Seasonal Variability of Golden Tides (Pylaiella littoralis, Phaeophyceae) and Nutrient Dynamics in a Potentially Eutrophic Intertidal Estuary
Abstract
:1. Introduction
2. Materials and Methods
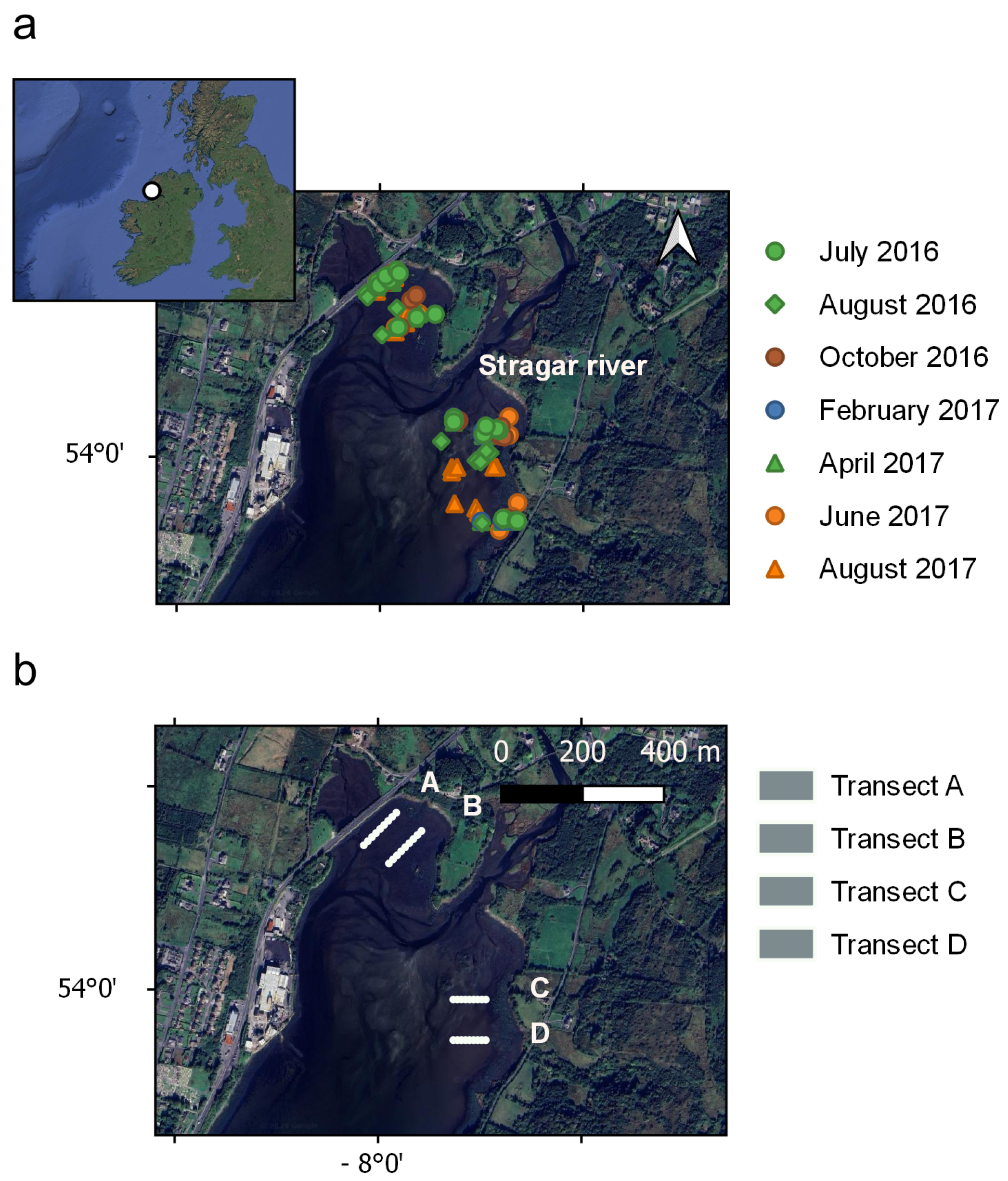
2.1. Study Area
2.2. Biomass Sample Collection
2.3. Tissue Nutrient Content and Isotopic Composition
2.4. Water Sample Collection
2.5. Spatiotemporal Patterns of NDVI from Sentinel-2A/B
2.6. Statistical Analysis
3. Results
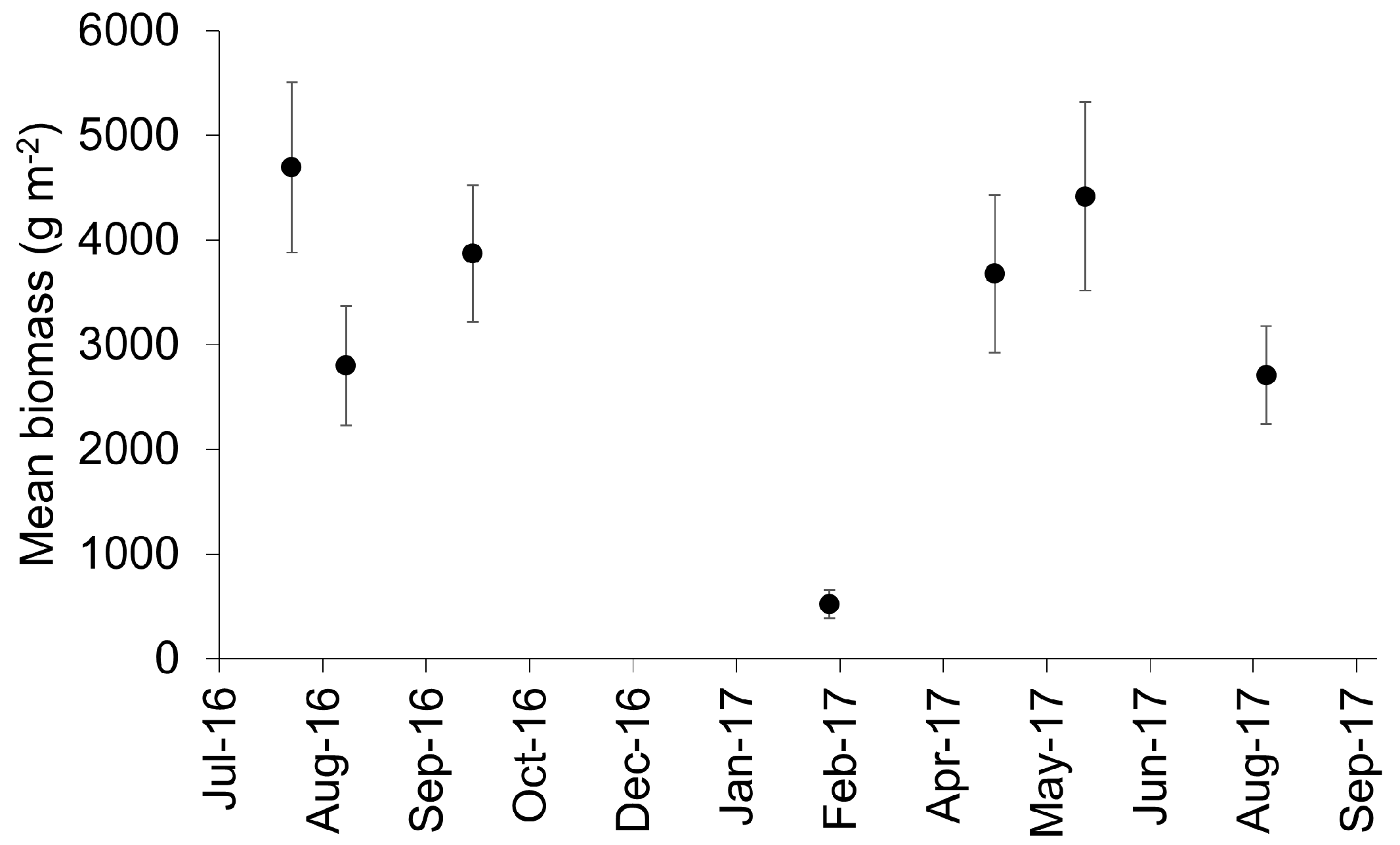
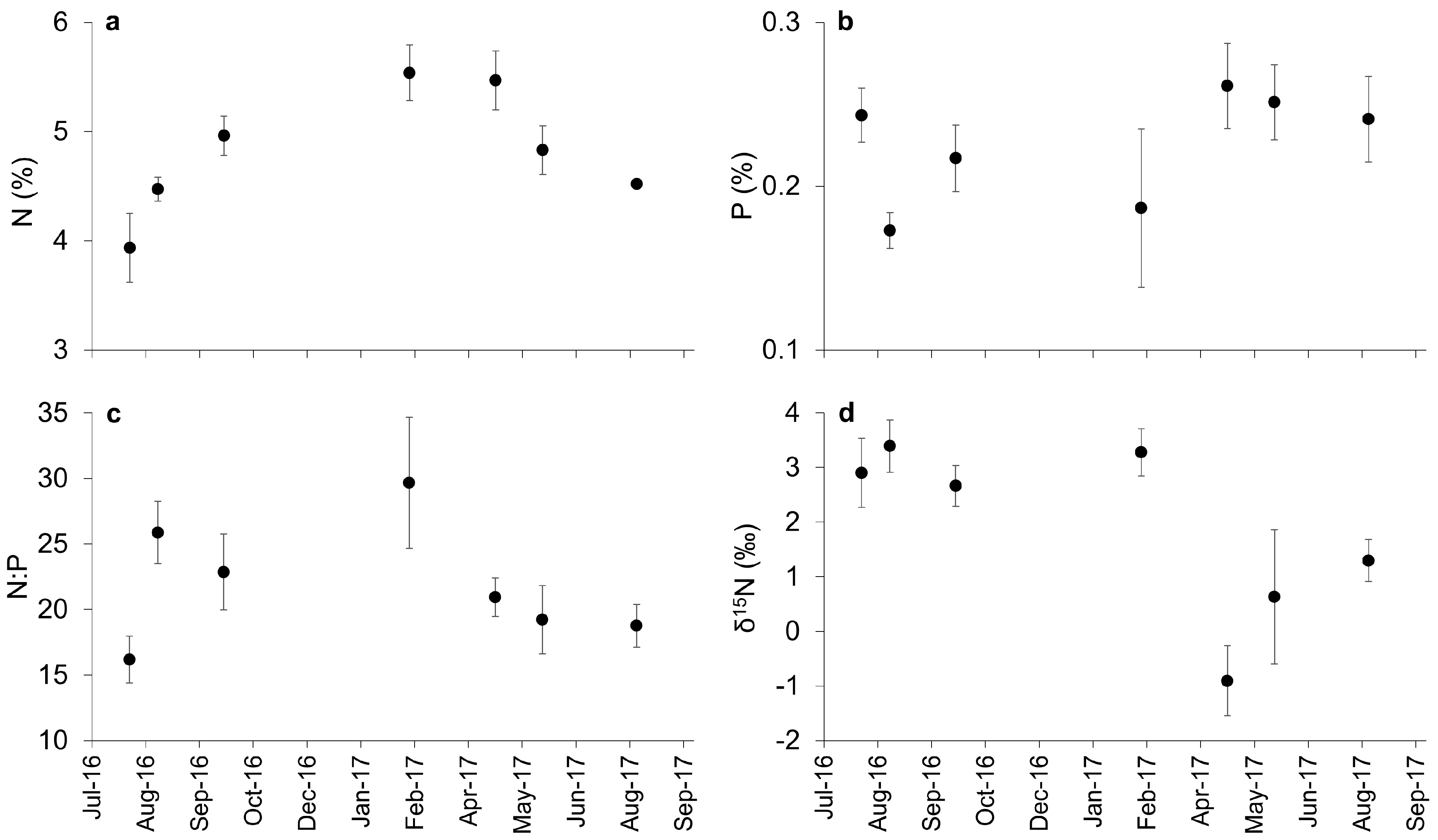
3.1. Biomonitoring Golden Tides: Biomass and Tissue Nutrient Content
3.2. Physical-Chemical Factors: Dissolved Seawater Nutrient Concentrations
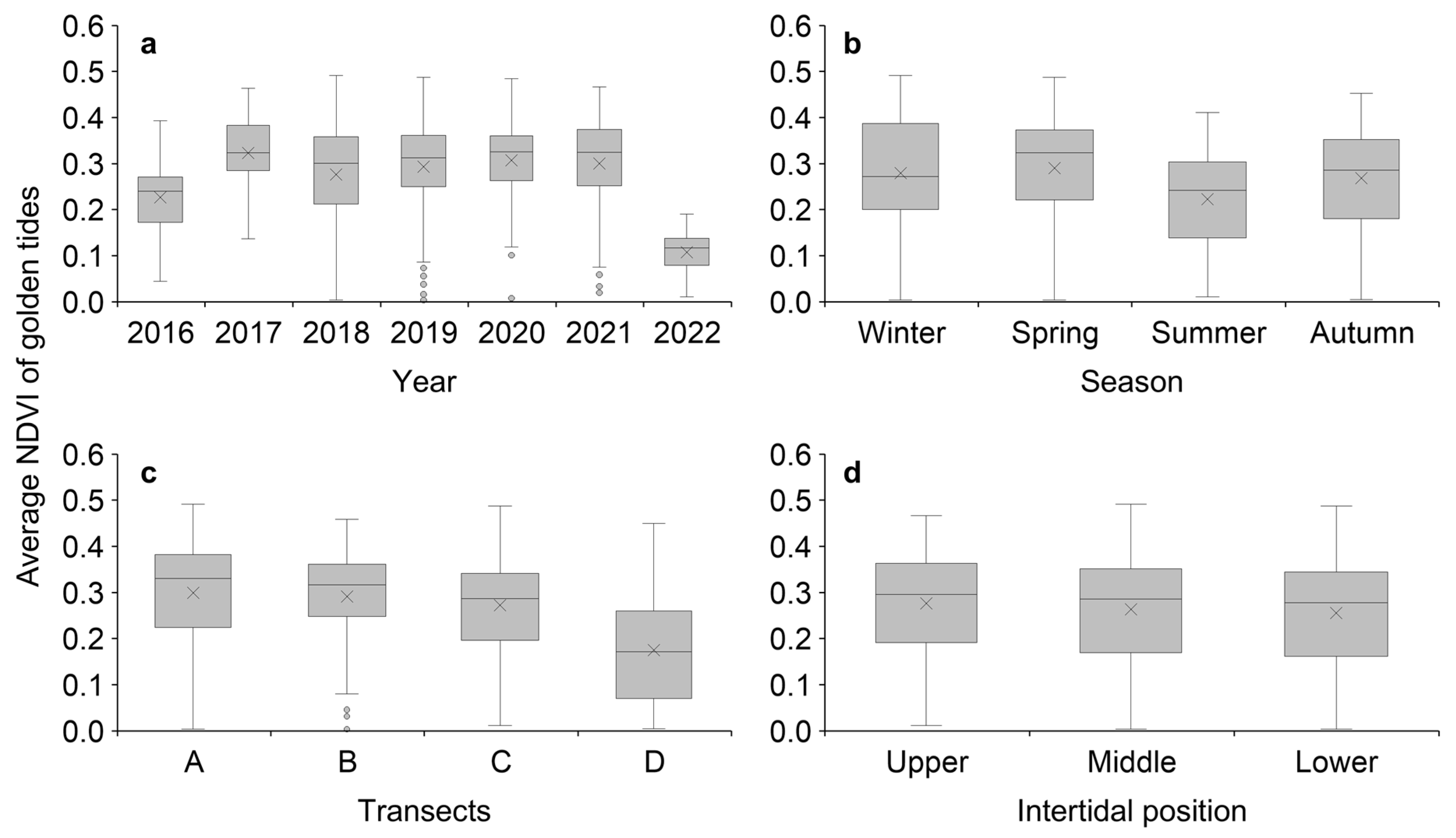
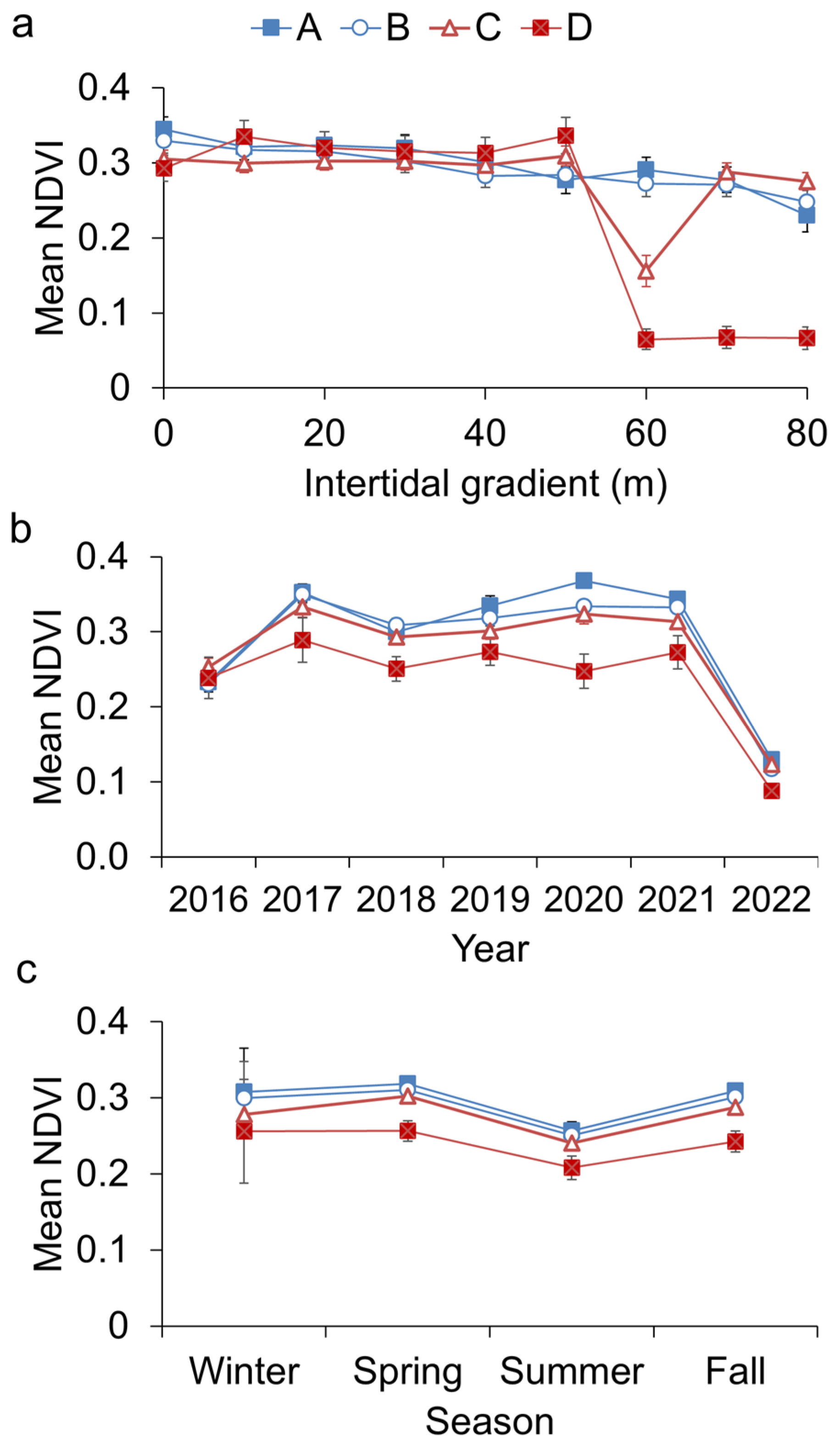
3.3. Spatiotemporal Variability of Golden Seaweed NDVI
4. Discussion
4.1. Identifying Nitrogen and Phosphorus Limitation, and Nitrogen Sources
4.2. Spatiotemporal Patterns of Golden Tides on the Shoreline
4.3. Nutrients in the Water Column
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennish, M.J. Environmental Threats and Environmental Future of Estuaries. Environ. Conserv. 2002, 29, 78–107. [Google Scholar] [CrossRef]
- Nelson, T.A.; Haberlin, K.; Nelson, A.V.; Ribarich, H.; Hotchkiss, R.; van Alstyne, K.L.; Buckingham, L.; Simunds, D.J.; Fredrickson, K. Ecological and Physiological Controls of Species Composition in Green Macroalgal Blooms. Ecology 2008, 89, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Gomes, L.; Pacheco, D.; Pereira, L. Ecosystem Services Provided by Seaweeds. Hydrobiology 2023, 2, 75–96. [Google Scholar] [CrossRef]
- Valiela, I.; McClelland, J.; Hauxwell, J.; Behr, P.J.; Hersh, D.; Foreman, K. Macroalgal Blooms in Shallow Estuaries: Controls and Ecophysiological and Ecosystem Consequences. Limnol. Oceanogr. 1997, 42, 1105–1118. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and Golden Seaweed Tides on the Rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, Harmful Algae and Biodiversity—Challenging Paradigms in a World of Complex Nutrient Changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef]
