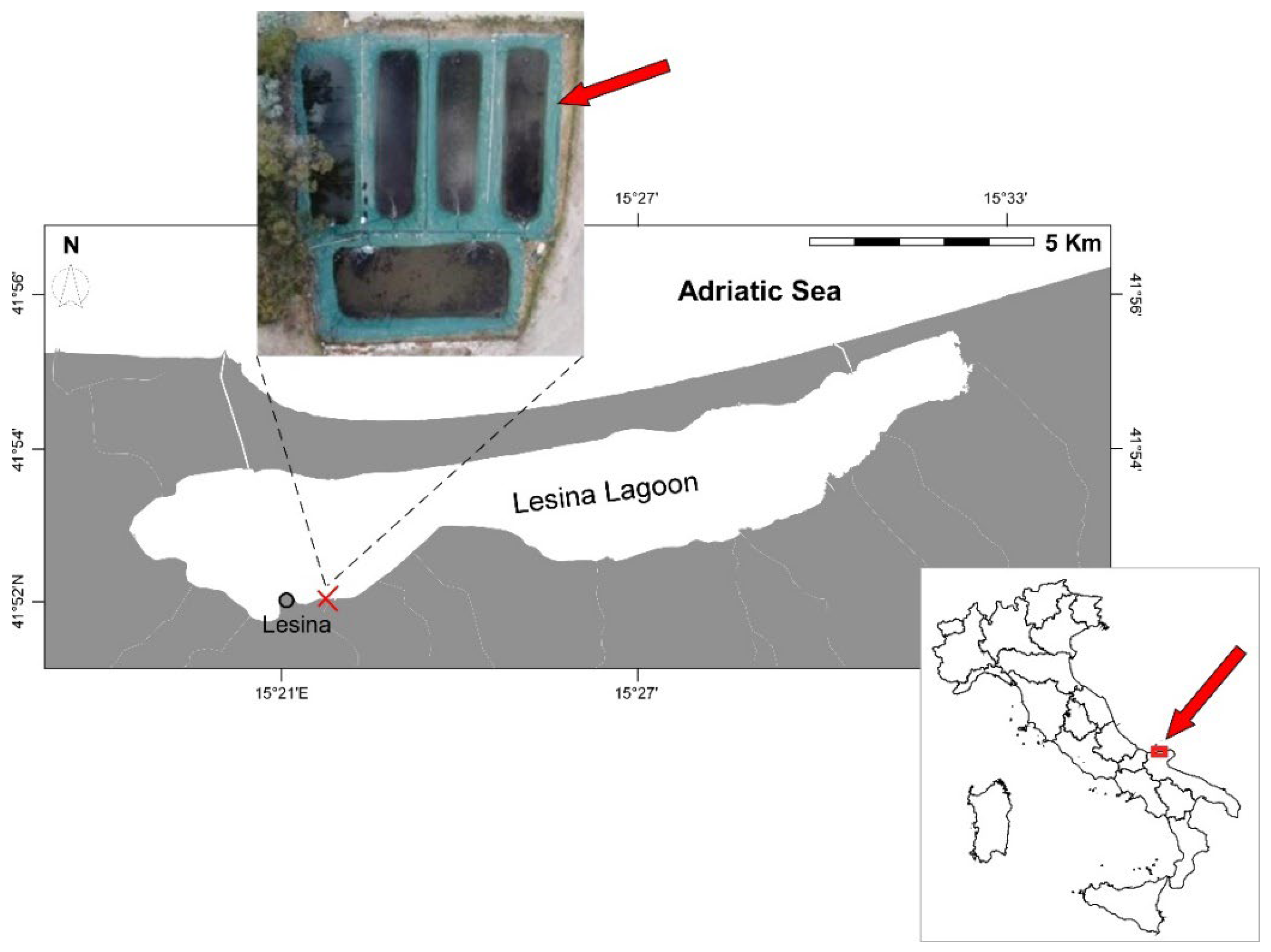
2.1. Study Area
2.2. Experimental Setup
2.3. Data Analysis
3. Results
3.1. Crab Sex Ratio and Biometry at the Start of the Trial
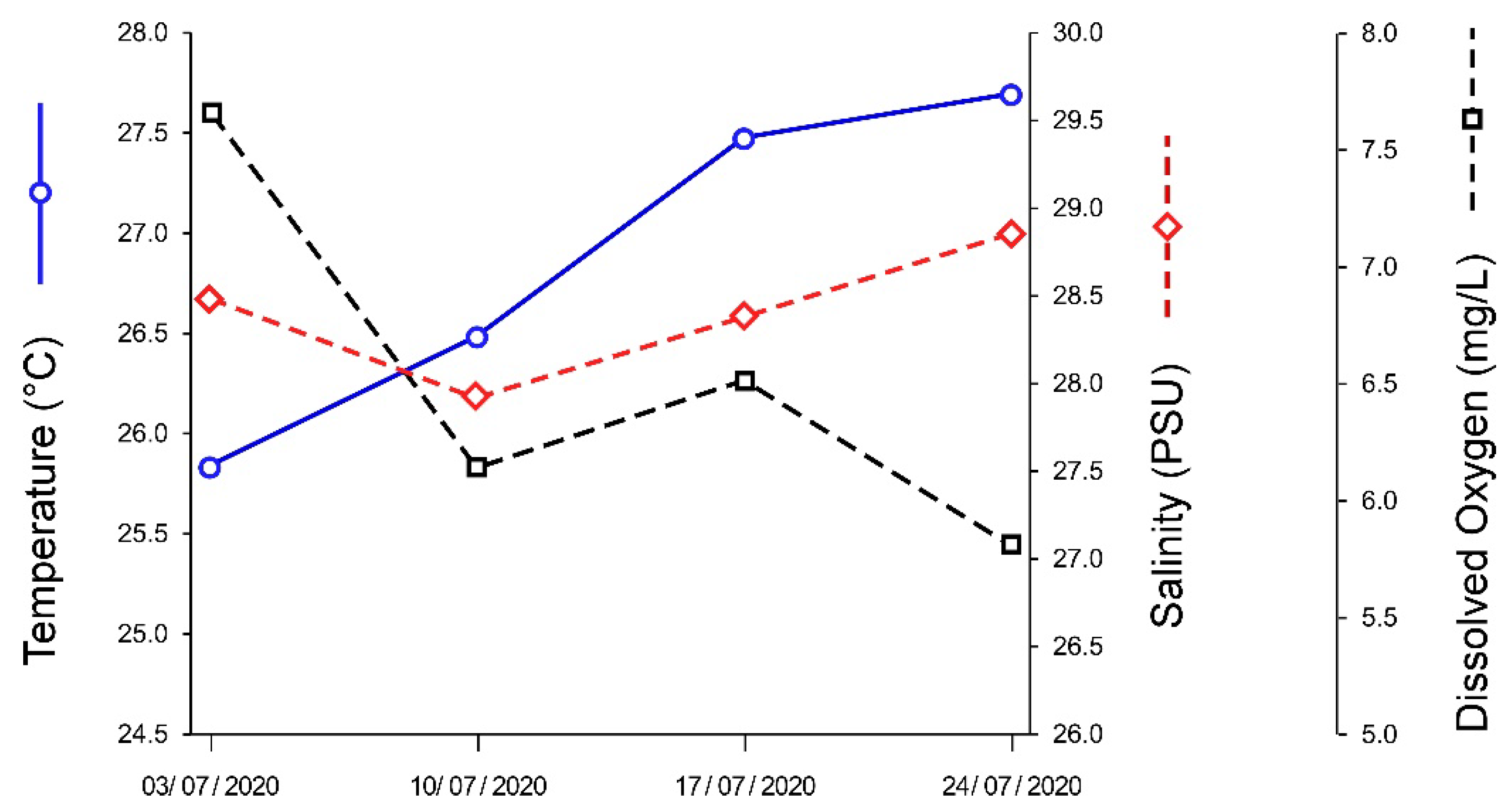
3.2. Pond Water Parameters
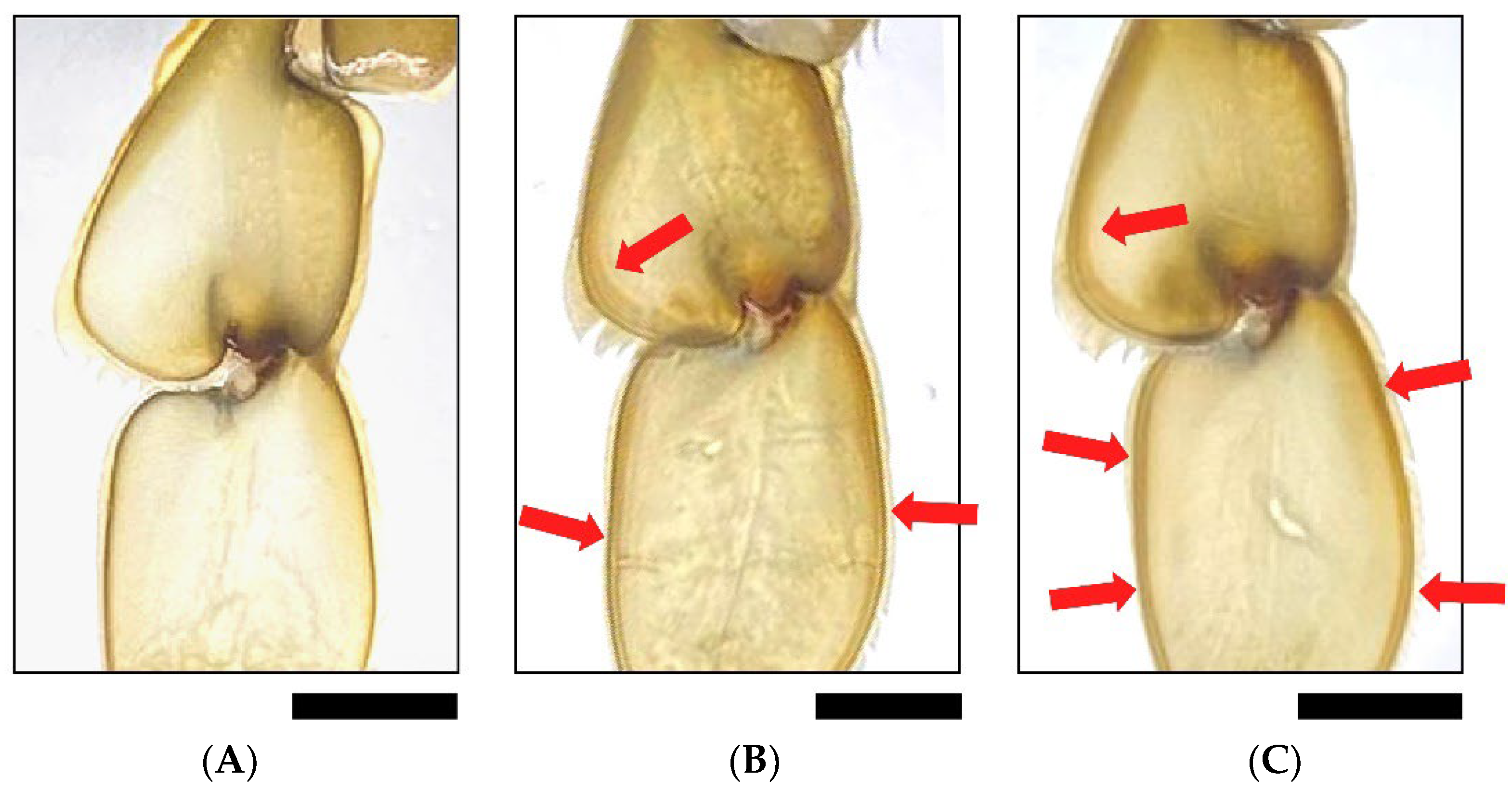
3.3. Crab Mortality and Molting during the Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- IPCC. Summary for Policymakers. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; IPCC, Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., et al., Eds.; IPCC: Geneva, Switzerland, 2019; p. 36. [Google Scholar]
- Dehnen-Schmutz, K.; Boivin, T.; Essl, F.; Groom, Q.J.; Harrison, L.; Touza, J.M.; Bayliss, H. Alien futures: What is on the horizon for biological invasions? Divers. Distrib. 2018, 24, 1149–1157. [Google Scholar] [CrossRef]
- Lieurance, D.; Canavan, S.; Behringer, D.C.; Kendig, A.E.; Minteer, C.R.; Reisinger, L.S.; Romagosa, C.M.; Flory, S.L.; Lockwood, J.L.; Anderson, P.J.; et al. Identifying invasive species threats, pathways, and impacts to improve biosecurity. Ecosphere 2023, 14, e4711. [Google Scholar] [CrossRef]
- Reaser, J.K.; Burgiel, S.W.; Kirkey, J.; Brantley, K.A.; Veatch, S.D.; Burgos-Rodríguez, J. The early detection of and rapid response (EDRR) to invasive species: A conceptual framework and federal capacities assessment. Biol. Invasions 2020, 22, 1–19. [Google Scholar] [CrossRef]
- Russell, J.C.; Binnie, H.R.; Oh, J.; Anderson, D.P.; Samaniego-Herrera, A. Optimizing confirmation of invasive species eradication with rapid eradication assessment. J. Appl. Ecol. 2017, 54, 160–169. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C.; et al. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef]
- Green, S.J.; Grosholz, E.D. Functional eradication as a framework for invasive species control. Front. Ecol. Environ. 2021, 19, 98–107. [Google Scholar] [CrossRef]
