mTOR Plays a Conserved Role in Regulation of Nutritional Metabolism in Bivalve Sinonovacula constricta
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence Analysis and Tissue Expression
2.2. Starvation and Re-Feeding Experiment
2.3. Challenge Experiments and Samples
2.4. Western Blotting
2.5. RNA Extraction and cDNA Synthesis
2.6. Statistical Analysis
3. Results
3.1. Molecular Characterization, Phylogenetic Relationship, and Tissue Expression Profiles of mTOR
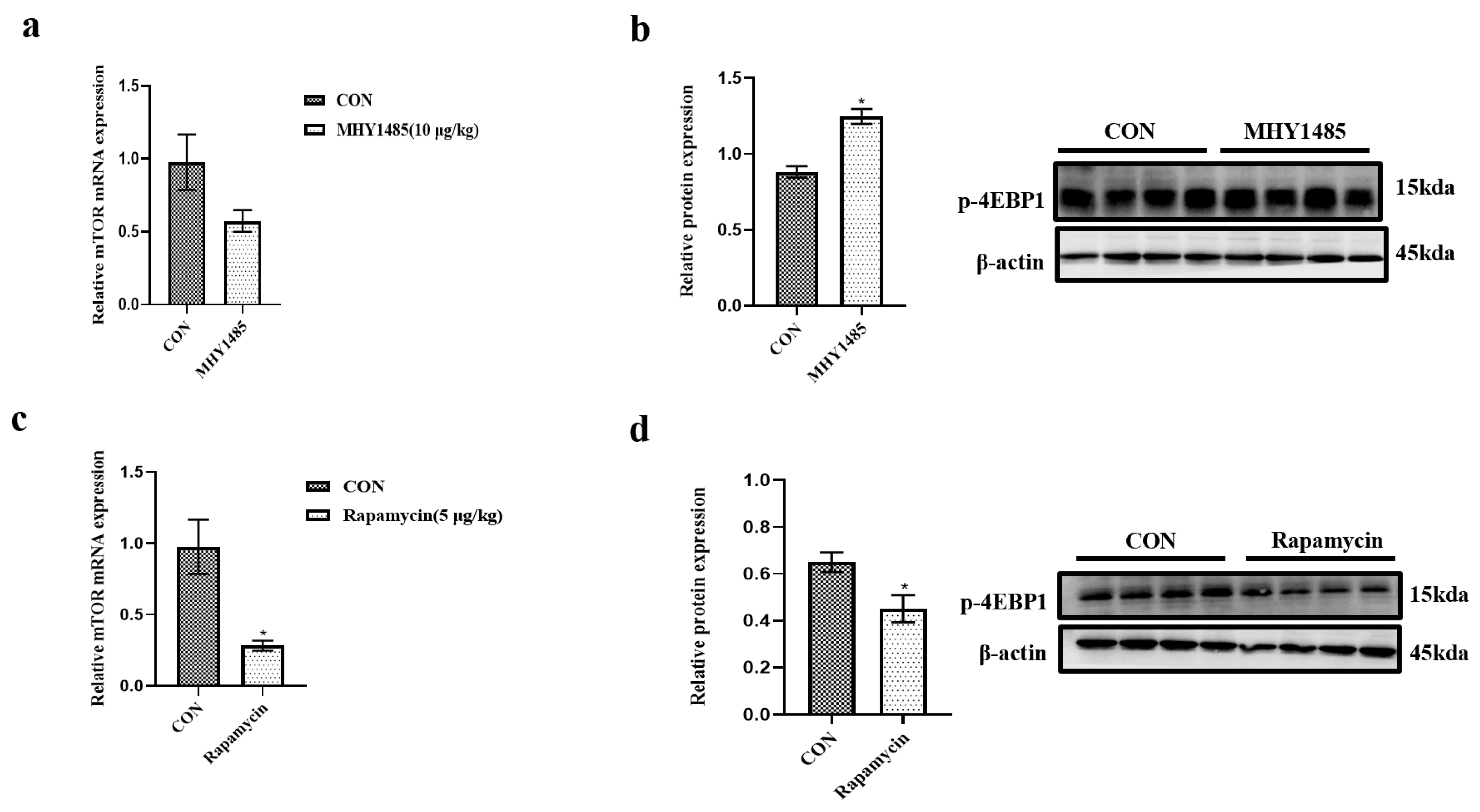
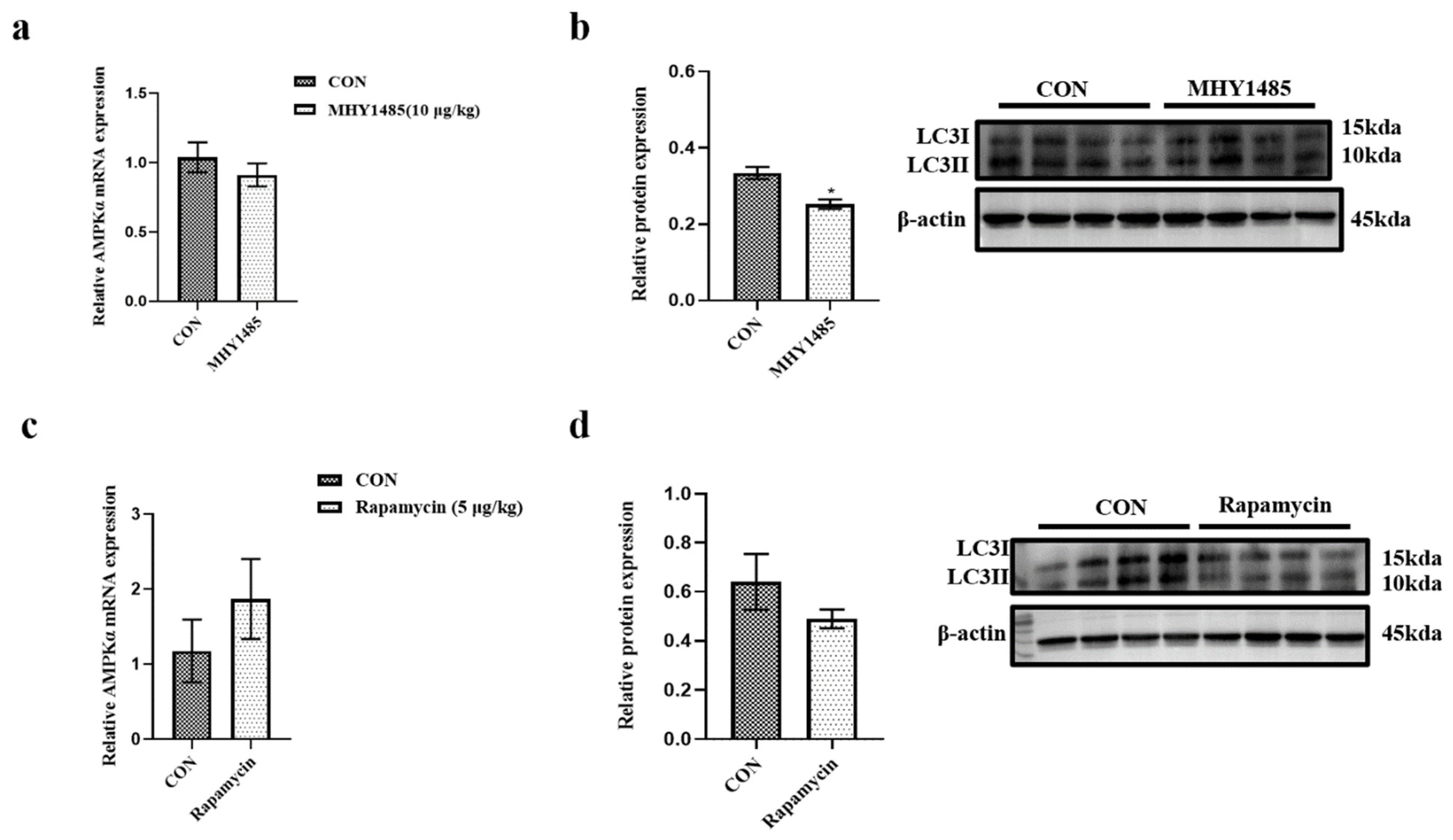
3.2. Effects of Starvation and Re-Feeding on the Expressions of mTOR, LC3, and AMPKα
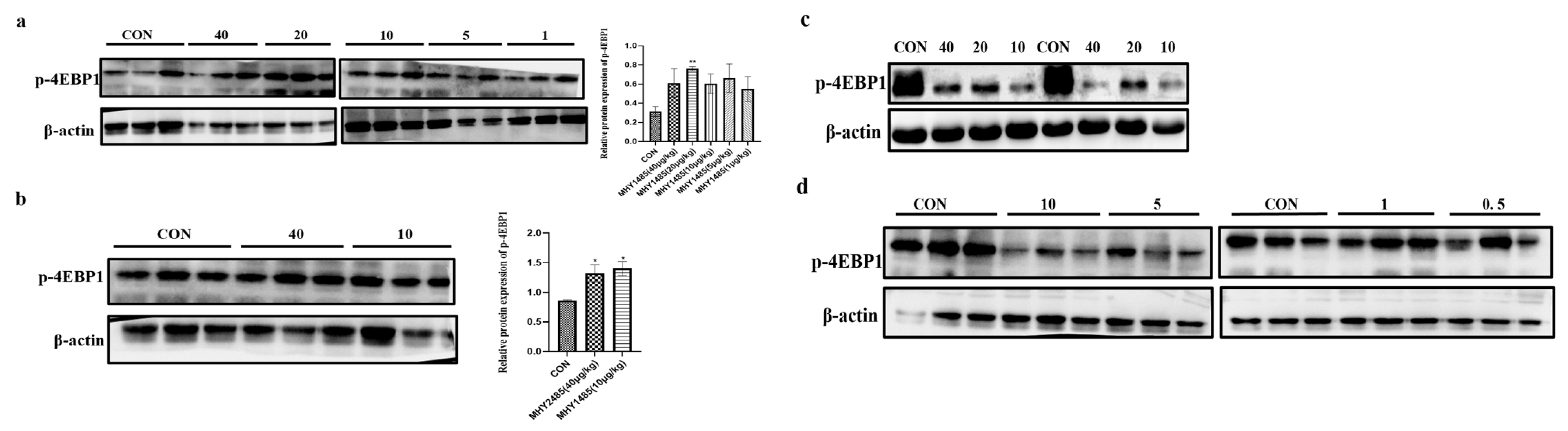
3.3. Dose Screening of Rapamycin and MHY1485
3.4. Effects of MHY1485 and Rapamycin on the Expression of mTOR and 4E-BP1
3.5. Effects of MHY1485 and Rapamycin on the Expression of AMPKα and LC3
3.6. Effects of MHY1485 and Rapamycin on the Expression of Genes Related to Nutritional Metabolism
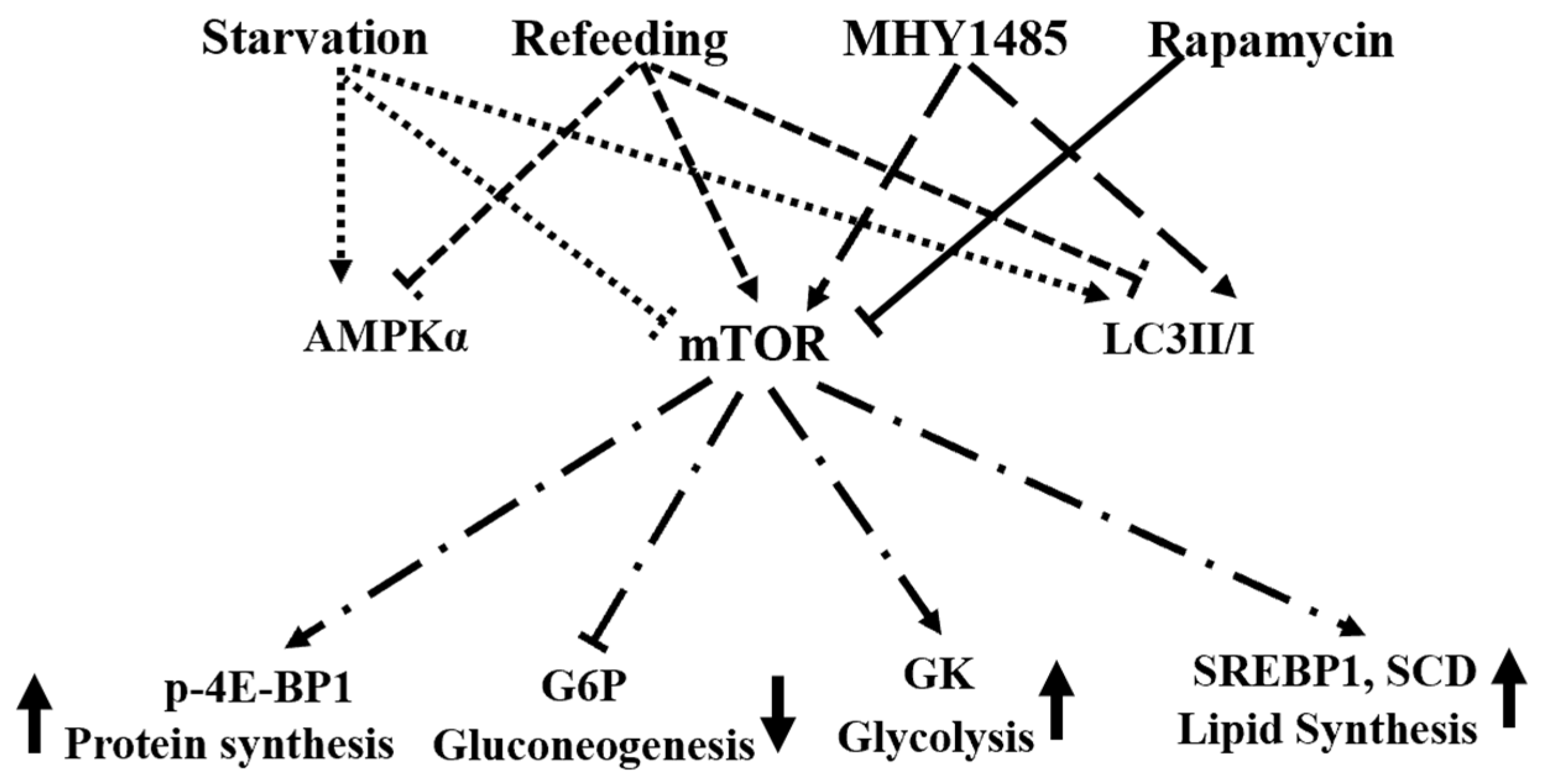
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, A.; Hall, M.N.; Lin, S.-C.; Hardie, D.G. AMPK and TOR: The yin and yang of cellular nutrient sensing and growth control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Loewith, R.; Jacinto, E.; Wullschleger, S.; Lorberg, A.; Crespo, J.L.; Bonenfant, D.; Oppliger, W.; Jenoe, P.; Hall, M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 2002, 10, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Nojima, H.; Tokunaga, C.; Eguchi, S.; Oshiro, N.; Hidayat, S.; Yoshino, K.I.; Hara, K.; Tanaka, N.; Avruch, J.; Yonezawa, K. The Mammalian Target of Rapamycin (mTOR) Partner, Raptor, Binds the mTOR Substrates p70 S6 Kinase and 4E-BP1 through Their TOR Signaling (TOS) Motif. J. Biol. Chem. 2003, 278, 15461–15464. [Google Scholar] [CrossRef]
- Gordan, J.D.; Thompson, C.B.; Simon, M.C. HIF and c-Myc: Sibling Rivals for Control of Cancer Cell Metabolism and Proliferation. Cancer Cell 2007, 12, 108–113. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.-L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Martina, J.A.; Chen, Y.; Gucek, M.; Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 2012, 8, 903–914. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Kadowaki, M.; Karim, M.R. Cytosolic LC3 ratio as a quantitative index of macroautophagy. Methods Enzymol. 2009, 452, 199–213. [Google Scholar]