- Corzo, A.; Van Bergeijk, S.A.; Garcia-Robledo, E. Effects of Green Macroalgal Blooms on Intertidal Sediments: Net Metabolism and Carbon and Nitrogen Contents. Mar. Ecol. Prog. Ser. 2009, 380, 81–93. [Google Scholar] [CrossRef]
- Hering, D.; Borja, A.; Carstensen, J.; Carvalho, L.; Elliott, M.; Feld, C.K.; Heiskanen, A.S.; Johnson, R.K.; Moe, J.; Pont, D.; et al. The European Water Framework Directive at the Age of 10: A Critical Review of the Achievements with Recommendations for the Future. Sci. Total Environ. 2010, 408, 4007–4019. [Google Scholar] [CrossRef]
- Xing, Q.; Guo, R.; Wu, L.; An, D.; Cong, M.; Qin, S.; Li, X. High-Resolution Satellite Observations of a New Hazard of Golden Tides Caused by Floating Sargassum in Winter in the Yellow Sea. IEEE Geosci. Remote Sens. Lett. 2017, 14, 1815–1819. [Google Scholar] [CrossRef]
- Schreyers, L.; van Emmerik, T.; Biermann, L.; Le Lay, Y.-F. Spotting Green Tides over Brittany from Space: Three Decades of Monitoring with Landsat Imagery. Remote Sens. 2021, 13, 1408. [Google Scholar] [CrossRef]
- Haro, S.; Morrison, L.; Caballero, I.; Figueroa, F.L.; Korbee, N.; Navarro, G.; Bermejo, R. Assessing Golden Tides from Space: Meteorological Drivers in the Accumulation of the Invasive Algae Rugulopteryx Okamurae on Coasts. Remote Sens. 2024, 16, 2689. [Google Scholar] [CrossRef]
- Bermejo, R.; Green-Gavrielidis, L.; Gao, G. Editorial: Macroalgal Blooms in a Global Change Context. Front. Mar. Sci. 2023, 10, 1204117. [Google Scholar] [CrossRef]
- Steele, D.H.; Whittick, A. Seasonal Variation in Pilayella littoralis (Phaeophyceae) and Its Consequences as a Food Source for the Amphipod, Gammarus lawrencianus, in the Intertidal of Newfoundland. J. Mar. Biol. Assoc. UK 1991, 71, 883–889. [Google Scholar] [CrossRef]
- Jeffrey, D.W.; Madden, B.; Rafferty, B. Beach Fouling by Ectocarpus Siliculosus in Dublin Bay. Mar. Pollut. Bull. 1993, 26, 51–53. [Google Scholar] [CrossRef]
- Lotze, H.K.; Schramm, W.; Schories, D.; Worm, B. Control of Macroalgal Blooms at Early Developmental Stages: Pilayella littoralis versus Enteromorpha spp. Oecologia 1999, 119, 46–54. [Google Scholar] [CrossRef]