- Grosholz, E.; Ashton, G.; Bradley, M.; Brown, C.; Ceballos-Osuna, L.; Chang, A.; de Rivera, C.; Gonzalez, J.; Heineke, M.; Marraffini, M.; et al. Stage-specific overcompensation, the hydra effect, and the failure to eradicate an invasive predator. Proc. Natl. Acad. Sci. USA 2021, 118, e2003955118. [Google Scholar] [CrossRef]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. On the Atlantic blue crab (Callinectes sapidus Rathbun 1896) in southern European coastal waters: Time to turn a threat into a resource? Fish. Res. 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Cerveira, I.; Baptista, V.; Teodósio, M.A.; Morais, P. What’s for dinner? Assessing the value of an edible invasive species and outreach actions to promote its consumption. Biol. Invasions 2022, 24, 815–829. [Google Scholar] [CrossRef]
- Ens, N.J.; Harvey, B.; Davies, M.M.; Thomson, H.M.; Meyers, K.J.; Yakimishyn, J.; Lee, L.C.; McCord, M.E.; Gerwing, T.G. The Green Wave: Reviewing the environmental impacts of the invasive European green crab (Carcinus maenas) and potential management approaches. Environ. Rev. 2022, 30, 306–322. [Google Scholar] [CrossRef]
- Millikin, M.R.; Williams, A.B. Synopsis of Biological Data on Blue Crab, Callinectes sapidus Rathbun; FAO Fisheries Synopsis 38; FAO: Rome, Italy, 1984; p. 39. [Google Scholar]
- Johnson, D.S. The savory swimmer swims north: A northern range extension of the blue crab Callinectes sapidus? J. Crust. Biol. 2015, 35, 105–110. [Google Scholar] [CrossRef]
- Mancinelli, G.; Bardelli, R.; Zenetos, A. A global occurrence database of the Atlantic blue crab Callinectes sapidus. Sci. Data 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Nehring, S. Invasion History and Success of the American Blue Crab Callinectes sapidus in European and Adjacent Waters. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Invading Nature—Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2011; Volume 6, pp. 607–624. [Google Scholar]