- Su, Y.; Wang, T.; Wu, N.; Li, D.; Fan, X.; Xu, Z.; Mishra, S.K.; Yang, M. Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging 2019, 11, 4183. [Google Scholar] [CrossRef]
- Potter, S.; Sifers, J.; Yocom, E.; Blümich, S.L.; Potter, R.; Nadolski, J.; Harrison, D.A.; Cooper, R.L. Effects of inhibiting mTOR with rapamycin on behavior, development, neuromuscular physiology and cardiac function in larval Drosophila. Biol. Open 2019, 8, bio046508. [Google Scholar] [CrossRef]
- Hansen, M.; Taubert, S.; Crawford, D.; Libina, N.; Lee, S.J.; Kenyon, C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 2007, 6, 95–110. [Google Scholar] [CrossRef]
- Wang, X.; Lei, X.-y.; Guo, Z.-x.; Wang, S.; Wan, J.-w.; Liu, H.-j.; Chen, Y.-k.; Wang, G.-q.; Wang, Q.-j.; Zhang, D.-m. The immuneoreaction and antioxidant status of Chinese mitten crab (Eriocheir sinensis) involve protein metabolism and the response of mTOR signaling pathway to dietary methionine levels. Fish Shellfish. Immunol. 2022, 127, 703–714. [Google Scholar] [CrossRef]
- MacLea, K.S.; Abuhagr, A.M.; Pitts, N.L.; Covi, J.A.; Bader, B.D.; Chang, E.S.; Mykles, D.L. Rheb, an activator of target of rapamycin, in the blackback land crab, Gecarcinus lateralis: Cloning and effects of molting and unweighting on expression in skeletal muscle. J. Exp. Biol. 2012, 215, 590–604. [Google Scholar] [CrossRef]
- Abuhagr, A.M.; MacLea, K.S.; Chang, E.S.; Mykles, D.L. Mechanistic target of rapamycin (mTOR) signaling genes in decapod crustaceans: Cloning and tissue expression of mTOR, Akt, Rheb, and p70 S6 kinase in the green crab, Carcinus maenas, and blackback land crab, Gecarcinus lateralis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 168, 25–39. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, H.; Zhu, W.; Yuan, X.; Shao, J. Effects of Time-Dependent Protein Restriction on Growth Performance, Digestibility, and mTOR Signaling Pathways in Juvenile White Shrimp Litopenaeus vannamei. Front. Physiol. 2021, 12, 661107. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Sun, G.; Wang, B.; Jiang, K.; Shao, J.; Qi, C.; Zhao, W.; Han, S.; Liu, M. Mechanistic target of rapamycin inhibition with rapamycin induces autophagy and correlative regulation in white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2018, 24, 1509–1520. [Google Scholar] [CrossRef]
