Dietary Fishmeal Replacement by Methanol-Extracted Cottonseed Meal with Amino Acid Supplementation for Juvenile Cobia Rachycentron canadum
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Formulations
2.2. Facilities, Feeding Trials and Fish
2.3. Sample Collection and Chemical Analyses
2.4. Response Measurements and Calculations
2.5. Statistical Analysis
3. Results
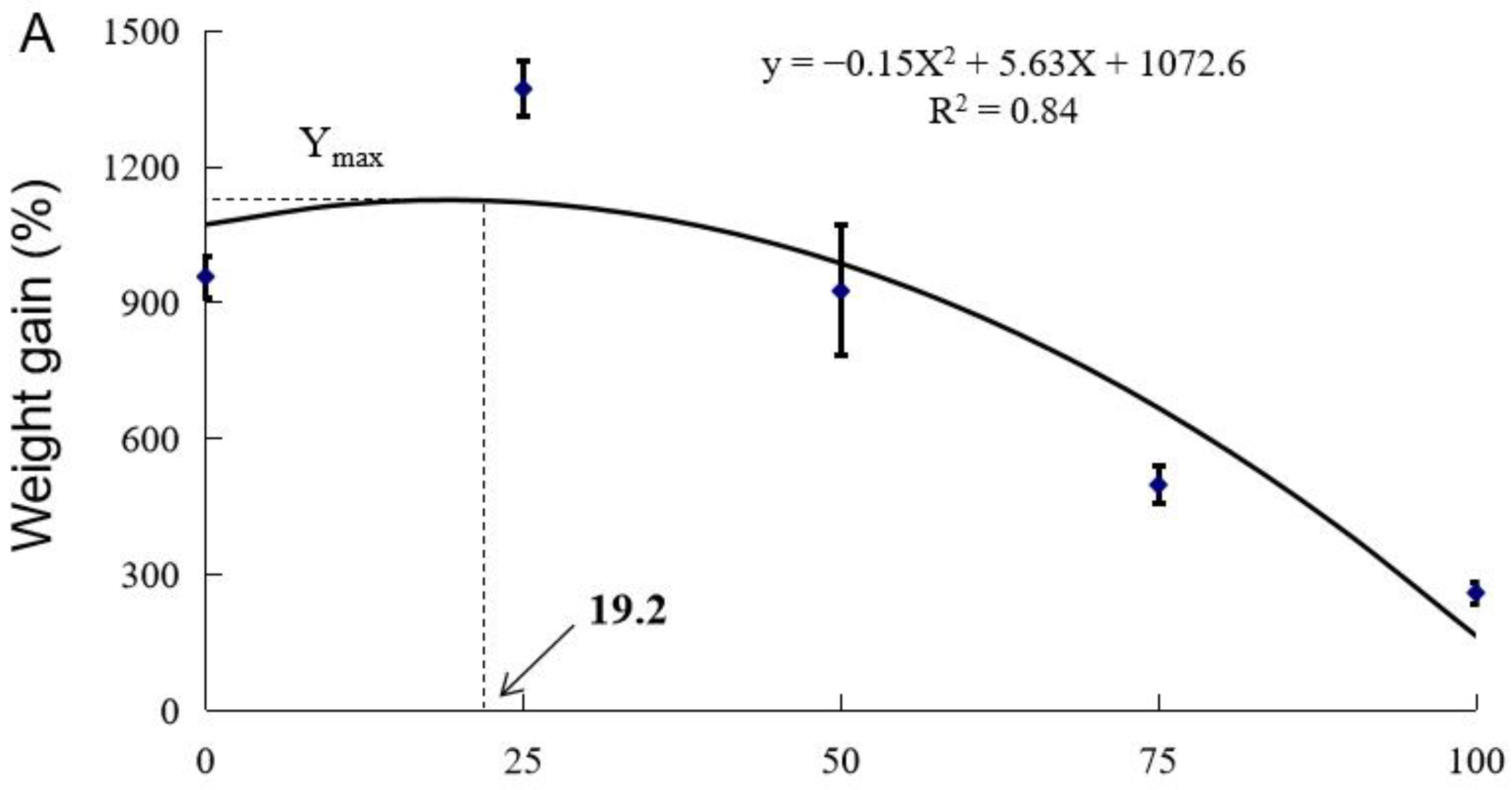
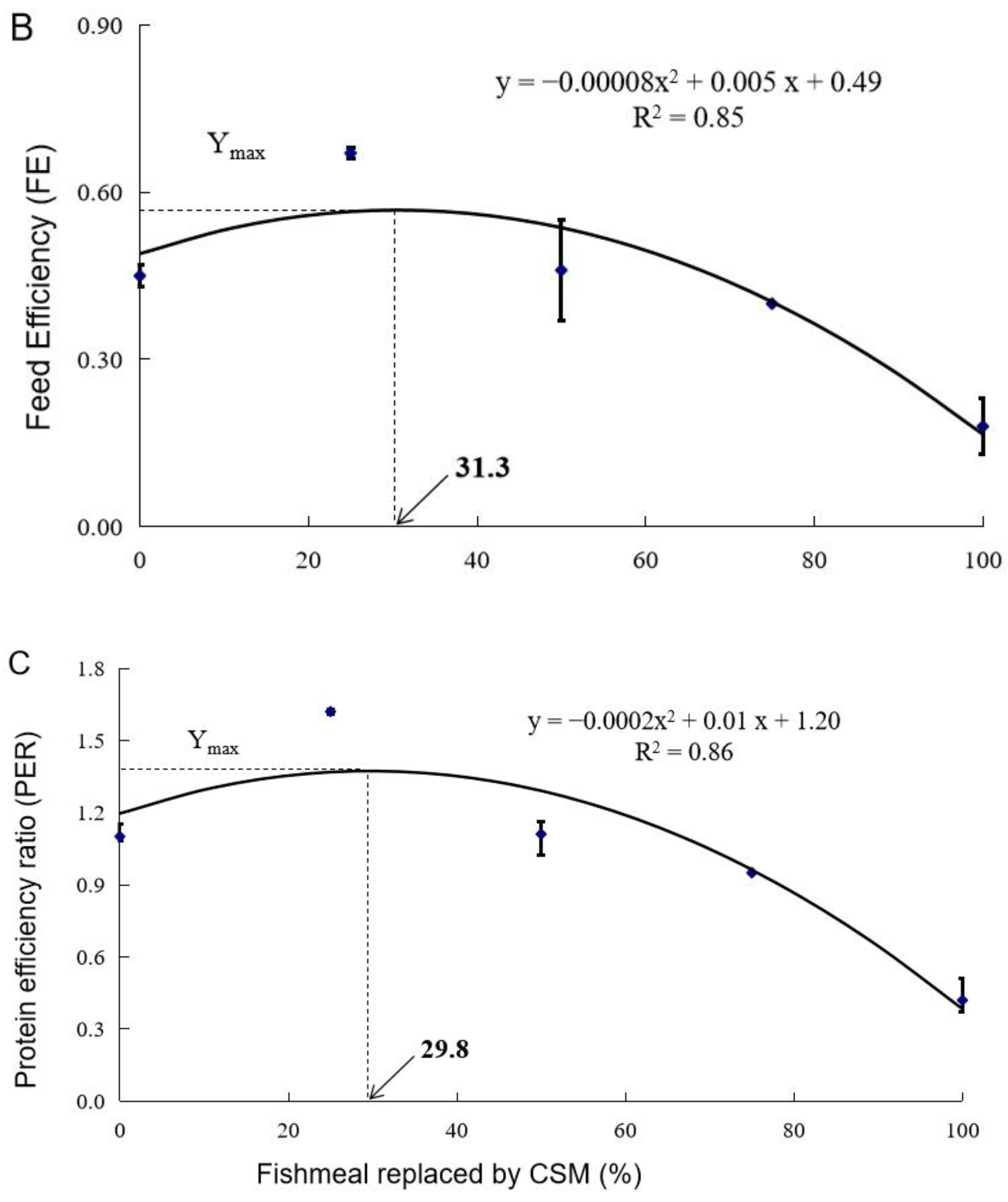
3.1. Growth Performance
3.2. Body Condition Indices and Whole-Body Composition
3.3. Amino Acid Profiles of Whole Bodies
3.4. Digestive Enzyme Activities
3.5. Antioxidant Enzyme Activities and Serum Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gatlin, D.M., III; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, Ǻ.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds—A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Indexmundi. Fishmeal Monthly Price. Available online: http://indexmundi.com/commodities/?commodity=fish-meal (accessed on 21 January 2015).