- Haro, S.; Bermejo, R.; Wilkes, R.; Bull, L.; Morrison, L. Monitoring Intertidal Golden Tides Dominated by Ectocarpus siliculosus Using Sentinel-2 Imagery. Int. J. Appl. Earth Obs. Geoinf. 2023, 122, 103451. [Google Scholar] [CrossRef]
- Pregnall, A.M.; Miller, S.L. Flux of Ammonium from Surf-Zone and Nearshore Sediments in Nahant Bay, Massachusetts, USA in Relation to Free-Living Pilayella littoralis. Mar. Ecol. Prog. Ser. 1988, 50, 161–167. [Google Scholar] [CrossRef]
- Berglund, J.; Mattila, J.; Rönnberg, O.; Heikkilä, J.; Bonsdorff, E. Seasonal and Inter-Annual Variation in Occurrence and Biomass of Rooted Macrophytes and Drift Algae in Shallow Bays. Estuar. Coast. Shelf Sci. 2003, 56, 1167–1175. [Google Scholar] [CrossRef]
- Lotze, H.K.; Worm, B.; Sommer, U. Strong Bottom-up and Top-down Control of Early Life Stages of Macroalgae. Limnol. Oceanogr. 2001, 46, 749–757. [Google Scholar] [CrossRef]
- Bermejo, R.; Heesch, S.; Donnell, M.O.; Golden, N.; Mac Monagail, M.; Edwards, M.; Curley, E.; Fenton, O.; Daly, E.; Morrison, L. Nutrient Dynamics and Ecophysiology of Opportunistic Macroalgal Blooms in Irish Estuaries and Coastal Bays (Sea-MAT). Environ. Prot. Agency Res. Rep. 2019, 56, 1–56. [Google Scholar]
- Bermejo, R.; Heesch, S.; Mac Monagail, M.; O’Donnell, M.; Daly, E.; Wilkes, R.J.; Morrison, L. Spatial and Temporal Variability of Biomass and Composition of Green Tides in Ireland. Harmful Algae 2019, 81, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, R.; MacMonagail, M.; Heesch, S.; Mendes, A.; Edwards, M.; Fenton, O.; Knöller, K.; Daly, E.; Morrison, L. The Arrival of a Red Invasive Seaweed to a Nutrient Over-Enriched Estuary Increases the Spatial Extent of Macroalgal Blooms. Mar. Environ. Res. 2020, 158, 104944. [Google Scholar] [CrossRef] [PubMed]
- Zoffoli, L.M.; Gernez, P.; Godet, L.; Peters, S.; Oiry, S.; Barillé, L. Decadal Increase in the Ecological Status of a North-Atlantic Intertidal Seagrass Meadow Observed with Multi-Mission Satellite Time-Series. Ecol. Indic. 2021, 130, 108033. [Google Scholar] [CrossRef]
- Li, Y.; Gundersen, H.; Poulsen, R.N.; Xie, L.; Ge, Z.; Hancke, K. Quantifying Seaweed and Seagrass Beach Deposits Using High-Resolution UAV Imagery. J. Environ. Manag. 2023, 331, 117171. [Google Scholar] [CrossRef]