- Picot, S.; Faury, N.; Arzul, I.; Chollet, B.; Renault, T.; Morga, B. Identification of the autophagy pathway in a mollusk bivalve, Crassostrea gigas. Autophagy 2020, 16, 2017–2035. [Google Scholar] [CrossRef]
- Gao, Z.; Pan, L.; Xu, R.; Zhou, Y.; Li, D. Insights into disruption of lipid metabolism in digestive gland of female scallop Chlamys farreri under B [a] P exposure. Environ. Pollut. 2022, 299, 118904. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, C.; Wang, S.; Ji, F.; Xie, Y. MHY1485 activates mTOR and protects osteoblasts from dexamethasone. Biochem. Biophys. Res. Commun. 2016, 481, 212–218. [Google Scholar] [CrossRef]
- Hao, E.-y.; Wang, D.-H.; Chang, L.-y.; Huang, C.-x.; Chen, H.; Yue, Q.-x.; Zhou, R.-Y.; Huang, R.-l. Melatonin regulates chicken granulosa cell proliferation and apoptosis by activating the mTOR signaling pathway via its receptors. Poult. Sci. 2020, 99, 6147–6162. [Google Scholar] [CrossRef]
- Liu, Q.; Miller, L.C.; Blecha, F.; Sang, Y. Reduction of infection by inhibiting mTOR pathway is associated with reversed repression of type I interferon by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2017, 98, 1316. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, Y.J.; Park, J.Y.; Jeong, H.O.; Kim, D.H.; Ha, Y.M.; Kim, J.M.; Song, Y.M.; Heo, H.-S.; Yu, B.P. Inhibitory effect of mTOR activator MHY1485 on autophagy: Suppression of lysosomal fusion. PLoS ONE 2012, 8, e43418. [Google Scholar] [CrossRef]
- Mikdache, A.; Boueid, M.-J.; van der Spek, L.; Lesport, E.; Delespierre, B.; Loisel-Duwattez, J.; Degerny, C.; Tawk, M. Rgs4 is a regulator of mTOR activity required for motoneuron axon outgrowth and neuronal development in zebrafish. Sci. Rep. 2021, 11, 13338. [Google Scholar] [CrossRef]
- Thomas, G.V. mTOR and cancer: Reason for dancing at the crossroads? Curr. Opin. Genet. Dev. 2006, 16, 78–84. [Google Scholar] [CrossRef]
- Johnson, S.C.; Yanos, M.E.; Bitto, A.; Castanza, A.; Gagnidze, A.; Gonzalez, B.; Gupta, K.; Hui, J.; Jarvie, C.; Johnson, B.M. Dose-dependent effects of mTOR inhibition on weight and mitochondrial disease in mice. Front. Genet. 2015, 6, 247. [Google Scholar] [CrossRef]