- Chaouti, A.; Belattmania, Z.; Nadri, A.; Serrão, E.; Encarnação, J.; Teodósio, M.; Reani, A.; Sabour, B. The invasive Atlantic blue crab Callinectes sapidus Rathbun, 1896 expands its distributional range southward to Atlantic African shores: First records along the Atlantic coast of Morocco. Bioinvasions Rec. 2022, 11, 227–237. [Google Scholar] [CrossRef]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. The Atlantic blue crab Callinectes sapidus in southern European coastal waters: Distribution, impact and prospective invasion management strategies. Mar. Pollut. Bull. 2017, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N.; et al. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Clavero, M.; Franch, N.; Bernardo-Madrid, R.; López, V.; Abelló, P.; Queral, J.M.; Mancinelli, G. Severe, rapid and widespread impacts of an Atlantic blue crab invasion. Mar. Pollut. Bull. 2022, 176, 113479. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.T.; Verani, J.R.; Nordi, N. Evaluation and management of blue crab Callinectes sapidus (Rathbun, 1896) (Decapoda-Portunidae) fishery in the Estuary of Cananéia, Iguape and Ilha Comprida, São Paulo, Brazil. Braz. J. Biol. 2010, 70, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Woodward, R.T.; Wilberg, M.J.; Tomberlin, D. Management evaluation for the Chesapeake Bay blue crab fishery: An integrated bioeconomic approach. N. Am. J. Fish Manag. 2015, 35, 216–228. [Google Scholar] [CrossRef]
- Hungria, D.B.; dos Santos Tavares, C.P.; Pereira, L.Â.; de Assis Teixeira da Silva, U.; Ostrensky, A. Global status of production and commercialization of soft-shell crabs. Aquacult. Int. 2017, 25, 2213–2226. [Google Scholar] [CrossRef]
- Prestes dos Santos Tavares, C.; Silva, U.A.T.; Pereira, L.A.; Ostrensky, A. Systems and techniques used in the culture of soft-shell swimming crabs. Rev. Aquacult. 2018, 10, 913–923. [Google Scholar] [CrossRef]
- Wickins, J.F.; O’C Lee, D. Crustacean Farming, Ranching and Culture; Wiley-Blackwell: Oxford, UK, 2002; p. 480. [Google Scholar]