- Karki, S.; Bermejo, R.; Wilkes, R.; Monagail, M.M.; Daly, E.; Healy, M.; Hanafin, J.; McKinstry, A.; Mellander, P.-E.; Fenton, O.; et al. Mapping Spatial Distribution and Biomass of Intertidal Ulva Blooms Using Machine Learning and Earth Observation. Front. Mar. Sci. 2021, 8, 633128. [Google Scholar] [CrossRef]
- Haro, S.; Jesus, B.; Oiry, S.; Papaspyrou, S.; Lara, M.; González, C.J.; Corzo, A. Microphytobenthos Spatio-Temporal Dynamics across an Intertidal Gradient Using Random Forest Classification and Sentinel-2 Imagery. Sci. Total Environ. 2022, 804, 149983. [Google Scholar] [CrossRef]
- Haro, S.; Jimenez-Reina, J.; Bermejo, R.; Morrison, L. SoftwareX BioIntertidal Mapper Software: A Satellite Approach for NDVI-Based Intertidal Habitat Mapping. SoftwareX 2023, 24, 101520. [Google Scholar] [CrossRef]
- Viaroli, P.; Bartoli, M.; Giordani, G.; Naldi, M. Community Shifts, Alternative Stable States, Biogeochemical Controls and Feedbacks in Eutrophic Coastal Lagoons: A Brief Overview. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 105–117. [Google Scholar] [CrossRef]
- Lalegerie, F.; Gager, L.; Stiger-Pouvreau, V.; Connan, S. The Stressful Life of Red and Brown Seaweeds on the Temperate Intertidal Zone: Effect of Abiotic and Biotic Parameters on the Physiology of Macroalgae and Content Variability of Particular Metabolites. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 95, pp. 247–287. ISBN 0065-2296. [Google Scholar]
- Zhang, D.; Zhang, Q.S.; Yang, X.Q. Plant Physiology and Biochemistry Seasonal Dynamics of Photosynthetic Activity in the Representive Brown Macroalgae Sagrassum thunbergii (Sargassaceae phaeophyta). Plant Physiol. Biochem. 2017, 120, 88–94. [Google Scholar] [CrossRef]
- Howarth, R.; Paerl, H.W. Coastal Marine Eutrophication: Control of Both Nitrogen and Phosphorus Is Necessary. Proc. Natl. Acad. Sci. USA 2008, 105, 2008. [Google Scholar] [CrossRef]
- Lapointe, B.E. Nutrient Thresholds for Bottom-up Control of Macroalgal Blooms on Coral Reefs in Jamaica and Southeast Florida. Limnol. Oceanogr. 1997, 42, 1119–1131. [Google Scholar] [CrossRef]