- Hirose, K.; Shiomi, T.; Hozumi, S.; Kikuchi, Y. Mechanistic target of rapamycin complex 1 signaling regulates cell proliferation, cell survival, and differentiation in regenerating zebrafish fins. BMC Dev. Biol. 2014, 14, 42. [Google Scholar] [CrossRef]
- Wang, A.; Mouser, J.; Pitt, J.; Promislow, D.; Kaeberlein, M. Rapamycin enhances survival in a Drosophila model of mitochondrial disease. Oncotarget 2016, 7, 80131. [Google Scholar] [CrossRef]
- Christie, G.R.; Hajduch, E.; Hundal, H.S.; Proud, C.G.; Taylor, P.M. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J. Biol. Chem. 2002, 277, 9952–9957. [Google Scholar] [CrossRef]
- Dikicioglu, D.; Dereli Eke, E.; Eraslan, S.; Oliver, S.G.; Kirdar, B. Saccharomyces cerevisiae adapted to grow in the presence of low-dose rapamycin exhibit altered amino acid metabolism. Cell Commun. Signal. 2018, 16, 85. [Google Scholar] [CrossRef]
- Xu, H.; Liu, L.; Su, Y.; Liang, Y.; Yang, J.J.A.B. Effects of rapamycin on life span and on expression of TOR and S6K in Brachionus calyciflorus (Rotifera). Aquat. Biol. 2017, 26, 49–56. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, X.; Liu, S.; Shi, W.; Han, Y.; Guo, C.; Jiang, J.; Wan, H.; Shen, T.; Liu, G. Effects of anthropogenic sound on digging behavior, metabolism, Ca2+/Mg2+ ATPase activity, and metabolism-related gene expression of the bivalve Sinonovacula constricta. Sci. Rep. 2016, 6, 24266. [Google Scholar] [CrossRef]
- Ha, J.; Kim, J. Novel pharmacological modulators of autophagy: An updated patent review (2012–2015). Expert Opin. Ther. Pat. 2016, 26, 1273–1289. [Google Scholar] [CrossRef]
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. mTOR kinase structure, mechanism and regulation. Nature 2013, 497, 217–223. [Google Scholar] [CrossRef]
- Su, B.; Jacinto, E. Mammalian TOR signaling to the AGC kinases. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 527–547. [Google Scholar] [CrossRef]
- Rodriguez Camargo, D.C.; Link, N.M.; Dames, S.A. The FKBP–rapamycin binding domain of human TOR undergoes strong conformational changes in the presence of membrane mimetics with and without the regulator phosphatidic acid. Biochemistry 2012, 51, 4909–4921. [Google Scholar] [CrossRef]