- Ayas, D.; Ozogul, Y. The effects of sex and seasonality on the metal levels of different muscle tissues of mature Atlantic blue crabs (Callinectes sapidus) in Mersin Bay, north-eastern Mediterranean. Int. J. Food Sci. Tech. 2011, 46, 2030–2034. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Antoniadou, C.; Avramoglou, K.; Efstathiadis, J.; Chintiroglou, C. Population structure of the blue crab Callinectes sapidus in Thermaikos Gulf (Methoni Bay). In Proceedings of the 15th Pan-Hellenic Congress of Ichthyologists, Thessaloniki, Greece, 10–13 October 2013; pp. 113–116. [Google Scholar]
- Kevrekidis, K.; Kevrekidis, T.; Mogias, A.; Boubonari, T.; Kantaridou, F.; Kaisari, N.; Malea, P.; Dounas, C.; Thessalou-Legaki, M. Fisheries biology and basic life-cycle characteristics of the invasive blue crab Callinectes sapidus Rathbun in the estuarine area of the Evros River (Northeast Aegean Sea, Eastern Mediterranean). J. Mar. Sci. Eng. 2023, 11, 462. [Google Scholar] [CrossRef]
- Ibarra, L.E.; Olivas, E.; Partida, A.L.; Paredes, D. Generation of added value through the process of Soft Shell Crab: A sustainable development option in the coastal region of Sonora. J. Manag. Sustain. 2015, 5, 57–68. [Google Scholar] [CrossRef]
- Florio, M.; Breber, P.; Scirocco, T.; Specchiulli, A.; Cilenti, L.; Lumare, L. Exotic species in Lesina and Varano lakes: Gargano National Park (Italy). Transit. Water Bull. 2008, 2, 69–79. [Google Scholar]
- Cilenti, L.; Pazienza, G.; Scirocco, T.; Fabbrocini, A.; D’Adamo, R. First record of ovigerous Callinectes sapidus (Rathbun, 1896) in the Gargano Lagoons (south-west Adriatic Sea). Bioinvasions Rec. 2015, 4, 281–287. [Google Scholar] [CrossRef]
- Bardelli, R.; Mancinelli, G.; Mazzola, A.; Vizzini, S. The Atlantic blue crab Callinectes sapidus spreading in the Tyrrhenian sea: Evidence of an established population in the Stagnone di Marsala (Sicily, southern Italy). Nase More 2023, 70, 177–183. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Antoniadou, C. Abundance and population structure of the blue crab (Decapoda, Portunidae) in Thermaikos Gulf (Methoni Bay), northern Aegean Sea. Crustaceana 2018, 91, 641–657. [Google Scholar] [CrossRef]