- Howarth, R.W.; Anderson, D.B.; Cloern, J.E.; Elfring, C.; Hopkinson, C.S.; Lapointe, B.; Maloney, T.J.; Marcus, N.; McGlathery, K.; Sharpley, A.N. Issues in Ecology: Nutrient Pollution in Coastal Rivers, Bays, and Seas; United States Geological Survey: Reston, VT, USA, 2000. [Google Scholar]
- Teichberg, M.; Fox, S.E.; Olsen, Y.S.; Valiela, I.; Martinetto, P.; Iribarne, O.; Muto, E.Y.; Petti, M.; Corbisier, T.N.; Soto-Jimenez, M.; et al. Eutrophication and Macroalgal Blooms in Temperate and Tropical Coastal Waters: Nutrient Enrichment Experiments with Ulva spp. Glob. Change Biol. 2010, 16, 2624–2637. [Google Scholar] [CrossRef]
- Costanzo, S.D.; O’Donohue, M.J.; Dennison, W.C. Gracilaria edulis (Rhodophyta) as a Biological Indicator of Pulsed Nutrients in Oligotrophic Waters. J. Phycol. 2000, 36, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, R.; Golden, N.; Schrofner, E.; Knöller, K.; Fenton, O.; Serrão, E.; Morrison, L. Biomass and Nutrient Dynamics of Major Green Tides in Ireland: Implications for Biomonitoring. Mar. Pollut. Bull. 2022, 175, 113318. [Google Scholar] [CrossRef]
- Licata, P.; Trombetta, D.; Cristani, M.; Martino, D.; Naccari, F. Organochlorine Compounds and Heavy Metals in the Soft Tissue of the Mussel Mytilus Galloprovincialis Collected from Lake Faro (Sicily, Italy). Environ. Int. 2004, 30, 805–810. [Google Scholar] [CrossRef]
- Viana, I.G.; Bode, A. Stable Nitrogen Isotopes in Coastal Macroalgae: Geographic and Anthropogenic Variability. Sci. Total Environ. 2013, 443, 887–895. [Google Scholar] [CrossRef]
- Viana, I.G.; Bode, A. Variability in δ 15 N of Intertidal Brown Algae along a Salinity Gradient: Differential Impact of Nitrogen Sources. Sci. Total Environ. 2015, 512–513, 167–176. [Google Scholar] [CrossRef]
- Lyngby, J.E.; Mortensen, S.; Ahrensberg, N. Bioassessment Techniques for Monitoring of Eutrophication and Nutrient Limitation in Coastal Ecosystems. Mar. Pollut. Bull. 1999, 39, 212–223. [Google Scholar] [CrossRef]
- Fong, P.; Boyer, K.E.; Zedler, J.B. Developing an Indicator of Nutrient Enrichment in Coastal Estuaries and Lagoons Using Tissue Nitrogen Content of the Opportunistic Alga, Enteromorpha intestinalis (L. Link). J. Exp. Mar. Bio. Ecol. 1998, 231, 63–79. [Google Scholar] [CrossRef]