- Oshiro, N.; Yoshino, K.i.; Hidayat, S.; Tokunaga, C.; Hara, K.; Eguchi, S.; Avruch, J.; Yonezawa, K. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 2004, 9, 359–366. [Google Scholar] [CrossRef]
- Sun, S.; Wang, B.; Jiang, K.; Sun, J.; Liu, M.; Wang, L. Target of rapamycin (TOR) in Fenneropenaeus chinensis: cDNA cloning, characterization, tissue expression and response to amino acids. Aquac. Nutr. 2015, 21, 1–9. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, L.; Liu, Y.; Jiang, W.-D.; Hu, K.; Li, S.-H.; Zhou, X.-Q. Mechanistic target of rapamycin in common carp: cDNA cloning, characterization, and tissue expression. Gene 2013, 512, 566–572. [Google Scholar] [CrossRef]
- Ekim, B.; Magnuson, B.; Acosta-Jaquez, H.A.; Keller, J.A.; Feener, E.P.; Fingar, D.C. mTOR kinase domain phosphorylation promotes mTORC1 signaling, cell growth, and cell cycle progression. Mol. Cell. Biol. 2011, 31, 2787–2801. [Google Scholar] [CrossRef]
- Li, F.; Fu, C.; Xie, Y.; Wang, A.; Li, J.; Gao, J.; Cui, X. Transcriptional responses to starvation stress in the hepatopancreas of oriental river prawn Macrobrachium nipponense. Environ. Pollut. 2019, 252, 14–20. [Google Scholar] [CrossRef]
- Viollet, B. The energy sensor AMPK: Adaptations to exercise, nutritional and hormonal signals. In Hormones, Metabolism and the Benefits of Exercise; Springer: Cham, Switzerland, 2017; pp. 13–24. [Google Scholar]
- Nazio, F.; Strappazzon, F.; Antonioli, M.; Bielli, P.; Cianfanelli, V.; Bordi, M.; Gretzmeier, C.; Dengjel, J.; Piacentini, M.; Fimia, G.M. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nature Cell Biol. 2013, 15, 406–416. [Google Scholar] [CrossRef]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Howell, J.J.; Manning, B.D. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 2011, 22, 94–102. [Google Scholar] [CrossRef]
- Peng, T.; Golub, T.R.; Sabatini, D.M. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol. Cell. Biol. 2002, 22, 5575–5584. [Google Scholar] [CrossRef]
- Bazer, F.W.; Wang, X.; Johnson, G.A.; Wu, G.J.A.N. Select nutrients and their effects on conceptus development in mammals. Anim. Nutr. 2015, 1, 85–95. [Google Scholar] [CrossRef]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.J.N. Absence of S6K1 protects against age-and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef]