- El-Saidy, D.M.S.; Gaber, M.M. Use of cottonseed meal supplemented with iron for detoxification of gossypol as a total replacement of fish meal in Nile tilapia, Oreochromis niloticus (L.) diets. Aquac. Res. 2004, 35, 859–865. [Google Scholar] [CrossRef]
- Li, M.H.; Robinson, E.H. Use of cottonseed meal in aquatic animal diets: A review. N. Am. J. Aquac. 2006, 68, 14–22. [Google Scholar] [CrossRef]
- Romano, G.B.; Scheffler, J.A. Lowering seed gossypol content in glanded cotton (Gossypium hirsutum L.) lines. Plant Breed. 2008, 127, 619–624. [Google Scholar] [CrossRef]
- Wang, J.; Clark, G.; Ju, M.; Castillo, S.; Gatlin, D.M., III. Effects of replacing menhaden fishmeal with cottonseed flour on growth performance, feed utilization and body composition of juvenile red drum Sciaenops ocellatus. Aquaculture 2020, 523, 735217. [Google Scholar] [CrossRef]
- Wu, G.D.; Lan, K.P.; Chen, X.; Wang, Y.; Zhou, C.P.; Lin, H.Z.; Ma, Z.H.; Wang, J. Effects of replacement of fish meal by fermented cottonseed meal on growth performance, feed utilization and intestinal bacteria community of juvenile golden pompano (Trachinotus ovatus). South China Fish. Sci. 2023, 19, 126–138, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Anderson, A.D.; Alam, M.S.; Watanabe, W.O.; Carroll, P.M.; Wedegaertner, T.C.; Dowd, M.K. Full replacement of menhaden fish meal protein by low-gossypol cottonseed flour protein in the diet of juvenile black sea bass Centropristis striata. Aquaculture 2016, 464, 618–628. [Google Scholar] [CrossRef]
- Bu, X.Y.; Chen, A.J.; Lian, X.Q.; Chen, F.Y.; Zhang, Y.; Muhammad, I.; Ge, X.P.; Yang, Y.H. An evaluation of replacing fish meal with cottonseed meal in the diet of juvenile Ussuri catfish Pseudobagrus ussuriensis: Growth, antioxidant capacity, nonspecific immunity and resistance to Aeromonas hydrophila. Aquaculture 2017, 479, 829–837. [Google Scholar] [CrossRef]
- Bian, F.; Zhou, H.; He, G.; Wang, C.; Peng, H.; Pu, X.; Jiang, H.; Wang, X.; Mai, K. Effects of replacing fishmeal with different cottonseed meals on growth, feed utilization, haematological indexes, intestinal and liver morphology of juvenile turbot (Scophthalmus maximus L.). Aquacult. Nutr. 2017, 23, 1429–1439. [Google Scholar] [CrossRef]
- Liu, H.K.; Yan, Q.G.; Han, D.; Jin, J.Y.; Zhu, X.M.; Yang, Y.X.; Xie, S.Q. Effect of dietary cottonseed meal on growth performance, physiological response, and gossypol accumulation in pre-adult grass carp, Ctenopharyngodon idellus. Chin. J. Oceanol. Limnol. 2016, 34, 992–1003. [Google Scholar] [CrossRef]
- Gaylord, T.G.; Gatlin, D.M., III. Determination of digestibility coefficients of various feedstuffs for red drum (Sciaenops ocellatus). Aquaculture 1996, 139, 303–314. [Google Scholar]
- Liu, Y.L.; Lu, Q.S.; Xi, L.W.; Gong, Y.L.; Su, J.Z.; Han, D.; Zhang, Z.M.; Liu, H.K.; Jin, J.Y.; Yang, Y.X.; et al. Effects of replacement of dietary fishmeal by cottonseed protein concentrate on growth performance, liver health, and intestinal histology of largemouth bass (Micropterus salmoides). Front. Physiol. 2021, 12, 764987. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Yang, H.; Zhang, C.Y.; Bian, Y.H.; Yao, W.X.; Xu, Z.; Wang, Y.Y.; Li, X.Q.; Leng, X.J. Effects of replacing fishmeal with cottonseed protein concentrate on growth performance, flesh quality and gossypol deposition of largemouth bass (Micropterus salmoides). Aquaculture 2022, 548, 737551. [Google Scholar] [CrossRef]