- Björnsäter, B.R.; Wheeler, P.A. Effect of Nitrogen and Phosphorus Supply on Growth and Tissue Composition of Ulva fenestrata and Enteromorpha intestinalis (Ulvales, Chlorophyta). J. Phycol. 1990, 26, 603–611. [Google Scholar] [CrossRef]
- Mariotti, A.; Germon, J.C.; Hubert, P.; Kaiser, P.; Letolle, R.; Tardieux, A.; Tardieux, P. Experimental Determination of Nitrogen Kinetic Isotope Fractionation: Some Principles; Illustration for the Denitrification and Nitrification Processes. Plant Soil 1981, 62, 413–430. [Google Scholar] [CrossRef]
- Lin, D.T.; Fong, P. Macroalgal Bioindicators (Growth, Tissue N, Δ15N) Detect Nutrient Enrichment from Shrimp Farm Effluent Entering Opunohu Bay, Moorea, French Polynesia. Mar. Pollut. Bull. 2008, 56, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.P.; Peralta, G.; Benavente, J.; Freitas, R.; Rodrigues, A.M.; Quintino, V.; Alvarez, O.; Valcárcel-pérez, N. Caulerpa Prolifera Stable Isotope Ratios Reveal Anthropogenic Nutrients within a Tidal Lagoon. Mar. Ecol. Prog. Ser. 2009, 390, 117–128. [Google Scholar] [CrossRef]
- Piñón-Gimate, A.; Espinosa-Andrade, N.; Sánchez, A.; Casas-Valdez, M. Nitrogen Isotopic Characterisation of Macroalgae Blooms from Different Sites within a Subtropical Bay in the Gulf of California. Mar. Pollut. Bull. 2017, 116, 130–136. [Google Scholar] [CrossRef]
- Viana, I.G.; Fernández, J.A.; Aboal, J.R.; Carballeira, A. Measurement of Δ15N in Macroalgae Stored in an Environmental Specimen Bank for Regional Scale Monitoring of Eutrophication in Coastal Areas. Ecol. Indic. 2011, 11, 888–895. [Google Scholar] [CrossRef]
- Gröcke, D.R.; Sauer, P.E.; Bridault, A.; Drucker, D.G.; Germonpré, M.; Bocherens, H. Hydrogen Isotopes in Quaternary Mammal Collagen from Europe. J. Archaeol. Sci. Rep. 2017, 11, 12–16. [Google Scholar] [CrossRef]
- Kjellman, F.R. Pylaiella Littoralis (Linnaeus) Kjellman, 1872. Bih. till Kongliga Sven. Vetenskaps-Akademiens Handl. 1872, 1, 1–24. [Google Scholar]
- Irish Waters Killybegs WWTP; AER 2011; D011. 2011. Available online: https://epawebapp.epa.ie/licences/lic_eDMS/090151b28044d75e.pdf (accessed on 28 October 2024).