- Kim, S.G.; Buel, G.R.; Blenis, J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol. Cells 2013, 35, 463–473. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Frias, M.A.; Chatterjee, A.; Yellen, P.; Foster, D.A. The enigma of rapamycin dosage. Mol. Cancer Ther. 2016, 15, 347–353. [Google Scholar] [CrossRef]
- Cheng, Y.; Kim, J.; Li, X.X.; Hsueh, A.J. Promotion of ovarian follicle growth following mTOR activation: Synergistic effects of AKT stimulators. PLoS ONE 2015, 10, e0117769. [Google Scholar] [CrossRef]
- Choo, A.Y.; Yoon, S.-O.; Kim, S.G.; Roux, P.P.; Blenis, J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. USA 2008, 105, 17414–17419. [Google Scholar] [CrossRef]
- Zhao, J.; Benakanakere, M.R.; Hosur, K.B.; Galicia, J.C.; Martin, M.; Kinane, D.F. Mammalian target of rapamycin (mTOR) regulates TLR3 induced cytokines in human oral keratinocytes. Mol. Immunol. 2010, 48, 294–304. [Google Scholar] [CrossRef]
- Wang, Q.; He, G.; Mai, K.; Xu, W.; Zhou, H.; Wang, X.; Mei, L. Chronic rapamycin treatment on the nutrient utilization and metabolism of juvenile turbot (Psetta maxima). Sci. Rep. 2016, 6, 28068. [Google Scholar] [CrossRef]
- Wan, X.; Mendoza, A.; Khanna, C.; Helman, L.J. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005, 65, 2406–2411. [Google Scholar] [CrossRef]
- Tran, T.A.; Kinch, L.; Peña-Llopis, S.; Kockel, L.; Grishin, N.; Jiang, H.; Brugarolas, J. Platelet-derived growth factor/vascular endothelial growth factor receptor inactivation by sunitinib results in Tsc1/Tsc2-dependent inhibition of TORC1. Mol. Cell. Biol. 2013, 33, 3762–3779. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Rosenberg, L.M.; Wang, X.; Zhou, Z.; Yue, P.; Fu, H.; Khuri, F.R. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005, 65, 7052–7058. [Google Scholar] [CrossRef]
- Massa, M.L.; Gagliardino, J.J.; Francini, F. Liver glucokinase: An overview on the regulatorymechanisms of its activity. IUBMB life 2011, 63, 1–6. [Google Scholar] [CrossRef]
- Feksa, L.R.; Cornelio, A.R.; Vargas, C.R.; de Souza Wyse, A.T.; Dutra-Filho, C.S.; Wajner, M.; Wannmacher, C.M.D. Alanine prevents the inhibition of pyruvate kinase activity caused by tryptophan in cerebral cortex of rats. Metab. Brain Dis. 2003, 18, 129–137. [Google Scholar] [CrossRef]