- Li, B.W.; Su, L.H.; Sun, Y.; Huang, H.; Deng, J.M.; Cao, Z.Y. Evaluation of cottonseed meal as an alternative to fish meal in diet for juvenile Asian red-tailed catfish Hemibagrus wyckioides. Aquacul. Nutri. 2023, 2023, 1741724. [Google Scholar] [CrossRef] [PubMed]
- Benetti, D.D.; Suarez, J.; Camperio, J.; Hoenig, R.H.; Tudela, C.E.; Daugherty, Z.; McGuigan, C.J.; Mathur, S.; Anchieta, L.; Buchalla, Y.; et al. A review on cobia, Rachycentron canadum, aquaculture. J. World Aquac. Soc. 2021, 52, 691–709. [Google Scholar] [CrossRef]
- Chou, R.L.; Su, M.S.; Chen, H.Y. Optimal dietary protein and lipid levels for juvenile cobia (Rachycentron canadum). Aquaculture 2001, 193, 81–89. [Google Scholar] [CrossRef]
- Wang, J.T.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Du, Z.Y.; Wang, Y.; Yang, H.J. Effect of dietary lipid level on growth performance, lipid deposition, hepatic lipogenesis in juvenile cobia (Rachycentron canadum). Aquaculture 2005, 249, 439–447. [Google Scholar] [CrossRef]
- Craig, S.R.; Schwarz, M.H.; McLean, E. Juvenile cobia (Rachycentron canadum) can utilize a wide range of protein and lipid levels without impacts on production characteristics. Aquaculture 2006, 261, 384–391. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Tan, B.P.; Mai, K.S.; Liu, Y.J. Apparent digestibility of selected feed ingredients for juvenile cobia Rachycentron canadum. Aquaculture 2004, 241, 441–451. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Wu, Z.H.; Tan, B.P.; Chi, S.Y.; Yang, Q.H. Optimal dietary methionine requirement for juvenile cobia (Rachycentron canadum). Aquaculture 2006, 258, 551–557. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Wu, Z.H.; Chi, S.Y.; Yang, Q.H. Dietary lysine requirement of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 273, 634–640. [Google Scholar] [CrossRef]
- Lunger, A.N.; Craig, S.; McLean, E. Replacement of fish m eal in cobia (Rachycentron canadum) diets using an organically certified protein. Aquaculture 2006, 257, 393–399. [Google Scholar] [CrossRef]
- Lunger, A.N.; McLean, E.; Craig, S.R. The effects of organic protein supplementation upon growth; feed conversion and texture quality parameters of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 264, 342–352. [Google Scholar] [CrossRef]
- Lunger, A.N.; McLean, E.; Gaylord, T.G.; Kuhn, D.; Craig, S.R. Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 271, 401–410. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Davies, S.J. Nutritional requirements of cobia, Rachycentron canadum (Linnaeus): A review. Aquac. Res. 2009, 40, 1219–1234. [Google Scholar] [CrossRef]