- Irish Water Annual Environmental Report. 2018. Available online: https://www.water.ie/sites/default/files/docs/aers/2018/Non-Priority-AERS/D0367-01_2018_aer.pdf (accessed on 28 October 2024).
- Bermejo, R.; Golden, N.; Haro, S.; Karki, S.; Macmonagail, M.; García-Poza, S.; Navarrete-Fernández, T.; Benedikt Brunner, K.K.; Mark Healy, O.; Fenton, P.-E.M.; et al. MACROalgal Blooms in Transitional and Coastal Waters; MANagement-Pressures, Policy and Solutions (MACRO-MAN). Environ. Prot. Agency Res. Rep. 2023, 3–14. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis. Bull. Fish. Res. Board Can. 1968, 167, 1–311. [Google Scholar]
- Fox, J. Applied Regression Analysis and Generalized Linear Models, 2nd ed.; SAGE Publications: New York, NY, USA, 2008. [Google Scholar]
- McArdle, B.H.; Anderson, M.J. Fitting Multivariate Models to Community Data: A Comment on Distance-Based Redundancy Analysis. Ecology 2001, 82, 290. [Google Scholar] [CrossRef]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.6-6 2023. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 25 October 2024).
- Martinez Arbizu, P. PairwiseAdonis: Pairwise Multilevel Comparison Using Adonis, R Package Version 0.4 2020. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 25 October 2024).
- Campbell, S. Ammonium Requirements of Fast-Growing Ephemeral Macroalgae in a Nutrient-Enriched Marine Embayment (Port Phillip Bay, Australia). Mar. Ecol. Prog. Ser. 2001, 209, 99–107. [Google Scholar] [CrossRef]
- Atkinson, M.J.; Smith, S.V. C:N:P Ratios of Benthic Marine Plants. Limnol. Oceanogr. 1983, 28, 568–574. [Google Scholar] [CrossRef]
- Duarte, C.M. Nutrient Concentration of Aquatic Plants: Patterns across Species. Limnol. Oceanogr. 1992, 37, 882–889. [Google Scholar] [CrossRef]
- McGillicuddy, D.J.; Morton, P.L.; Brewton, R.A.; Hu, C.; Kelly, T.B.; Solow, A.R.; Lapointe, B.E. Nutrient and Arsenic Biogeochemistry of Sargassum in the Western Atlantic. Nat. Commun. 2023, 14, 6205. [Google Scholar] [CrossRef]
- Huang, S.X.; Jiang, Q.; Ding, Y.F.; Wang, F.J.; Zhu, C. Arsenic Contents and Speciation at Different Growth Stages of Sargassum fusiforme [Harv.] Setchell (Hijiki), an Edible Seaweed. Appl. Ecol. Environ. Res. 2020, 18, 1941–1952. [Google Scholar] [CrossRef]
- Villares, R.; Carballeira, A. Nutrient Limitation in Macroalgae (Ulva and Enteromorpha) from the Rías Baixas (NW Spain). Mar. Ecol. 2004, 25, 225–243. [Google Scholar] [CrossRef]
- Bermejo, R.; Galindo-Ponce, M.; Golden, N.; Linderhoff, C.; Heesch, S.; Hernández, I.; Morrison, L. Two Bloom-forming Species of Ulva (Chlorophyta) Show Different Responses to Seawater Temperature and No Antagonistic Interaction. J. Phycol. 2023, 59, 167–178. [Google Scholar] [CrossRef]
- Hernández, I.; Pérez-Pastor, A.; Mateo, J.J.; Megina, C.; Vergara, J.J. Growth Dynamics of Ulva rotundata (Chlorophyta) in a Fish Farm: Implications for Biomitigation at a Large Scale. J. Phycol. 2008, 44, 1080–1089. [Google Scholar] [CrossRef]
- Pedersen, M.F.; Johnsen, K.L. Nutrient (N and P) Dynamics of the Invasive Macroalga Gracilaria Vermiculophylla: Nutrient Uptake Kinetics and Nutrient Release through Decomposition. Mar. Biol. 2017, 164, 1–12. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D. Fertilizer Nitrogen Isotope Signatures. Isotopes Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef]
- Cohen, R.A.; Fong, P. Using Opportunistic Green Macroalgae as Indicators of Nitrogen Supply and Sources to Estuaries. Ecol. Appl. 2006, 16, 1405–1420. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Chapter 9: The Marine Environment; Routledge: London, UK, 2024. [Google Scholar]
- Environmental Protection Agency. Water Quality in Ireland 2016–2021; Environmental Protection Agency: Wexford, Ireland, 2022. [Google Scholar]
- Jeffrey, D.W.; Brennan, M.T.; Jennings, E.; Madden, B.; Wilson, J.G. Nutrient Sources for In-Shore Nuisance Macroalgae: The Dublin Bay Case. Ophelia 1995, 42, 147–161. [Google Scholar] [CrossRef]
- Salovius, S.; Bonsdorff, E. Effects of Depth, Sediment and Grazers on the Degradation of Drifting Filamentous Algae (Cladophora glomerata and Pilayella littoralis). J. Exp. Mar. Bio. Ecol. 2004, 298, 93–109. [Google Scholar] [CrossRef]