- Mancinelli, G.; Glamuzina, B.; Petrić, M.; Carrozzo, L.; Glamuzina, L.; Zotti, M.; Raho, D.; Vizzini, S. The trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in the food web of Parila Lagoon (South Eastern Adriatic, Croatia): A first assessment using stable isotopes. Mediterr. Mar. Sci. 2016, 17, 634–643. [Google Scholar] [CrossRef]
- Mancinelli, G.; Guerra, M.T.; Alujević, K.; Raho, D.; Zotti, M.; Vizzini, S. Trophic flexibility of the Atlantic blue crab Callinectes sapidus in invaded coastal systems of the Apulia region (SE Italy): A stable isotope analysis. Estuar. Coast. Shelf Sci. 2017, 198, 421–431. [Google Scholar] [CrossRef]
- Kampouris, T.E.; Porter, J.S.; Sanderson, W.G. Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae): An assessment on its diet and foraging behaviour, Thermaikos Gulf, NW Aegean Sea, Greece: Evidence for ecological and economic impacts. Crustac. Res. 2019, 48, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Prado, P.; Ibáñez, C.; Chen, L.; Caiola, N. Feeding habits and short-term mobility patterns of blue crab, Callinectes sapidus, across invaded habitats of the Ebro Delta subjected to contrasting salinity. Estuar. Coasts 2021, 45, 839–855. [Google Scholar] [CrossRef]
- Mancinelli, G.; Rossi, L. Indirect, size-dependent effects of crustacean mesograzers on the Rhodophyta Gracilaria verrucosa (Hudson) Papenfuss: Evidence from a short-term study in the Lesina Lagoon (Italy). Mar. Biol. 2001, 138, 1163–1173. [Google Scholar]
- Roselli, L.; Fabbrocini, A.; Manzo, C.; D’Adamo, R. Hydrological heterogeneity, nutrient dynamics and water quality of a non-tidal lentic ecosystem (Lesina Lagoon, Italy). Estuar. Coast. Shelf Sci. 2009, 84, 539–552. [Google Scholar] [CrossRef]
- Manzo, C.; Fabbrocini, A.; Roselli, L.; D’Adamo, R. Characterization of the fish assemblage in a Mediterranean coastal lagoon: Lesina Lagoon (central Adriatic Sea). Reg. Stud. Mar. Sci. 2016, 8, 192–200. [Google Scholar] [CrossRef]
- Smith, S.G.; Chang, E.C. Molting and Growth. In The Blue Crab: Callinectes sapidus; Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant College: College Park, MD, USA, 2007; pp. 197–255. [Google Scholar]