- Salze, G.; Craig, S.R.; Smith, B.H.; Smith, E.P.; Mclean, E. Morphological development of larval cobia Rachycentron canadum and the influence of dietary taurine supplementation. J. Fish Biol. 2011, 78, 1470–1491. [Google Scholar] [CrossRef] [PubMed]
- Salze, G.; McLean, E.; Craig, S.R. Dietary taurine enhances growth and digestive enzyme activities in larval cobia. Aquaculture 2012, 362, 44–49. [Google Scholar] [CrossRef]
- Ren, M.; Ai, Q.; Mai, K. Dietary arginine requirement of juvenile cobia (Rachycentron canadum). Aquac. Res. 2014, 45, 225–233. [Google Scholar] [CrossRef]
- Chi, S.; He, Y.; Zhu, Y.; Tan, B.; Dong, X.; Yang, Q.; Zhang, S. Dietary methionine affects growth and the expression of key genes involved in hepatic lipogenesis and glucose metabolism in cobia (Rachycentron canadum). Aquacult. Nutr. 2020, 26, 123–133. [Google Scholar] [CrossRef]
- Wang, J.; Lan, K.P.; Wu, G.D.; Wang, Y.; Zhou, C.P.; Lin, H.Z.; Ma, Z.H. Effect of dietary carbohydrate level on growth, feed utilization, energy retention, body composition, and digestive and metabolic enzymes activities of juvenile cobia, Rachycentron canadum. Aquacul. Rep. 2022, 25, 101211. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Chen, M.J. Carbohydrate utilization by Tilapia (Oreochromis niloticus × O. aureus) as influenced by different chromium sources. J. Nutr. 1993, 123, 1747–1753. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 18th ed.; AOAC: Arlington, TX, USA, 1995. [Google Scholar]
- Hidalgo, M.C.; Urea, E.; Sanz, A. Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture 1999, 170, 267–283. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.G.; Zuo, R.T.; Mai, K.S.; Xu, W.; Ai, Q.H. Effects of oxidised dietary fish oil and high-dose vitamin E supplementation on growth performance, feed utilisation and antioxidant defence enzyme activities of juvenile large yellow croaker (Larmichthys crocea). Brit. J. Nutr. 2016, 115, 1531–1538. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, J.; Yao, J.; Xue, H.Y.; Zhang, H.T.; Wu, Y. Determination of gossypol in cottonseed, cotton leaves and cotton stalk by LC-MS with internal standard method. Food Sci. Technol. 2016, 38, 280–284. [Google Scholar]
- Davis, M.J. Contrast coding in multiple regression analysis: Strengths, weaknesses, and utility of popular coding structures. J. Data Sci. 2010, 8, 61–73. [Google Scholar] [CrossRef]
- Robinson, E.H. Improvement of cottonseed meal protein with supplemental lysine in feeds for channel catfish. J. Appl. Aquacult. 1991, 1, 1–14. [Google Scholar] [CrossRef]
- Luo, L.; Xue, M.; Wu, X.; Cai, X.; Cao, H.; Liang, Y. Partial or total replacement of fishmeal by solvent-extracted cottonseed meal in diets for juvenile rainbow trout (Oncorhynchus mykiss). Aquacult. Nutr. 2006, 12, 418–424. [Google Scholar] [CrossRef]
- Wilson, R.P.; Robinson, E.H.; Poe, W.E. Apparent and true availability of amino acids from common feed ingredients for channel catfish. J. Nutr. 1981, 111, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Li, X.Q.; Leng, X.J.; Shan, L.L.; Zhao, J.X.; Wang, Y.T. Effects of dietary cottonseed meal level on the growth, hematological indices, liver and gonad histology of juvenile common carp (Cyprinus carpio). Aquaculture 2014, 428, 79–87. [Google Scholar] [CrossRef]