- Hayden, H.S.; Blomster, J.; Maggs, C.A.; Silva, P.C.; Stanhope, M.J.; Waaland, J.R. Linnaeus Was Right All along: Ulva and Enteromorpha Are Not Distinct Genera. Eur. J. Phycol. 2003, 38, 277–294. [Google Scholar] [CrossRef]
- Råberg, S.; Berger-Jönsson, R.; Björn, A.; Granéli, E.; Kautsky, L. Effects of Pilayella littoralis on Fucus Vesiculosus Recruitment: Implications for Community Composition. Mar. Ecol. Prog. Ser. 2005, 289, 131–139. [Google Scholar] [CrossRef]
- Zoffoli, L.M.; Gernez, P.; Rosa, P.; Le, A.; Brando, V.E.; Barillé, A.; Harin, N.; Peters, S.; Poser, K.; Spaias, L.; et al. Remote Sensing of Environment Sentinel-2 Remote Sensing of Zostera Noltei-Dominated Intertidal Seagrass Meadows. Remote Sens. Environ. 2020, 251, 112020. [Google Scholar] [CrossRef]
- Jacobs, P.; Pitarch, J.; Kromkamp, J.C.; Philippart, C.J.M. Assessing Biomass and Primary Production of Microphytobenthos in Depositional Coastal Systems Using Spectral Information. PLoS ONE 2021, 16, e0246012. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, B.; Wang, Y.; Liu, D.; Zhou, Y.; van der Wal, D. Mapping Depth-Integrated Microphytobenthic Biomass on an Estuarine Tidal Flat Using Sentinel Satellite Data. Int. J. Appl. Earth Obs. Geoinf. 2023, 122, 103417. [Google Scholar] [CrossRef]
- Ní Longphuirt, S.; O’Boyle, S.; Stengel, D.B. Environmental Response of an Irish Estuary to Changing Land Management Practices. Sci. Total Environ. 2015, 521–522, 388–399. [Google Scholar] [CrossRef]
- Longphuirt, S.N.; Mockler, E.M.; O’Boyle, S.; Wynne, C.; Stengel, D.B. Linking Changes in Nutrient Source Load to Estuarine Responses: An Irish Perspective. Biol. Environ. Proc. R. Irish Acad. 2016, 116B, 295. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Global Nitrogen and Phosphorus Fertilizer Use for Agriculture Production in the Past Half Century: Shifted Hot Spots and Nutrient Imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- Trodd, W.; O’Boyle, S. Water Quality in 2020; EPA: Wexford, Ireland, 2021. [Google Scholar]

| Factors | Df | Sum of Sqs | R2 | F | Pr (>F) |
|---|---|---|---|---|---|
| Year | 6 | 4.69 | 0.320 | 188.23 | 0.001 |
| Transects | 3 | 2.25 | 0.154 | 181.16 | 0.001 |
| Season | 3 | 0.88 | 0.060 | 70.51 | 0.001 |
| Year:Season | 9 | 0.79 | 0.054 | 21.15 | 0.001 |
| Year:Season:Transects | 27 | 0.41 | 0.028 | 3.64 | 0.001 |
| Transects:Intertidal | 6 | 0.31 | 0.021 | 12.62 | 0.001 |
| Year:Transects | 18 | 0.28 | 0.019 | 3.69 | 0.001 |
| Year:Season:Transects:Intertidal | 52 | 0.21 | 0.014 | 0.98 | 0.533 |
| Year:Season:Intertidal | 18 | 0.19 | 0.013 | 2.52 | 0.001 |
| Season:Transects | 9 | 0.18 | 0.012 | 4.85 | 0.001 |
| Year:Transects:Intertidal | 36 | 0.01 | 0.009 | 0.85 | 0.707 |
| Season:Transects:Intertidal | 16 | 0.12 | 0.008 | 1.76 | 0.003 |
| Intertidal | 2 | 0.11 | 0.008 | 13.32 | 0.001 |
| Season:Intertidal | 6 | 0.09 | 0.006 | 3.47 | 0.002 |
| Residual | 953 | 3.95 | 0.320 | ||
| Total | 1176 | 14.66 | 0.154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haro, S.; Bermejo, R.; Healy, M.G.; Knöeller, K.; Fenton, O.; Heesch, S.; Morrison, L. Seasonal Variability of Golden Tides (Pylaiella littoralis, Phaeophyceae) and Nutrient Dynamics in a Potentially Eutrophic Intertidal Estuary. J. Mar. Sci. Eng. 2024, 12, 2336. https://doi.org/10.3390/jmse12122336
Haro S, Bermejo R, Healy MG, Knöeller K, Fenton O, Heesch S, Morrison L. Seasonal Variability of Golden Tides (Pylaiella littoralis, Phaeophyceae) and Nutrient Dynamics in a Potentially Eutrophic Intertidal Estuary. Journal of Marine Science and Engineering. 2024; 12(12):2336. https://doi.org/10.3390/jmse12122336
Chicago/Turabian StyleHaro, Sara, Ricardo Bermejo, Mark G. Healy, Kay Knöeller, Owen Fenton, Svenja Heesch, and Liam Morrison. 2024. "Seasonal Variability of Golden Tides (Pylaiella littoralis, Phaeophyceae) and Nutrient Dynamics in a Potentially Eutrophic Intertidal Estuary" Journal of Marine Science and Engineering 12, no. 12: 2336. https://doi.org/10.3390/jmse12122336
APA StyleHaro, S., Bermejo, R., Healy, M. G., Knöeller, K., Fenton, O., Heesch, S., & Morrison, L. (2024). Seasonal Variability of Golden Tides (Pylaiella littoralis, Phaeophyceae) and Nutrient Dynamics in a Potentially Eutrophic Intertidal Estuary. Journal of Marine Science and Engineering, 12(12), 2336. https://doi.org/10.3390/jmse12122336