- Oesterling, M.J. Manual for Handling and Shedding Blue Crabs (Callinectes sapidus); Virgina Sea Grant Program; Virginia Institute of Marine Science, College of William & Mary: Gloucester Point, VA, USA, 1988; p. 102. [Google Scholar]
- Campbell, I. Chi-squared and Fisher–Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T.E. The analysis of 2 × 2 contingency tables—Yet again. Stat. Med. 2011, 30, 890. [Google Scholar] [CrossRef]
- Anderson, M.J. Permanova: A Fortran Computer Program for Permutational Multivariate Analysis of Variance; University of Auckland: Auckland, New Zealand, 2005. [Google Scholar]
- Malone, R.F.; Burden, D.G. Design of Recirculating Blue Crab Shedding Systems; Louisiana Sea Grant College Program, Center for Wetland Resources: Baton Rouge, LA, USA, 1988; p. 86. [Google Scholar]
- Wehrtmann, I.S.; Mena-Castañeda, D. Molt sign description of the Pacific blue crab Callinectes arcuatus Ordway, 1863 (Decapoda, Portunidae). Nauplius 2003, 11, 135–139. [Google Scholar]
- Prestes dos Santos Tavares, C.; Assis Teixeira da Silva, U.; Pereira, L.A.; Ostrensky, A. Evaluation of different induced molting methods in Callinectes ornatus (Crustacea, Decapoda, Portunidae) as a tool for the commercial production of soft-shell crabs. Anais Acad. Bras. Cienc. 2021, 93, e20190580. [Google Scholar] [CrossRef]
- Prestes dos Santos Tavares, C.; Zhao, M.; Lopes Vogt, É.; Felipe Argenta Model, J.; Sommer Vinagre, A.; de Assis Teixeira da Silva, U.; Ostrensky, A.; James Schott, E. High prevalence of CsRV2 in cultured Callinectes danae: Potential impacts on soft-shell crab production in Brazil. J. Invertebr. Pathol. 2022, 190, 107739. [Google Scholar] [CrossRef]
- Spitznagel, M.I.; Small, H.J.; Lively, J.A.; Shields, J.D.; Schott, E.J. Investigating risk factors for mortality and reovirus infection in aquaculture production of soft-shell blue crabs (Callinectes sapidus). Aquaculture 2019, 502, 289–295. [Google Scholar] [CrossRef]