- Mbahinzireki, G.B.; Dabrowski, K.; Lee, K.J.; El-Saidy, D.; Wisner, E.R. Growth, feed utilization and body composition of tilapia (Oreochromis sp.) fed with cottonseed meal-based diets in a recirculating system. Aquac. Nutr. 2000, 7, 189–200. [Google Scholar] [CrossRef]
- Dorsa, W.J.; Robinette, H.R.; Robinson, E.H.; Poe, W.E. Effects of dietary cottonseed meal and gossypol on growth of young channel catfish. Trans. Am. Fish. Soc. 1982, 111, 651–655. [Google Scholar] [CrossRef]
- Alam, M.S.; Watanabe, W.O.; Carroll, P.M.; Gabel, J.E.; Corum, M.A.; Seaton, P.; Wedegaertner, T.C.; Rathore, K.S.; Dowd, M.K. Evaluation of genetically-improved (glandless) and genetically-modified low-gossypol cottonseed meal as alternative protein sources in the diet of juvenile southern flounder Paralichthys lethostigma reared in a recirculating aquaculture system. Aquaculture 2018, 489, 36–45. [Google Scholar] [CrossRef]
- Malbrouck, C.; Trausch, G.; Devos, P.; Kestemont, P. Hepatic accumulation and effects of microcystin-LR on juvenile goldfish Carassius auratus L. Comp. Biochem. Physiol. Part C 2003, 135, 39–48. [Google Scholar] [CrossRef]
- Zhang, H.J.; Dai, J.H.; Cai, M.L.; Cheng, K.M.; Hu, Y.; Luo, Z. Effects of dietary replacement of fishmeal by cottonseed meal on the growth performance, immune and antioxidant responses, and muscle quality of juvenile crayfish Procambarus clarkii. Aquac. Rep. 2023, 31, 101639. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant. Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.H.; Ye, J.Y.; Zhang, Y.X.; Xu, P.; Xie, J. Effects of dietary reduced glutathione on growth performance, non-specific immunity, antioxidant capacity and expression levels of IGF-I and HSP70 mRNA of grass carp (Ctenopharyngodon idella). Aquaculture 2015, 438, 39–46. [Google Scholar] [CrossRef]
| Ingredient | Diet Designation (Cottonseed Meal/% Fishmeal Replacement) | ||||
|---|---|---|---|---|---|
| Control (0) | 25 | 50 | 75 | 100 | |
| Fishmeal 1 | 35.00 | 26.25 | 17.50 | 8.75 | 0.00 |
| Cottonseed meal 2 | 0.00 | 9.92 | 19.83 | 29.75 | 39.67 |
| Soy Protein Conc. | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Menhaden Oil | 5.00 | 5.50 | 6.00 | 6.50 | 6.80 |
| Premix 3,4 | 29.3 | 29.3 | 29.3 | 29.3 | 29.3 |
| Bone meal 5 | 6.95 | 5.05 | 3.16 | 1.3 | 0 |
| L-Lysine | 0.00 | 0.15 | 0.30 | 0.45 | 0.60 |
| DL-Methionine | 0.00 | 0.15 | 0.30 | 0.45 | 0.60 |
| Analyzed proximate composition (% dry weight) 6 | |||||
| Moisture | 10.5 | 10.4 | 10.6 | 10.2 | 10.0 |
| Crude protein | 41.1 | 41.1 | 41.3 | 42.6 | 42.0 |
| Crude lipid | 10.5 | 10.4 | 11.7 | 11.2 | 10.6 |
| Ash | 17.0 | 14.7 | 12.3 | 9.3 | 9.4 |
| Metabolized energy (kJ/g) 7 | 19.3 | 19.6 | 20.3 | 20.8 | 20.6 |
| Free gossypol (mg·kg−1) | 0 | 31.04 | 62.00 | 92.91 | 123.32 |
| Amino Acid | Fish Meal | Cottonseed Meal | Control | CSM25 | CSM50 | CSM75 | CSM100 |
|---|---|---|---|---|---|---|---|
| His | 2.10 | 1.71 | 1.11 | 1.13 | 1.15 | 1.20 | 1.17 |