- Ferre, T.; Riu, E.; Bosch, F.; Valera, A. Evidence from transgenic mice that glucokinase is rate limiting for glucose utilization in the liver. FASEB J. 1996, 10, 1213–1218. [Google Scholar] [CrossRef]
- Lan, R.; Wan, Z.; Xu, Y.; Wang, Z.; Fu, S.; Zhou, Y.; Lin, X.; Han, X.; Luo, Z.; Miao, J. Taurine reprograms mammary-gland metabolism and alleviates inflammation induced by Streptococcus uberis in mice. Front. Immunol. 2021, 12, 696101. [Google Scholar] [CrossRef]
- Sipula, I.J.; Brown, N.F.; Perdomo, G. Rapamycin-mediated inhibition of mammalian target of rapamycin in skeletal muscle cells reduces glucose utilization and increases fatty acid oxidation. Metabolism 2006, 55, 1637–1644. [Google Scholar] [CrossRef]
- Taha, C.; Liu, Z.; Jin, J.; Al-Hasani, H.; Sonenberg, N.; Klip, A. Opposite translational control of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin: Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in GLUT1 mRNA translation. J. Biol. Chem. 1999, 274, 33085–33091. [Google Scholar] [CrossRef]
- Sul, H.S.; Wang, D. Nutritional and hormonal regulation of enzymes in fat synthesis: Studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu. Rev. Nutr. 1998, 18, 331–351. [Google Scholar] [CrossRef]
- Dai, W.; Panserat, S.; Plagnes-Juan, E.; Seiliez, I.; Skiba-Cassy, S. Amino acids attenuate insulin action on gluconeogenesis and promote fatty acid biosynthesis via mTORC1 signaling pathway in trout hepatocytes. Cell. Physiol. Biochem. 2015, 36, 1084–1100. [Google Scholar] [CrossRef]
- Yellen, P.; Saqcena, M.; Salloum, D.; Feng, J.; Preda, A.; Xu, L.; Rodrik-Outmezguine, V.; Foster, D.A. High-dose rapamycin induces apoptosis in human cancer cells by dissociating mTOR complex 1 and suppressing phosphorylation of 4E-BP1. Cell Cycle 2011, 10, 3948–3956. [Google Scholar] [CrossRef]
- Luyimbazi, D.; Akcakanat, A.; McAuliffe, P.F.; Zhang, L.; Singh, G.; Gonzalez-Angulo, A.M.; Chen, H.; Do, K.-A.; Zheng, Y.; Hung, M.-C. Rapamycin Regulates Stearoyl CoA Desaturase 1 Expression in Breast CancerRapamycin Regulates SCD1 Expression. Mol. Cancer Ther. 2010, 9, 2770–2784. [Google Scholar] [CrossRef]
- Brown, N.F.; Stefanovic-Racic, M.; Sipula, I.J.; Perdomo, G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism 2007, 56, 1500–1507. [Google Scholar] [CrossRef]