- Ostrensky, A.; Ventura, R.; Araújo Corrêa, A.M.; Vieira Santos, G.; Castilho-Westphal, G.G. Improving production of soft-shelled swimming crabs: Macroscopic signs of molting as a tool for selection and management of Callinectes danae and Callinectes exasperatus (Portunidae, Decapoda, Crustacea). Arch. Vet. Sci. 2015, 20, 122–131. [Google Scholar] [CrossRef][Green Version]
- Chaves, J.C.; Eggleston, D.B. Blue crab mortality in the North Carolina soft-shell industry: Biological and operational effects. J. Shellfish Res. 2003, 22, 241–249. [Google Scholar]
- Guillory, V. A review of incidental fishing mortalities of blue crabs. In Proceedings of the Blue Crab Mortality Symposium, Lafayette, LA, USA, 28–29 May 1999; pp. 28–41. [Google Scholar]
- Pellizzato, M. Pesca ed allevamento di Carcinus aestuarii, Nardo 1847 nel contesto della attivita alieutiche lagunari e delle tradizioni Venete. In Proceedings of the Convegno: La Risorsa Crostacei nel Mediterraneo: Ricerca, Produzione e Mercato, Corte Benedettina, Legnaro, Italy, 25–26 November 2010; pp. 136–145. [Google Scholar]
- Lazzarini, R.; Vendramini, A.; Cruciani, L.; Galvan, T. Moleche in laguna di Venezia: Dati di produzione ed efficienza del sistema. Il Pesce 2023, 2, 1–7. [Google Scholar]
- Cataudella, S.; Crosetti, D.; Massa, F. Mediterranean Coastal Lagoons: Sustainable Management and Interactions Among Aquaculture, Capture Fisheries and the Environment; Food and Agricolture Organization of the United Nations: Rome, Italy, 2015; Volume 95, p. 293. [Google Scholar]
- Sundet, J.H.; Hoel, A.H. The Norwegian management of an introduced species: The Arctic red king crab fishery. Mar. Policy 2016, 72, 278–284. [Google Scholar] [CrossRef]

| Treatment | Sex | CW (mm) | CL (mm) | WW (g) |
|---|---|---|---|---|
| C group | F (7) | 108.29 (12.13) | 53.86 (4.85) | 100.54 (27.83) |
| M (3) | 114.33 (19.35) | 52.01 (4.01) | 132.71 (58.39) | |
| W group | F (16) | 113.44 (11.87) | 55.06 (4.99) | 108.64 (28.05) |
| M (4) | 102.01 (12.08) | 53.01 (4.76) | 92.39 (34.97) | |
| R group | F (11) | 102.45 (11.94) | 50.18 (4.85) | 84.51 (23.59) |