| Arg | 3.49 | 7.19 | 2.25 | 2.65 | 3.02 | 3.33 | 3.59 |
| Thr | 2.41 | 1.81 | 1.81 | 1.78 | 1.77 | 1.77 | 1.69 |
| Lys | 4.23 | 2.28 | 2.54 | 2.46 | 2.42 | 2.43 | 2.30 |
| Met | 1.57 | 0.81 | 0.76 | 0.72 | 0.62 | 0.53 | 0.78 |
| Val | 2.87 | 2.45 | 1.81 | 1.78 | 1.77 | 1.77 | 1.69 |
| Ile | 2.41 | 1.75 | 1.58 | 1.51 | 1.46 | 1.42 | 1.32 |
| Leu | 4.18 | 3.21 | 2.78 | 2.70 | 2.61 | 2.59 | 2.41 |
| Phe | 2.31 | 3.16 | 1.76 | 1.85 | 1.94 | 2.05 | 2.05 |
| Tyr | 2.09 | 1.18 | 1.13 | 1.11 | 1.1 | 1 | 1.01 |
| Asp | 6.31 | 5.32 | 3.64 | 3.65 | 3.67 | 3.75 | 3.62 |
| Ser | 2.79 | 2.57 | 1.69 | 1.73 | 1.75 | 1.81 | 1.77 |
| Glu | 8.28 | 10.86 | 6.81 | 7.28 | 7.75 | 8.3 | 8.39 |
| Pro | 2.98 | 2.32 | 1.89 | 1.88 | 1.85 | 1.87 | 1.77 |
| Gly | 4.34 | 2.39 | 2.08 | 1.96 | 1.85 | 1.78 | 1.61 |
| Ala | 3.96 | 2.22 | 2.09 | 1.95 | 1.83 | 1.74 | 1.56 |
| Diet Designation | Survival (%) | Final Weight (g) | WG (%) | FI (g fish−1 d−1) | FE | PER |
|---|---|---|---|---|---|---|
| Control | 90.0 ± 0.0 a | 120.1 ± 5.7 ab | 957.6 ± 46.1 ab | 3.79 ± 0.16 bc | 0.45 ± 0.02 b | 1.10 ± 0.05 b |
| CSM25 | 96.7 ± 1.7 a | 166.7 ± 5.7 a | 1371.8 ± 61.3 a | 3.69 ± 0.12 bc | 0.67 ± 0.01 a | 1.62 ± 0.01 a |
| CSM50 | 88.3 ± 10.1 a | 115.4 ± 16.4 bc | 926.2 ± 44.1 bc | 3.95 ± 0.43 c | 0.46 ± 0.09 b | 1.11 ± 0.05 b |
| CSM75 | 90.0 ± 5.0 a | 68.6 ± 4.2 cd | 498.5 ± 41.8 cd | 2.21 ± 0.13 a | 0.40 ± 0.00 b | 0.95 ± 0.01 b |
| CSM100 | 37.5 ± 7.5 b | 40.8 ± 3.0 d | 260.4 ± 23.8 d | 2.90 ± 0.42 ab | 0.18 ± 0.05 c | 0.42 ± 0.09 c |
| ANOVA (Pr > F) 2 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 |
| Linear Trend (Pr > F) | 0.000 | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 |
| Quadratic Trend (Pr > F) | 0.004 | 0.003 | 0.003 | 0.705 | 0.000 | 0.001 |
| Diet Designation | Condition Factor (CF) | Hepatosomatic Index (HSI) (%) | Moisture (%) | Protein (%) | Lipid (%) | Ash (%) |
|---|---|---|---|---|---|---|
| Control | 0.94 ± 0.03 ab | 2.43 ± 0.54 ab | 71.2 ± 0.4 a | 16.78 ± 0.19 ab | 7.80 ± 0.36 a | 3.30 ± 0.03 b |
| CSM25 | 1.03 ± 0.02 a | 2.47 ± 0.10 a | 70.1 ± 0.4 a | 17.32 ± 0.10 a | 8.63 ± 0.28 a | 3.28 ± 0.08 b |
| CSM50 | 0.95 ± 0.05 ab | 2.04 ± 0.20 abc | 70.8 ± 0.9 a | 16.93 ± 0.25 a | 7.66 ± 1.07 ab | 3.81 ± 0.24 ab |
| CSM75 | 0.90 ± 0.07 ab | 1.96 ± 0.10 bc | 72.1 ± 1.8 a | 16.78 ± 0.22 ab | 6.11 ± 1.25 ab | 3.83 ± 0.19 ab |
| CSM100 | 0.82 ± 0.02 b | 1.80 ± 0.10 c | 74.8 ± 0.3 b | 15.81 ± 0.23 b | 4.57 ± 0.01 b | 4.01 ± 0.04 a |
| ANOVA (Pr > F) 2 | 0.000 | 0.009 | 0.000 | 0.009 | 0.018 | 0.012 |
| Linear Trend (Pr > F) | 0.001 | 0.001 | 0.000 | 0.006 | 0.004 | 0.001 |
| Quadratic Trend (Pr > F) | 0.003 | 0.554 | 0.006 | 0.005 | 0.05 | 0.166 |
| Amino Acid | Control | CSM25 | CSM50 | CSM75 | CSM100 | ANOVA (Pr > F) 2 | Linear Trend (Pr > F) |
|---|---|---|---|---|---|---|---|
| His | 1.35 ± 0.05 | 1.33 ± 0.01 | 1.30 ± 0.00 | 1.38 ± 0.06 | 1.40 ± 0.04 | 0.130 | 0.067 |
| Arg | 3.59 ± 0.06 | 3.48 ± 0.02 | 3.47 ± 0.01 | 3.66 ± 0.18 | 3.84 ± 0.05 | 0.063 | 0.024 |
| Thr | 2.35 ± 0.09 | 2.28 ± 0.01 | 2.31 ± 0.06 | 2.45 ± 0.12 | 2.47 ± 0.06 | 0.087 | 0.027 |