- Shao, Y.; Che, Z.; Chen, K.; Li, C.; Zhao, X. Target of rapamycin signaling inhibits autophagy in sea cucumber Apostichopus japonicus. Fish Shellfish. Immunol. 2020, 102, 480–488. [Google Scholar]
- Huang, J.; Brumell, J.H. Bacteria–autophagy interplay: A battle for survival. Nat. Rev. Microbiol. 2014, 12, 101–114. [Google Scholar] [CrossRef]



| Title 1 | Title | Title 3 |
|---|---|---|
| β-actin | F | CCATCTACGAAGGTTACGCCC |
| R | TCGTAGTGAAGGAGTAGCCTCTTT | |
| mTOR | F R | CAGCGGTCTGGTAATGTGAAGG TAGCAACTTAGGTGACACATACTGG |
| AMPK | F | ACCACAGGCACCTCAGTAAACA |
| R | AGGATGCGTGCGTGAAGTTA | |
| PK | F R | TCGTGTAATGGCAATAATCG GTAGAAGCATCGTTCAAGTC |
| GK | F R | GTTCGCCCGTTTATGCTTGG CAAGTCCAGGGCGAGAAAGT |
| GLUT1 | F R | CGTTATCCTCGTCGCTTCCA CCACCATTGCTTCTGTTGGC |
| G6P | F R | CCCTCGTCTTGTCTGGCATT CAGCATCCCTGTACACAGCA |
| PEPCK | F R | GGGAGGACAAGAAGGGAGT ATTGTATCCCATGAAAGGTCTC |
| SREBP | F R | GCTCCTACTCTGTTATCCGATTGTG TCCTGAGACTGGCGAGATCCTT |
| ACC | F R | TGGATGGCAATGTTGATGA GGCACTGATGGTAGAGAAG |
| SCD | F R | CACCGCATCCCCGAAAAATC AGGCGCAAATTATGGTTGCC |
| CS | F R | CAGTTCAGTGCTGCCATA CAAGTTACGGTAGATGATAGAC |
| NADP | F R | ATGTTGCTAAGGATGTTACC TTAGGAGATGGACTGTTCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, Y.; Liao, K.; Chen, D.; Qiu, Y.; Yan, X.; Xu, J. mTOR Plays a Conserved Role in Regulation of Nutritional Metabolism in Bivalve Sinonovacula constricta. J. Mar. Sci. Eng. 2023, 11, 1040. https://doi.org/10.3390/jmse11051040
Zhang Q, Li Y, Liao K, Chen D, Qiu Y, Yan X, Xu J. mTOR Plays a Conserved Role in Regulation of Nutritional Metabolism in Bivalve Sinonovacula constricta. Journal of Marine Science and Engineering. 2023; 11(5):1040. https://doi.org/10.3390/jmse11051040
Chicago/Turabian StyleZhang, Qian, Yanrong Li, Kai Liao, Deshui Chen, Yangyang Qiu, Xiaojun Yan, and Jilin Xu. 2023. "mTOR Plays a Conserved Role in Regulation of Nutritional Metabolism in Bivalve Sinonovacula constricta" Journal of Marine Science and Engineering 11, no. 5: 1040. https://doi.org/10.3390/jmse11051040
APA StyleZhang, Q., Li, Y., Liao, K., Chen, D., Qiu, Y., Yan, X., & Xu, J. (2023). mTOR Plays a Conserved Role in Regulation of Nutritional Metabolism in Bivalve Sinonovacula constricta. Journal of Marine Science and Engineering, 11(5), 1040. https://doi.org/10.3390/jmse11051040