| M (9) | 103.22 (14.78) | 52.89 (7.15) | 97.36 (47.08) |
| Factor | Degree of Freedom * | FCW | FCL | FWW |
|---|---|---|---|---|
| Sex (1) | 1 | 0.13 (0.72) | 0.05 (0.82) | 0.73 (0.39) |
| Treatment (2) | 2 | 1.41 (0.25) | 0.87 (0.43) | 1.72 (0.19) |
| 1 × 2 | 2 | 1.37 (0.26) | 0.98 (0.38) | 1.41 (0.25) |
| Parameter | Pond | Optimal Conditions |
|---|---|---|
| Temperature (°C) | 26.9 ± 0.9 | 21.1–26.7 |
| Salinity (PSU) | 28.4 ± 0.4 | ±5 PSU of harvesting water |
| Dissolved Oxygen (mg/L) | 6.5 ± 0.8 | >5 |
| Treatment | Dead | Molting | Non-Molting | Days to Molting |
|---|---|---|---|---|
| C group | 2 (20%) | 1 (10%) | 7 (70%) | 17 |
| W group | 2 (10%) | 13 (65%) | 5 (25%) | 13.07 ± 6.91 |
| R group | 3 (15%) | 17 (85%) | 0 | 4 ± 1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cilenti, L.; Lago, N.; Lillo, A.O.; Li Veli, D.; Scirocco, T.; Mancinelli, G. Soft-Shell Production of the Invasive Atlantic Blue Crab Callinectes sapidus in the Lesina Lagoon (SE Italy): A First Assessment. J. Mar. Sci. Eng. 2024, 12, 310. https://doi.org/10.3390/jmse12020310
Cilenti L, Lago N, Lillo AO, Li Veli D, Scirocco T, Mancinelli G. Soft-Shell Production of the Invasive Atlantic Blue Crab Callinectes sapidus in the Lesina Lagoon (SE Italy): A First Assessment. Journal of Marine Science and Engineering. 2024; 12(2):310. https://doi.org/10.3390/jmse12020310
Chicago/Turabian StyleCilenti, Lucrezia, Nicola Lago, Antonio Oscar Lillo, Daniel Li Veli, Tommaso Scirocco, and Giorgio Mancinelli. 2024. "Soft-Shell Production of the Invasive Atlantic Blue Crab Callinectes sapidus in the Lesina Lagoon (SE Italy): A First Assessment" Journal of Marine Science and Engineering 12, no. 2: 310. https://doi.org/10.3390/jmse12020310
APA StyleCilenti, L., Lago, N., Lillo, A. O., Li Veli, D., Scirocco, T., & Mancinelli, G. (2024). Soft-Shell Production of the Invasive Atlantic Blue Crab Callinectes sapidus in the Lesina Lagoon (SE Italy): A First Assessment. Journal of Marine Science and Engineering, 12(2), 310. https://doi.org/10.3390/jmse12020310