| Lys | 4.00 ± 0.10 | 3.92 ± 0.03 | 3.86 ± 0.03 | 4.08 ± 0.19 | 4.15 ± 0.16 | 0.146 | 0.084 |
| Met | 1.46 ± 0.05 bc | 1.43 ± 0.01 c | 1.43 ± 0.03 c | 1.54 ± 0.06 b | 1.55 ± 0.03 a | 0.041 | 0.011 |
| Val | 2.50 ± 0.07 ab | 2.43 ± 0.07 b | 2.40 ± 0.10 b | 2.59 ± 0.02 a | 2.63 ± 0.06 a | 0.041 | 0.023 |
| Iso | 2.09 ± 0.05 ab | 2.02 ± 0.06 b | 2.01 ± 0.08 b | 2.19 ± 0.02 ab | 2.21 ± 0.05 a | 0.019 | 0.010 |
| Leu | 3.73 ± 0.10 ab | 3.63 ± 0.02 b | 3.65 ± 0.11 b | 3.91 ± 0.12 a | 3.89 ± 0.11 a | 0.044 | 0.018 |
| Phe | 2.04 ± 0.06 ab | 1.98 ± 0.2 b | 1.99 ± 0.06 b | 2.13 ± 0.78 a | 2.14 ± 0.04 a | 0.038 | 0.016 |
| Diets | Control | CSM25 | CSM50 | CSM75 | CSM100 | ANOVA (Pr > F) 2 | Linear Trend (P) | Quadratic Trend (P) |
|---|---|---|---|---|---|---|---|---|
| Trypsin (U mg prot−1) | 810.18 ± 83.01 | 904.40 ± 164.48 | 676.23 ± 20.73 | 620.05 ± 190.05 | 587.25 ± 84.16 | 0.268 | 0.051 | 0.603 |
| Amylase (U mg prot−1) | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.00 | 0.12 ± 0.00 | 0.071 | 0.055 | 0.845 |
| Lipase (U g prot−1) | 26.05 ± 0.86 | 29.35 ± 1.40 | 24.93 ± 0.51 | 24.22 ± 0.72 | 26.97 ± 0.86 | 0.058 | 0.628 | 0.585 |
| SOD (U mg prot−1) | 82.26 ± 5.12 ab | 77.03 ± 4.52 b | 70.09 ± 3.46 b | 105.11 ± 5.22 a | 85.08 ± 5.79 ab | 0.050 | 0.217 | 0.569 |
| GSH-Px (μmol L−1) | 363.18± 98.76 ab | 459.10 ± 102.93 a | 151.27 ± 8.61 bc | 201.98 ± 5.55 abc | 98.81 ± 25.54 c | 0.039 | 0.008 | 0.494 |
| MDA (nmol mg prot−1) | 14.73 ± 3.13 a | 15.13 ± 1.19 a | 2.22 ± 0.43 b | 1.38 ± 0.33 b | 5.38 ± 1.23 b | 0.000 | 0.000 | 0.222 |
| ALT (U L−1) | 4.00 ± 0.00 | 4.20 ± 0.74 | 2.50 ± 0.29 | 2.50 ± 0.29 | 3.33 ± 0.33 | 0.069 | 0.057 | 0.168 |
| AST/(U L−1) | 52.67 ± 13.69 | 67.75 ± 12.29 | 48.50 ± 9.23 | 53.00 ± 7.91 | 51.50 ± 10.50 | 0.715 | 0.594 | 0.834 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wu, G.; Gatlin, D.M., III; Lan, K.; Wang, Y.; Zhou, C.; Ma, Z. Dietary Fishmeal Replacement by Methanol-Extracted Cottonseed Meal with Amino Acid Supplementation for Juvenile Cobia Rachycentron canadum. J. Mar. Sci. Eng. 2024, 12, 235. https://doi.org/10.3390/jmse12020235
Wang J, Wu G, Gatlin DM III, Lan K, Wang Y, Zhou C, Ma Z. Dietary Fishmeal Replacement by Methanol-Extracted Cottonseed Meal with Amino Acid Supplementation for Juvenile Cobia Rachycentron canadum. Journal of Marine Science and Engineering. 2024; 12(2):235. https://doi.org/10.3390/jmse12020235
Chicago/Turabian StyleWang, Jun, Guangde Wu, Delbert M. Gatlin, III, Kunpeng Lan, Yun Wang, Chuanpeng Zhou, and Zhenhua Ma. 2024. "Dietary Fishmeal Replacement by Methanol-Extracted Cottonseed Meal with Amino Acid Supplementation for Juvenile Cobia Rachycentron canadum" Journal of Marine Science and Engineering 12, no. 2: 235. https://doi.org/10.3390/jmse12020235
APA StyleWang, J., Wu, G., Gatlin, D. M., III, Lan, K., Wang, Y., Zhou, C., & Ma, Z. (2024). Dietary Fishmeal Replacement by Methanol-Extracted Cottonseed Meal with Amino Acid Supplementation for Juvenile Cobia Rachycentron canadum. Journal of Marine Science and Engineering, 12(2), 235. https://doi.org/10.3390/jmse12020235








